14.4 Gluconeogenesis
The central role of glucose in metabolism arose early in evolution, and this sugar remains the nearly universal fuel and building block in modern organisms, from microbes to humans. In mammals, some tissues depend almost completely on glucose for their metabolic energy. For the human brain and nervous system, as well as the erythrocytes, testes, renal medulla, and embryonic tissues, glucose from the blood is the sole or major fuel source. The brain alone requires about 120 g of glucose each day — more than half of all the glucose stored as glycogen in muscle and liver. However, the supply of glucose from these stores is not always sufficient; between meals and during longer fasts, or after vigorous exercise, glycogen is depleted. For these times, organisms need a method for synthesizing glucose from noncarbohydrate precursors. This is accomplished by a pathway called gluconeogenesis (“new formation of sugar”), which converts pyruvate and related three- and four-carbon compounds to glucose.
Gluconeogenesis occurs in all animals, plants, fungi, and microorganisms. The reactions are essentially the same in all tissues and all species. The important precursors of glucose in animals are three-carbon compounds such as lactate, pyruvate, and glycerol, as well as certain amino acids (Fig. 14-15). In mammals, gluconeogenesis takes place mainly in the liver, and to a lesser extent in the renal cortex and in the epithelial cells that line the small intestine. The glucose produced passes into the blood to supply other tissues. After vigorous exercise, lactate produced by anaerobic glycolysis in skeletal muscle returns to the liver and is converted to glucose, which moves back to muscle and is converted to glycogen — a circuit called the Cori cycle (see Fig. 23-17). In plant seedlings, stored fats and proteins are converted, via paths that include gluconeogenesis, to the disaccharide sucrose for transport throughout the developing plant. Glucose and its derivatives are precursors for the synthesis of plant cell walls, nucleotides and coenzymes, and a variety of other essential metabolites. In many microorganisms, gluconeogenesis starts from simple organic compounds of two or three carbons, such as acetate, lactate, and propionate, in their growth medium.
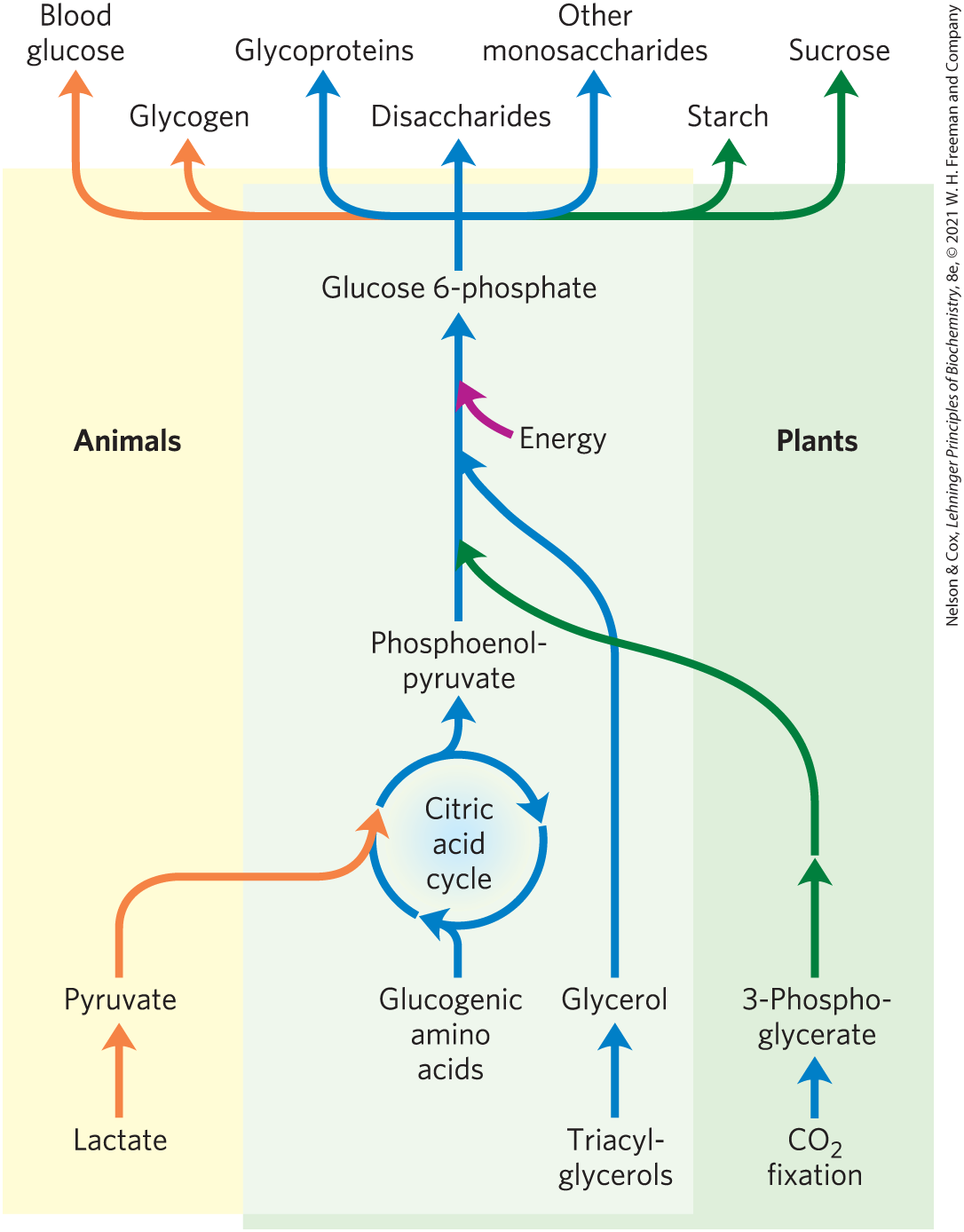
FIGURE 14-15 Carbohydrate synthesis from simple precursors. The pathway from phosphoenolpyruvate to glucose 6-phosphate is common to the biosynthetic conversion of many different precursors of carbohydrates in animals and plants. The path from pyruvate to phosphoenolpyruvate leads through oxaloacetate, an intermediate of the citric acid cycle, which we discuss in Chapter 16. Any compound that can be converted to either pyruvate or oxaloacetate can therefore serve as starting material for gluconeogenesis. This includes alanine and aspartate, which are convertible to pyruvate and oxaloacetate, respectively, and other amino acids that can also yield three- or four-carbon fragments, the so-called glucogenic amino acids. Plants and photosynthetic bacteria are uniquely able to convert to carbohydrates, using the Calvin cycle, as we shall see in Section 20.4.
Although the reactions of gluconeogenesis are the same in all organisms, the metabolic context and the regulation of the pathway differ from one species to another and from tissue to tissue. In this section we focus on gluconeogenesis as it occurs in the mammalian liver. In Chapter 20 we show how photosynthetic organisms use this pathway to convert the primary products of photosynthesis into glucose, to be stored as sucrose or starch.
Gluconeogenesis and glycolysis are not identical pathways running in opposite directions, although they do share several steps (Fig. 14-16); 7 of the 10 enzymatic reactions of gluconeogenesis are the reverse of glycolytic reactions. However, three reactions of glycolysis are essentially irreversible in vivo and cannot be used in gluconeogenesis: the conversion of glucose to glucose 6-phosphate by hexokinase, the phosphorylation of fructose 6-phosphate to fructose 1,6-bisphosphate by phosphofructokinase-1, and the conversion of phosphoenolpyruvate to pyruvate by pyruvate kinase. In cells, these three reactions are characterized by a large negative free-energy change, whereas other glycolytic reactions have a near 0 (Table 14-2). In gluconeogenesis, the three irreversible steps are bypassed by a separate set of enzymes, catalyzing reactions that are sufficiently exergonic to be effectively irreversible in the direction of glucose synthesis. Thus, both glycolysis and gluconeogenesis are irreversible processes in cells. In animals, both pathways occur largely in the cytosol, necessitating their reciprocal and coordinated regulation, described in Section 14.5.
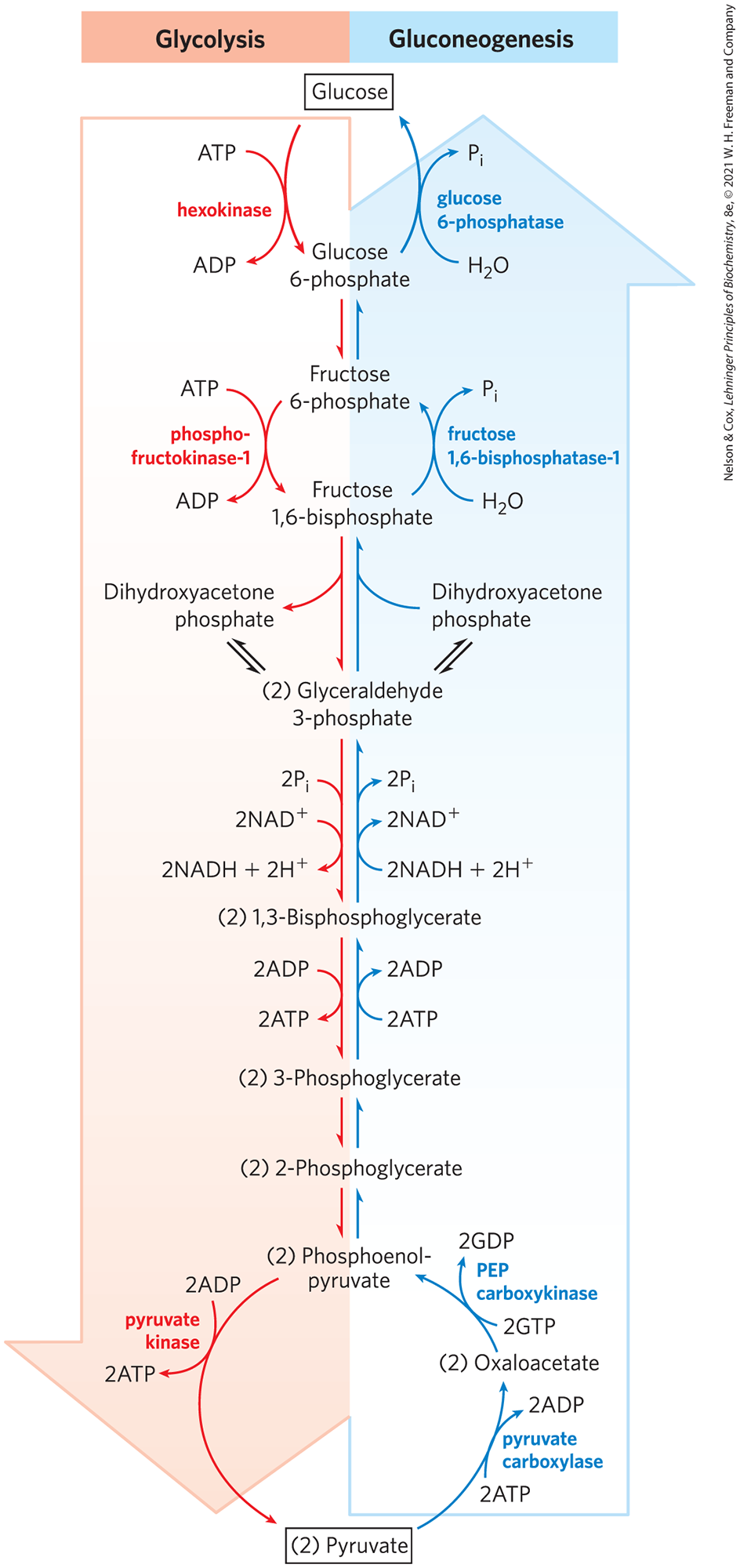
FIGURE 14-16 Opposing pathways of glycolysis and gluconeogenesis in liver. The reactions of glycolysis are on the left side, in red; the opposing pathway of gluconeogenesis is on the right, in blue. The major sites of regulation of gluconeogenesis shown here are discussed in Section 14.5.
Glycolytic reaction step |
||
|---|---|---|
|
||
|
1.7 |
0 to 25 |
|
||
|
23.8 |
|
|
7.5 |
0 to 4 |
|
6.3 |
|
|
0 to 2 |
|
|
4.4 |
0 to 0.8 |
|
7.5 |
0 to 3.3 |
|
We begin by considering the three bypass reactions of gluconeogenesis. (Keep in mind that “bypass” refers throughout to the bypass of irreversible glycolytic reactions.)
The First Bypass: Conversion of Pyruvate to Phosphoenolpyruvate Requires Two Exergonic Reactions
The first of the bypass reactions in gluconeogenesis is the conversion of pyruvate to phosphoenolpyruvate (PEP). This reaction cannot occur by simple reversal of the pyruvate kinase reaction of glycolysis (p. 521), which has a large, negative free-energy change and is therefore irreversible under the conditions prevailing in intact cells (Table 14-2, step ). Instead, the phosphorylation of pyruvate is achieved by a roundabout sequence of reactions that in eukaryotes requires enzymes in both the cytosol and mitochondria. As we shall see, the pathway shown in Figure 14-16 and described in detail here is one of two routes from pyruvate to PEP; it is the predominant path when pyruvate or alanine is the glucogenic precursor. A second pathway, described later, predominates when lactate is the glucogenic precursor.
Pyruvate is first transported from the cytosol into mitochondria or is generated from alanine within mitochondria by transamination, in which the α-amino group is transferred from alanine (leaving pyruvate) to an α-keto carboxylic acid (transamination reactions are discussed in detail in Chapter 18). Then pyruvate carboxylase, a mitochondrial enzyme that requires the coenzyme biotin, converts the pyruvate to oxaloacetate:
(14-4)
The carboxylation reaction involves biotin as a carrier of activated bicarbonate, as shown in Figure 14-17; the reaction mechanism is shown in Figure 16-16. (Note that is formed by ionization of carbonic acid formed from .) is phosphorylated by ATP to form a mixed anhydride (a carboxyphosphate); then biotin displaces the phosphate in the formation of carboxybiotin.
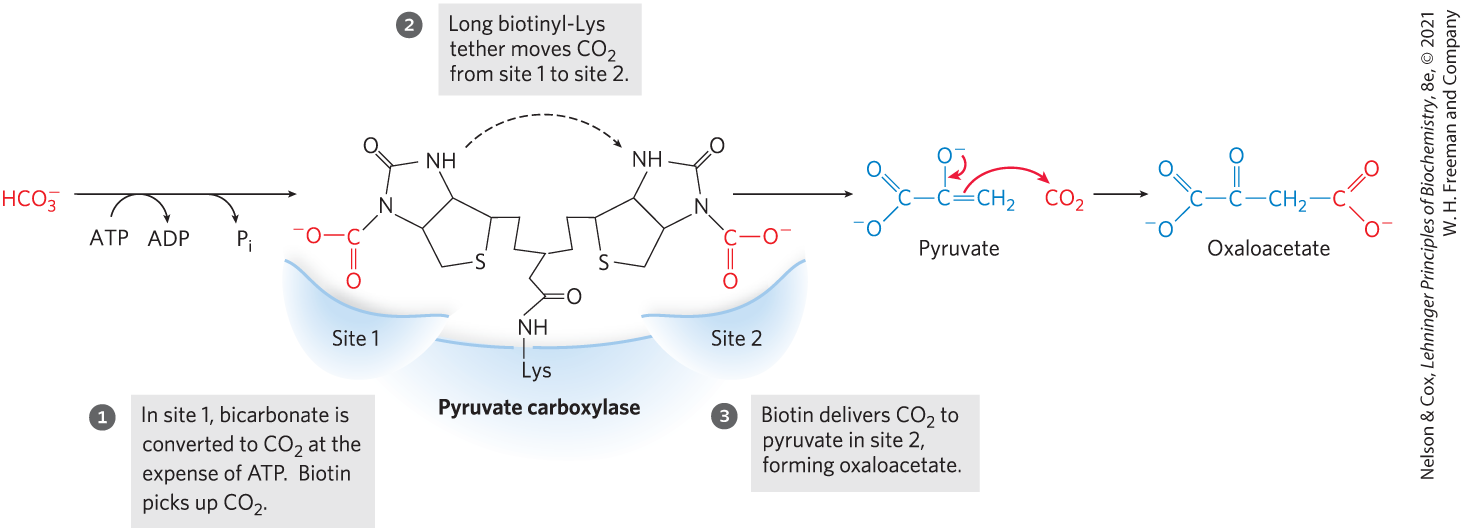
FIGURE 14-17 Role of biotin in the pyruvate carboxylase reaction. The cofactor biotin is covalently attached to pyruvate carboxylase through an amide linkage to the ε-amino group of a Lys residue, forming a biotinyl-enzyme. The reaction takes place in two phases, which occur at two different sites in the enzyme. The long biotinyl-Lys arm carries the substrate from one site to the other.
Pyruvate carboxylase is the first regulatory enzyme in the gluconeogenic pathway, requiring acetyl-CoA as a positive effector. Acetyl-CoA is produced by fatty acid oxidation (Chapter 17), and its accumulation signals the availability of fatty acids as fuel. As we shall see in Chapter 16, the pyruvate carboxylase reaction can replenish intermediates in another central metabolic pathway, the citric acid cycle.
Because the mitochondrial membrane has no transporter for oxaloacetate, before export to the cytosol the oxaloacetate formed from pyruvate must be reduced to malate by mitochondrial malate dehydrogenase, at the expense of NADH:
(14-5)
The standard free-energy change for this reaction is quite high, but under physiological conditions (including a very low concentration of oxaloacetate) and the reaction is readily reversible. Mitochondrial malate dehydrogenase functions in both gluconeogenesis and the citric acid cycle, but the overall flow of metabolites in the two processes is in opposite directions.
Malate leaves the mitochondrion through a specific transporter in the inner mitochondrial membrane (see Fig. 19-31), and in the cytosol it is reoxidized to oxaloacetate, with the production of cytosolic NADH:
(14-6)
The oxaloacetate is then converted to PEP by phosphoenolpyruvate carboxykinase (Fig. 14-18). This -dependent reaction requires GTP as the phosphoryl group donor:
(14-7)
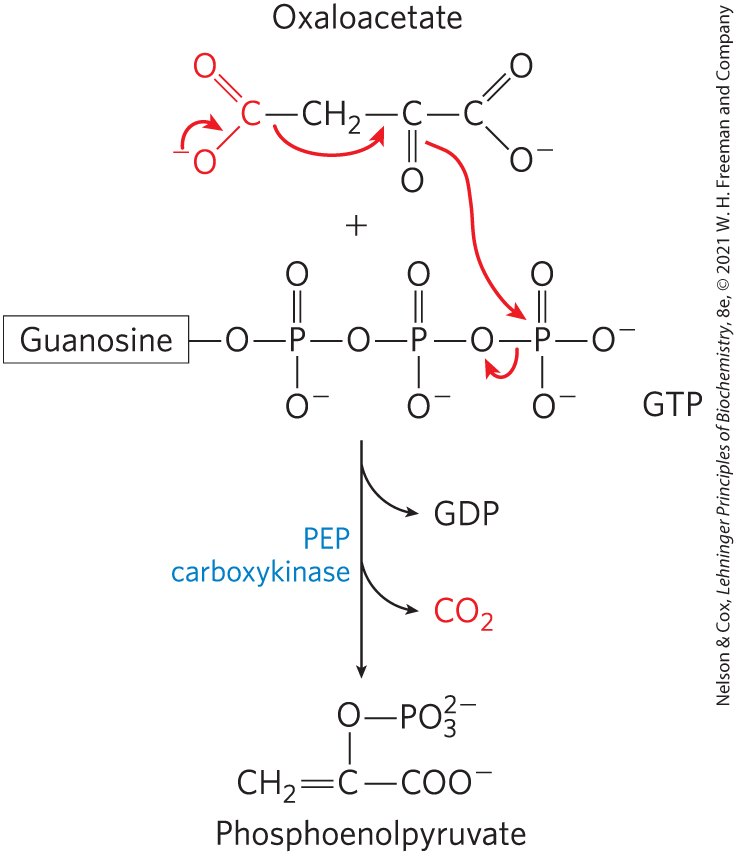
FIGURE 14-18 Synthesis of phosphoenolpyruvate from oxaloacetate. In the cytosol, oxaloacetate is converted to phosphoenolpyruvate by PEP carboxykinase. The incorporated in the pyruvate carboxylase reaction is lost here as . The decarboxylation leads to a rearrangement of electrons that facilitates attack of the carbonyl oxygen of the pyruvate moiety on the γ phosphate of GTP.
The reaction is reversible under intracellular conditions; the formation of one high-energy phosphate compound (PEP) is balanced by the hydrolysis of another (GTP). The overall equation for this set of bypass reactions, the sum of Equations 14-4 through 14-7, is
(14-8)
Two high-energy phosphate equivalents (one from ATP and one from GTP), each yielding about under cellular conditions, must be expended to phosphorylate one molecule of pyruvate to PEP. In contrast, when PEP is converted to pyruvate during glycolysis, only one ATP is generated from ADP. Although the standard free-energy change of the two-step path from pyruvate to PEP is 0.9 kJ/mol, the actual free-energy change , calculated from measured cellular concentrations of intermediates, is very strongly negative ; this results from the ready consumption of PEP in other reactions such that its concentration remains relatively low. The reaction is thus effectively irreversible in the cell.
Note that the added to pyruvate in the pyruvate carboxylase step (Fig. 14-17) is the same molecule that is lost in the PEP carboxykinase reaction (Fig. 14-18). This carboxylation-decarboxylation sequence represents a way of “activating” pyruvate, in that the decarboxylation of oxaloacetate facilitates PEP formation. In Chapter 21 we shall see how a similar carboxylation-decarboxylation sequence is used to activate acetyl-CoA for fatty acid biosynthesis (see Fig. 21-1).
There is a logic to the route of these reactions through the mitochondrion. The ratio in the cytosol is several orders of magnitude lower than in mitochondria. Because cytosolic NADH is consumed in gluconeogenesis (in the conversion of 1,3-bisphosphoglycerate to glyceraldehyde 3-phosphate; Fig. 14-16), glucose biosynthesis cannot proceed unless NADH is available. The transport of malate from the mitochondrion to the cytosol and its reconversion there to oxaloacetate effectively moves reducing equivalents to the cytosol, where they are scarce. This path from pyruvate to PEP therefore provides an important balance between NADH produced and consumed in the cytosol during gluconeogenesis.
A second pyruvate → PEP bypass predominates when lactate is the glucogenic precursor (Fig. 14-19). This pathway makes use of lactate produced by glycolysis in erythrocytes or anaerobic muscle, for example, and it is particularly important in large vertebrates after vigorous exercise (Box 14-2). The conversion of lactate to pyruvate in the cytosol of hepatocytes yields NADH, and the export of reducing equivalents (as malate) from mitochondria is therefore unnecessary. After the pyruvate produced by the lactate dehydrogenase reaction is transported into the mitochondrion (by a transporter in the inner mitochondrial membrane specific for pyruvate), it is converted to oxaloacetate by pyruvate carboxylase, as described above. This oxaloacetate, however, is converted directly to PEP by a mitochondrial isozyme of PEP carboxykinase, and the PEP is transported out of the mitochondrion to continue on the gluconeogenic path. The mitochondrial and cytosolic isozymes of PEP carboxykinase are encoded by separate genes in the nuclear chromosomes, providing another example of two distinct enzymes catalyzing the same reaction but having different cellular locations or metabolic roles (recall the isozymes of hexokinase).

FIGURE 14-19 Alternative paths from pyruvate to phosphoenolpyruvate. The relative importance of the two pathways depends on the availability of lactate or pyruvate and the cytosolic requirements for NADH for gluconeogenesis. The path on the right predominates when lactate is the precursor, because cytosolic NADH is generated in the lactate dehydrogenase reaction and does not have to be shuttled out of the mitochondrion (see text).
The Second and Third Bypasses Are Simple Dephosphorylations by Phosphatases
The second glycolytic reaction that cannot participate in gluconeogenesis is the phosphorylation of fructose 6-phosphate by PFK-1 (Table 14-2, step ). Because this reaction is highly exergonic and therefore irreversible in intact cells, the generation of fructose 6-phosphate from fructose 1,6-bisphosphate (Fig. 14-16) is catalyzed by a different enzyme, -dependent fructose 1,6-bisphosphatase (FBPase-1), which promotes the essentially irreversible hydrolysis of the C-1 phosphate (not phosphoryl group transfer to ADP):
FBPase-1 is so named to distinguish it from another, similar enzyme (FBPase-2) with a regulatory role, which we discuss in Section 14.5.
The third bypass is the final reaction of gluconeogenesis, the dephosphorylation of glucose 6-phosphate to yield glucose (Fig. 14-16). Reversal of the hexokinase reaction (p. 514) would require phosphoryl group transfer from glucose 6-phosphate to ADP, forming ATP, an energetically unfavorable reaction (Table 14-2, step ). The reaction catalyzed by glucose 6-phosphatase does not require synthesis of ATP; it is a simple hydrolysis of a phosphate ester:
This -activated enzyme is a membrane protein in the lumen of the endoplasmic reticulum of hepatocytes, renal cells, and epithelial cells of the small intestine (see Fig. 15-6), but not in other tissues, which are therefore unable to supply glucose to the blood. If other tissues had glucose 6-phosphatase, this enzyme’s activity would hydrolyze the glucose 6-phosphate needed within those tissues for glycolysis. Glucose produced by gluconeogenesis in the liver or kidney or ingested in the diet is delivered to these other tissues, including brain and muscle, through the bloodstream.
Gluconeogenesis Is Energetically Expensive, But Essential
The sum of the biosynthetic reactions leading from pyruvate to free blood glucose (Table 14-3) is
(14-9)
For each molecule of glucose formed from pyruvate, six high-energy phosphate groups are required: four from ATP and two from GTP. In addition, two molecules of NADH are required for the reduction of two molecules of 1,3-bisphosphoglycerate. Clearly, Equation 14-9 is not simply the reverse of the equation for conversion of glucose to pyruvate by glycolysis, which would require only two molecules of ATP:
This makes the synthesis of glucose from pyruvate a relatively expensive process. Much of this high energy cost is necessary to ensure the irreversibility of gluconeogenesis. Under intracellular conditions, the overall free-energy change of glycolysis is at least . Under the same conditions the overall of gluconeogenesis is . Thus both glycolysis and gluconeogenesis are essentially irreversible processes in cells. A second advantage to investing energy to convert pyruvate to glucose is that if pyruvate were instead excreted, its considerable potential for ATP production by complete, aerobic oxidation would be lost (more than 10 ATP are produced per pyruvate, as we shall see in Chapter 16).
The biosynthetic pathway to glucose described above allows the net synthesis of glucose not only from pyruvate but also from the four-, five-, and six-carbon intermediates of the citric acid cycle (Chapter 16). The citric acid cycle intermediates can undergo oxidation to oxaloacetate (see Fig. 16-7). Some or all of the carbon atoms of most amino acids derived from proteins are ultimately catabolized to pyruvate or to intermediates of the citric acid cycle. Such amino acids can therefore undergo net conversion to glucose and are said to be glucogenic (Table 14-4). Alanine and glutamine, the principal molecules that transport amino groups from extrahepatic tissues to the liver (see Fig. 18-9), are particularly important glucogenic amino acids in mammals. After removal of their amino groups in liver mitochondria, the carbon skeletons remaining (pyruvate and α-ketoglutarate, respectively) are readily funneled into gluconeogenesis.
Pyruvate |
Succinyl-CoA |
|---|---|
Alanine |
Isoleucinea |
Cysteine |
Methionine |
Glycine |
Threonine |
Serine |
Valine |
Threonine |
Fumarate |
Tryptophana |
Phenylalaninea |
α-Ketoglutarate |
Tyrosinea |
Arginine |
Oxaloacetate |
Glutamate |
Asparagine |
Glutamine |
Aspartate |
Histidine |
|
Proline |
Mammals Cannot Convert Fatty Acids to Glucose; Plants and Microorganisms Can
No net conversion of fatty acids to glucose occurs in mammals. As we shall see in Chapter 17, the catabolism of most fatty acids yields only acetyl-CoA. Mammals cannot use acetyl-CoA as a precursor of glucose, because the pyruvate dehydrogenase reaction is irreversible and cells have no other pathway to convert acetyl-CoA to pyruvate. Plants, yeast, and many bacteria do have a pathway (the glyoxylate cycle; see Fig. 20-45) for converting acetyl-CoA to oxaloacetate, so these organisms can use fatty acids as the starting material for gluconeogenesis. This is important during the germination of seedlings, for example; before leaves develop and photosynthesis can provide energy and carbohydrates, the seedling relies on stored seed oils for energy production and cell wall biosynthesis.
Although mammals cannot convert fatty acids to carbohydrate, they can use the small amount of glycerol produced from the breakdown of fats (triacylglycerols) for gluconeogenesis. Phosphorylation of glycerol by glycerol kinase, followed by oxidation of the central carbon, yields dihydroxyacetone phosphate, an intermediate in gluconeogenesis in liver.
As we shall see in Chapter 21, glycerol phosphate is an essential intermediate in triacylglycerol synthesis in adipocytes, but these cells lack glycerol kinase and so cannot simply phosphorylate glycerol. Instead, adipocytes carry out a truncated version of gluconeogenesis, known as glyceroneogenesis: the conversion of pyruvate to dihydroxyacetone phosphate via the early reactions of gluconeogenesis, followed by reduction of the dihydroxyacetone phosphate to glycerol 3-phosphate (see Fig. 21-21).
SUMMARY 14.4 Gluconeogenesis
- Gluconeogenesis is a multistep process in which glucose is produced from lactate, pyruvate, or oxaloacetate, or any compound (including citric acid cycle intermediates) that can be converted to one of these intermediates. Seven steps are the reversal of glycolytic reactions; three differ and must be bypassed with exergonic reactions.
- In the first bypass, pyruvate is converted to PEP via oxaloacetate in two steps catalyzed by pyruvate carboxylase (which uses ATP) and PEP carboxykinase (which uses GTP).
- In the second bypass, FBPase-1 removes a phosphate group from fructose 1,6-bisphosphate, producing fructose 6-phosphate. In the third bypass, glucose 6-phosphatase converts glucose 6-phosphate to glucose.
- In mammals, gluconeogenesis in the liver, kidney, and small intestine provides glucose for use by the brain, muscles, and erythrocytes. Formation of one molecule of glucose from pyruvate requires four ATP, two GTP, and two NADH; it is expensive.
- Animals cannot convert acetyl-CoA derived from fatty acids into glucose; they lack the enzymatic machinery to convert acetyl-CoA to pyruvate. Plants and microorganisms have the glyoxylate pathway, which allows them to make glucose from fatty acids.
 Gluconeogenesis occurs in all animals, plants, fungi, and microorganisms. The reactions are essentially the same in all tissues and all species. The important precursors of glucose in animals are three-carbon compounds such as lactate, pyruvate, and glycerol, as well as certain amino acids (
Gluconeogenesis occurs in all animals, plants, fungi, and microorganisms. The reactions are essentially the same in all tissues and all species. The important precursors of glucose in animals are three-carbon compounds such as lactate, pyruvate, and glycerol, as well as certain amino acids (








 ). Instead, the phosphorylation of pyruvate is achieved by a roundabout sequence of reactions that in eukaryotes requires enzymes in both the cytosol and mitochondria. As we shall see, the pathway shown in
). Instead, the phosphorylation of pyruvate is achieved by a roundabout sequence of reactions that in eukaryotes requires enzymes in both the cytosol and mitochondria. As we shall see, the pathway shown in  ). Because this reaction is highly exergonic and therefore irreversible in intact cells, the generation of fructose 6-phosphate from fructose 1,6-bisphosphate (
). Because this reaction is highly exergonic and therefore irreversible in intact cells, the generation of fructose 6-phosphate from fructose 1,6-bisphosphate ( ). The reaction catalyzed by glucose 6-phosphatase does not require synthesis of ATP; it is a simple hydrolysis of a phosphate ester:
). The reaction catalyzed by glucose 6-phosphatase does not require synthesis of ATP; it is a simple hydrolysis of a phosphate ester: Gluconeogenesis is a multistep process in which glucose is produced from lactate, pyruvate, or oxaloacetate, or any compound (including citric acid cycle intermediates) that can be converted to one of these intermediates. Seven steps are the reversal of glycolytic reactions; three differ and must be bypassed with exergonic reactions.
Gluconeogenesis is a multistep process in which glucose is produced from lactate, pyruvate, or oxaloacetate, or any compound (including citric acid cycle intermediates) that can be converted to one of these intermediates. Seven steps are the reversal of glycolytic reactions; three differ and must be bypassed with exergonic reactions.