14.5 Coordinated Regulation of Glycolysis and Gluconeogenesis
Glycolysis (the conversion of glucose to pyruvate) and gluconeogenesis (the conversion of pyruvate to glucose) generally do not both occur at the same time in the same tissues. In mammals, gluconeogenesis occurs primarily in the liver, where its role is to provide glucose for export to other tissues when glycogen stores are exhausted and when no dietary glucose is available. Glycolysis occurs in most tissues, including, brain, kidney, muscle and liver. Glycolysis provides ATP to support all of the energy-requiring activities of cells: active transport of ions; synthesis of macromolecules and of their precursors; synthesis of lipids and storage compounds like glycogen; and muscle contraction.
At each of the three points where glycolytic reactions are bypassed by alternative, gluconeogenic reactions (Fig. 14-16), simultaneous operation of both pathways would consume ATP without accomplishing any chemical or biological work. For example, PFK-1 and FBPase-1 catalyze opposing reactions:
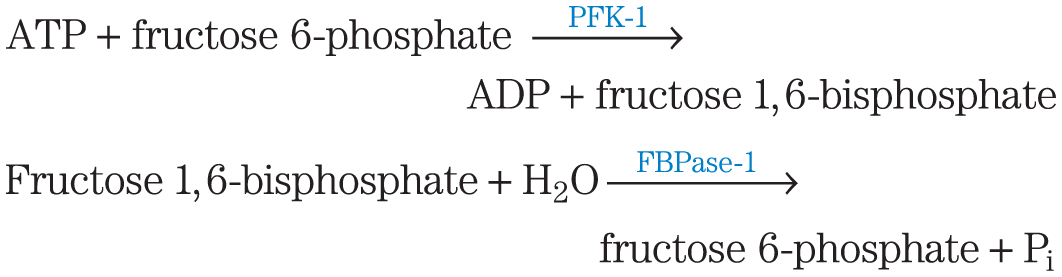
The sum of these two reactions is
that is, hydrolysis of ATP without any useful metabolic work being done. Clearly, if these two reactions were allowed to proceed simultaneously at a high rate in the same cell, a large amount of chemical energy would be dissipated as heat.
We look now in some detail at the mechanisms that regulate glycolysis and gluconeogenesis at the three points where these pathways diverge.
Hexokinase Isozymes Are Affected Differently by Their Product, Glucose 6-Phosphate
Hexokinase, which catalyzes the entry of glucose into the glycolytic pathway, is a regulatory enzyme. As noted in Section 14.1, humans have four isozymes of hexokinase (designated I to IV), encoded by four different genes (Box 14-3). The different hexokinase isozymes of liver and muscle reflect the different roles of these organs in carbohydrate metabolism: muscle consumes glucose, using it for energy production; liver maintains blood glucose homeostasis by consuming or producing glucose, depending on the prevailing blood glucose concentration.
The predominant hexokinase isozyme of myocytes (hexokinase II) has a high affinity for glucose — it is half-saturated at about 0.1 mm. Because glucose entering myocytes from the blood (where the glucose concentration is 4 to 5 mm) produces an intracellular glucose concentration high enough to saturate hexokinase II, the muscle enzyme normally acts at or near its . Muscle hexokinase I and hexokinase II are allosterically inhibited by their product, glucose 6-phosphate, so whenever the cellular concentration of glucose 6-phosphate rises above its normal level, these isozymes are temporarily and reversibly inhibited, bringing the rate of glucose 6-phosphate formation into balance with the rate of its utilization and reestablishing the steady state.
The predominant hexokinase isozyme of liver is hexokinase IV (also called glucokinase), which differs in three important respects from hexokinases I, II, and III of muscle. First, the glucose concentration at which hexokinase IV is half-saturated (about 10 mm) is higher than the usual concentration of glucose in the blood. Because an efficient glucose transporter in hepatocytes (GLUT2) rapidly equilibrates the glucose concentrations in cytosol and blood, the high of hexokinase IV allows its direct regulation by the blood glucose concentration (Fig. 14-20). When blood glucose is high, as it is after a meal rich in carbohydrates, excess glucose is transported into hepatocytes, where hexokinase IV converts it to glucose 6-phosphate. Because hexokinase IV is not saturated at 10 mm glucose, its activity continues to increase as the glucose concentration rises to 10 mm or more. Under conditions of low blood glucose, the glucose concentration in a hepatocyte is low relative to the of hexokinase IV, and the glucose generated by gluconeogenesis leaves the cell before being trapped by phosphorylation.
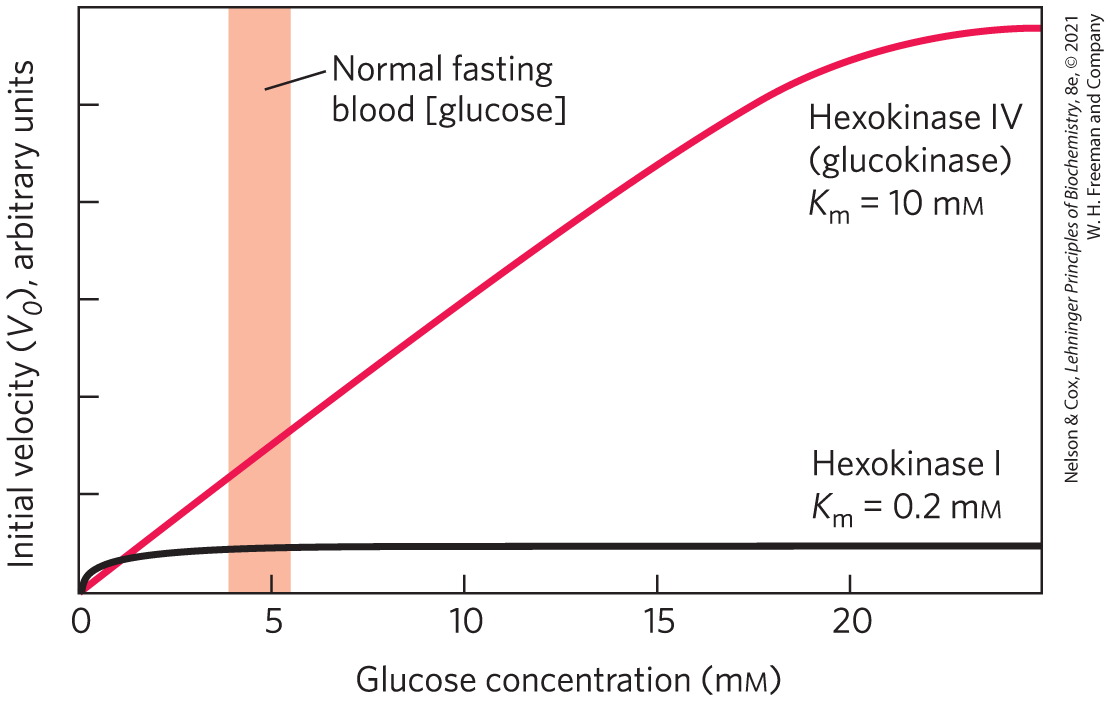
FIGURE 14-20 Comparison of the kinetic properties of hexokinase IV (glucokinase) and hexokinase I. Note the much lower for hexokinase I. When blood glucose rises above 5 mm, hexokinase IV activity increases, but hexokinase I is already operating near and cannot respond to an increase in glucose concentration. Hexokinases I, II, and III have similar kinetic properties.
Second, hexokinase IV is not inhibited by glucose 6-phosphate, and it can therefore continue to operate when the accumulation of glucose 6-phosphate completely inhibits hexokinases I, II, and III. Third, hexokinase IV is subject to inhibition by the reversible binding of a regulatory protein specific to liver (Fig. 14-21). The binding is much tighter in the presence of the allosteric effector fructose 6-phosphate. Glucose competes with fructose 6-phosphate for binding and causes dissociation of the regulatory protein from the hexokinase, relieving the inhibition. Immediately after a carbohydrate-rich meal, when blood glucose is high, glucose enters the hepatocyte via GLUT2 and activates hexokinase IV by this mechanism. During a fast, when blood glucose drops below 5 mm, fructose 6-phosphate triggers the inhibition of hexokinase IV by the regulatory protein, so the liver does not compete with other organs for the scarce glucose. The mechanism of inhibition by the regulatory protein is interesting: the protein anchors hexokinase IV inside the nucleus, where it is segregated from the other enzymes of glycolysis in the cytosol. When the glucose concentration in the cytosol rises, it equilibrates with glucose in the nucleus by transport through the nuclear pores. Glucose causes dissociation of the regulatory protein, and hexokinase IV enters the cytosol and begins to phosphorylate glucose.
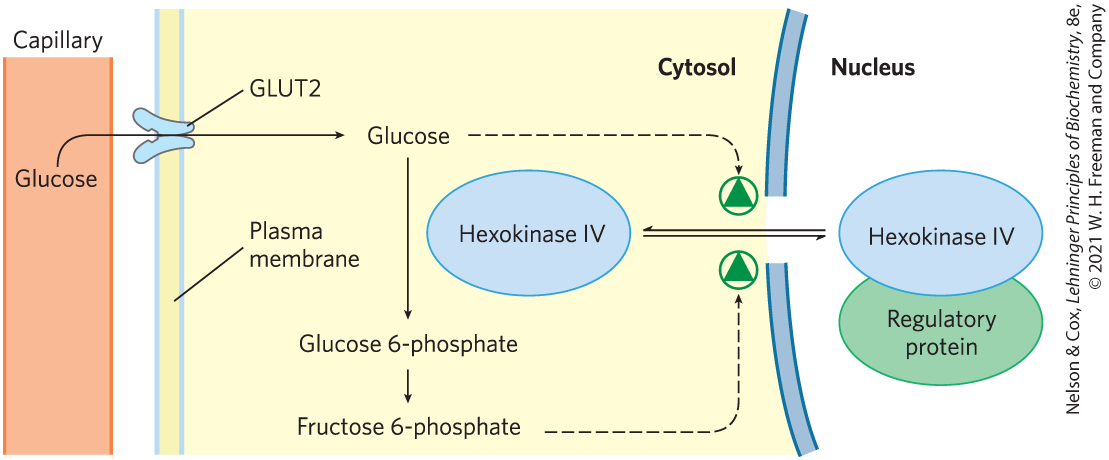
FIGURE 14-21 Regulation of hexokinase IV (glucokinase) by sequestration in the nucleus. The protein inhibitor of hexokinase IV is a nuclear binding protein that draws hexokinase IV into the nucleus when the fructose 6-phosphate concentration in liver is high and releases it to the cytosol when the glucose concentration is high.
Hexokinase IV is also regulated at the level of protein synthesis. Circumstances that call for greater energy production (low [ATP], high [AMP], vigorous muscle contraction) or for greater glucose consumption (high blood [glucose], for example) cause increased transcription of the hexokinase IV gene. Glucose 6-phosphatase, the gluconeogenic enzyme that bypasses the hexokinase step of glycolysis, is transcriptionally regulated by factors that call for increased production of glucose (low blood glucose, glucagon signaling). The transcriptional regulation of these two enzymes (along with other enzymes of glycolysis and gluconeogenesis) is described below.
Phosphofructokinase-1 and Fructose 1,6-Bisphosphatase Are Reciprocally Regulated
Glucose 6-phosphate can flow either into glycolysis or through any of several other pathways, including glycogen synthesis and the pentose phosphate pathway. The metabolically irreversible reaction catalyzed by PFK-1 is the step that commits glucose to glycolysis. In addition to its substrate-binding sites, this complex enzyme has several regulatory sites at which allosteric activators or inhibitors bind.
ATP is not only a substrate for PFK-1 but also an end product of the glycolytic pathway. When high cellular [ATP] signals that ATP is being produced faster than it is being consumed, ATP inhibits PFK-1 by binding to an allosteric site and lowering the affinity of the enzyme for its substrate fructose 6-phosphate (Fig. 14-22). ADP and AMP, which increase in concentration as consumption of ATP outpaces production, act allosterically to relieve this inhibition by ATP. These effects combine to produce higher enzyme activity when ADP or AMP accumulates and lower activity when ATP accumulates.
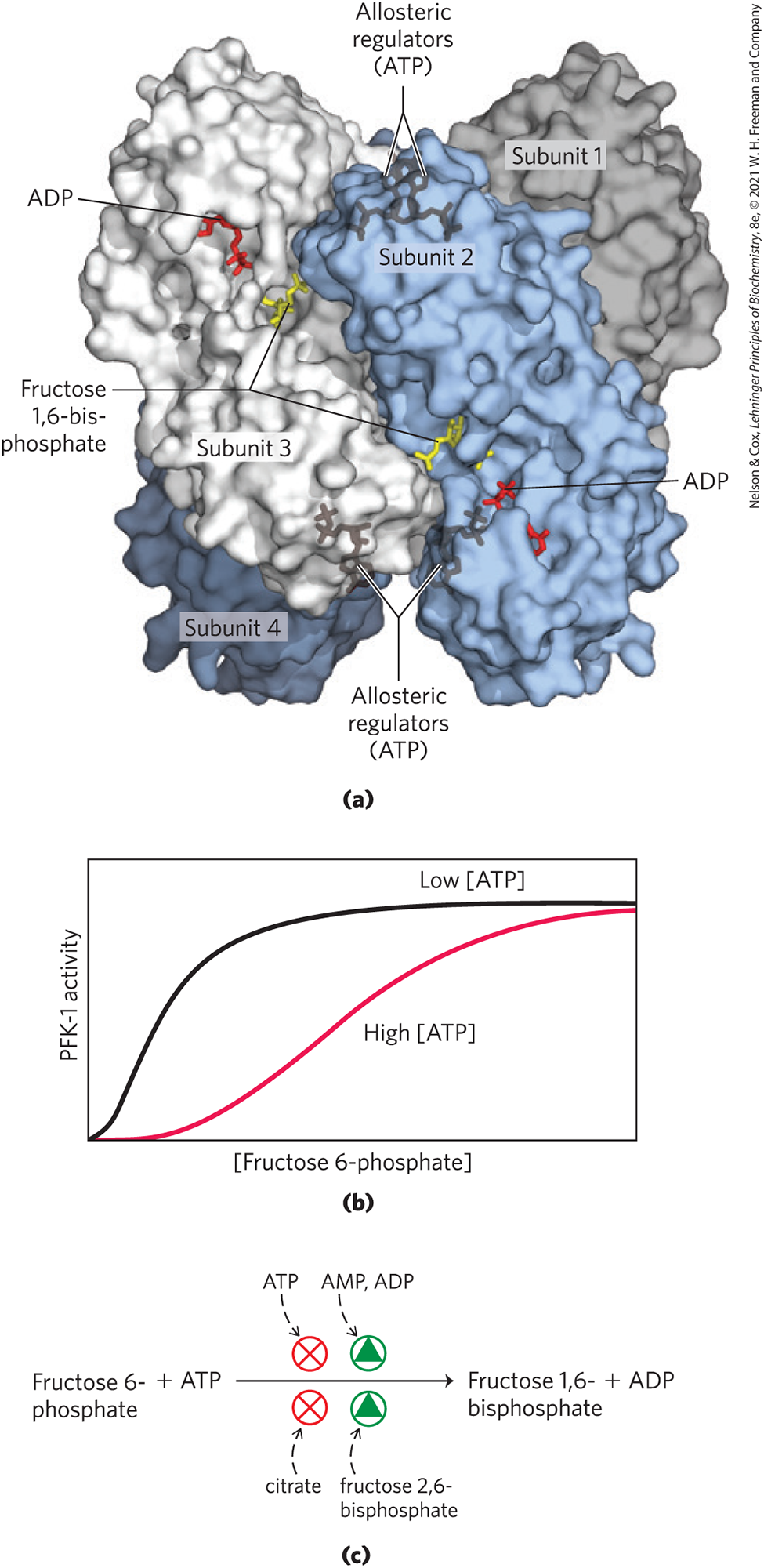
FIGURE 14-22 Phosphofructokinase-1 (PFK-1) and its regulation. (a) Surface contour image of E. coli PFK-1, showing portions of its four identical subunits. Each subunit has its own catalytic site, where the products ADP and fructose 1,6-bisphosphate (red and yellow stick structures, respectively) are almost in contact, and its own binding sites for the allosteric regulator ATP, buried in the protein in the positions indicated. (b) Allosteric regulation of muscle PFK-1 by ATP, shown by a substrate-activity curve. At low [ATP], the for fructose 6-phosphate is relatively low, enabling the enzyme to function at a high rate at relatively low [fructose 6-phosphate]. (Recall from Chapter 6 that is the term for regulatory enzymes; when is larger, the binding is weaker.) When [ATP] is high, for fructose 6-phosphate is greatly increased, as indicated by the sigmoid relationship between substrate concentration and enzyme activity. (c) Summary of the regulators affecting PFK-1 activity. [(a) Data from PDB ID 1PFK, Y. Shirakihara and P. R. Evans, J. Mol. Biol. 204:973, 1988.]
Citrate (the ionized form of citric acid), a key intermediate in the aerobic oxidation of pyruvate, fatty acids, and amino acids, is also an allosteric regulator of PFK-1. High citrate concentration increases the inhibitory effect of ATP, further reducing the flow of glucose through glycolysis. In this case, as in several others encountered later, citrate serves as an intracellular signal that the cell is meeting its current needs for energy-yielding metabolism by the oxidation of fats and proteins.
The corresponding step in gluconeogenesis is the conversion of fructose 1,6-bisphosphate to fructose 6-phosphate (Fig. 14-23). The enzyme that catalyzes this reaction, FBPase-1, is strongly inhibited (allosterically) by AMP; when the cell’s supply of ATP is low (corresponding to high [AMP]), the ATP-requiring synthesis of glucose slows.
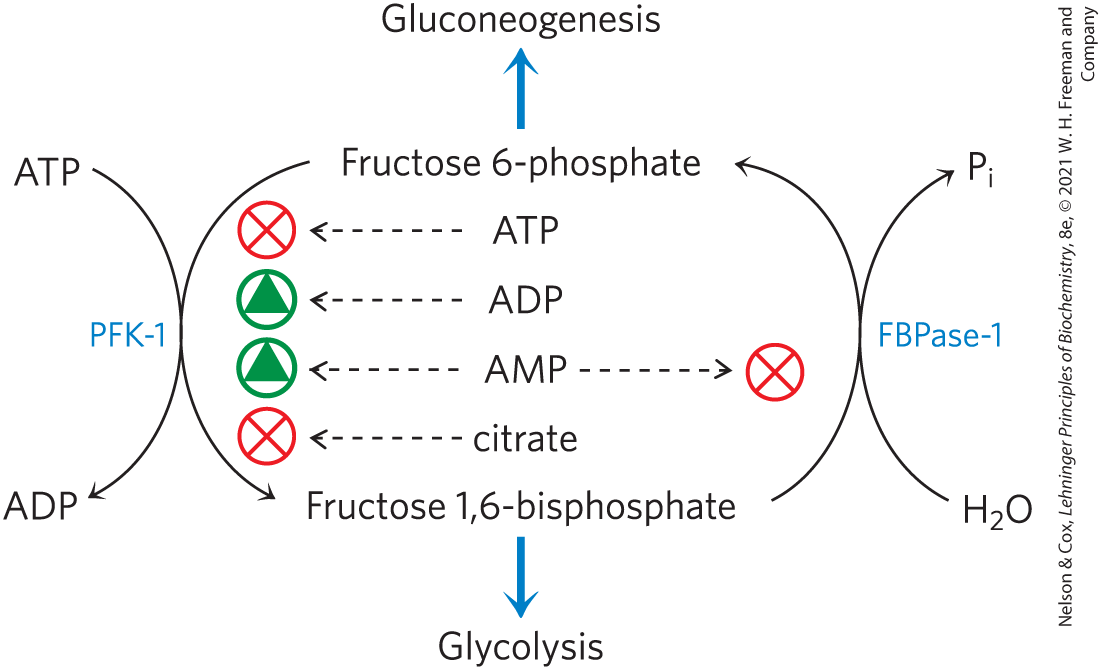
FIGURE 14-23 Regulation of phosphofructokinase-1 (PFK-1) and fructose 1,6-bisphosphatase (FBPase-1).
Thus, these opposing steps in the glycolytic and gluconeogenic pathways — those catalyzed by PFK-1 and FBPase-1 — are regulated in a coordinated and reciprocal manner. In general, when sufficient concentrations of acetyl-CoA or citrate (the product of acetyl-CoA condensation with oxaloacetate) are present, or when a high proportion of the cell’s adenylate is in the form of ATP, gluconeogenesis is favored. When the concentration of AMP increases, it promotes glycolysis by stimulating PFK-1 (and, as we shall see in Section 15.3, promotes glycogen degradation by activating glycogen phosphorylase).
Fructose 2,6-Bisphosphate Is a Potent Allosteric Regulator of PFK-1 and FBPase-1
The special role of the liver in maintaining a constant blood glucose level requires additional regulatory mechanisms to coordinate glucose production and consumption. When the blood glucose level decreases, the hormone glucagon signals the liver to produce and release more glucose and to stop consuming it for its own needs. One source of glucose is glycogen stored in the liver; another source is gluconeogenesis, using pyruvate, lactate, glycerol, or certain amino acids as starting material. When blood glucose is high, insulin signals the liver to use glucose as a fuel and as a precursor for the synthesis and storage of glycogen and triacylglycerol.
The rapid hormonal regulation of glycolysis and gluconeogenesis is mediated by fructose 2,6-bisphosphate, an allosteric effector for the enzymes PFK-1 and FBPase-1:
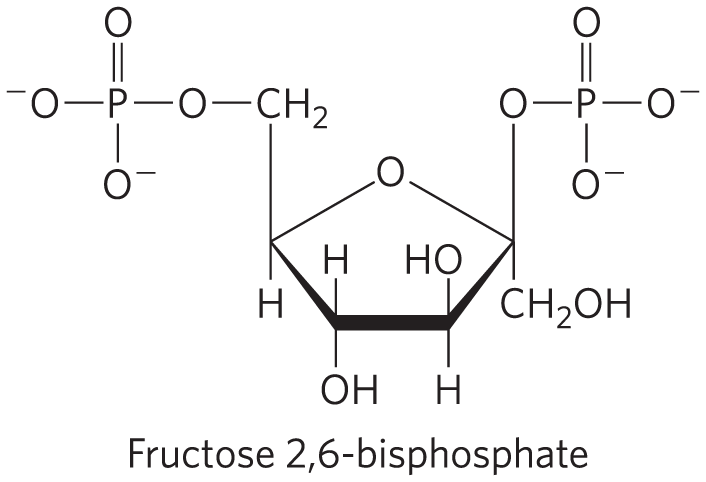
When fructose 2,6-bisphosphate binds to its allosteric site on PFK-1, it increases the enzyme’s affinity for its substrate fructose 6-phosphate (Fig. 14-24a) and reduces its affinity for the allosteric inhibitors ATP and citrate. At the physiological concentrations of its substrates, ATP and fructose 6-phosphate, and of its other positive and negative effectors (ATP, AMP, citrate), PFK-1 is virtually inactive in the absence of fructose 2,6-bisphosphate. Fructose 2,6-bisphosphate has the opposite effect on FBPase-1: it reduces its affinity for its substrate (Fig. 14-24b), thereby slowing gluconeogenesis.
![A three-part figure shows a graph plotting [fructose 6-phosphate] against P F K-1 activity in part a, a graph plotting [fructose 1,6-bisphosphate] against F B Pase-1 activity in part b, and a summary of regulation by F 2 6 B P in part c.](../images/8e_14_24_308261.png)
FIGURE 14-24 Role of fructose 2,6-bisphosphate in regulation of glycolysis and gluconeogenesis. Fructose 2,6-bisphosphate (F26BP) has opposite effects on the enzymatic activities of phosphofructokinase-1 (PFK-1, a glycolytic enzyme) and fructose 1,6-bisphosphatase (FBPase-1, a gluconeogenic enzyme). (a) PFK-1 activity in the absence of F26BP (blue curve) is half-maximal when the concentration of fructose 6-phosphate is 2 mm (that is, ). When 0.13 F26BP is present (red curve), the for fructose 6-phosphate is only 0.08 mm. Thus F26BP activates PFK-1 by increasing its apparent affinity for fructose 6-phosphate (see Fig. 14-23b). (b) FBPase-1 activity is inhibited by as little as F26BP and is strongly inhibited by 25 . In the absence of this inhibitor (blue curve), the for fructose 1,6-bisphosphate is , but in the presence of F26BP (red curve), the is . Fructose 2,6-bisphosphate also makes FBPase-1 more sensitive to inhibition by another allosteric regulator, AMP. (c) Summary of regulation by F26BP.
The cellular concentration of the allosteric regulator fructose 2,6-bisphosphate is set by the relative rates of its formation and breakdown (Fig. 14-25a). It is formed by phosphorylation of fructose 6-phosphate, catalyzed by phosphofructokinase-2 (PFK-2), and broken down by fructose 2,6-bisphosphatase (FBPase-2). (Note that these enzymes are distinct from PFK-1 and FBPase-1, which catalyze the formation and breakdown, respectively, of fructose 1,6-bisphosphate.) PFK-2 and FBPase-2 are two separate enzymatic activities of a single, bifunctional protein. The balance of these two activities in the liver, which determines the cellular level of fructose 2,6-bisphosphate, is set by glucagon and insulin (Fig. 14-25b).
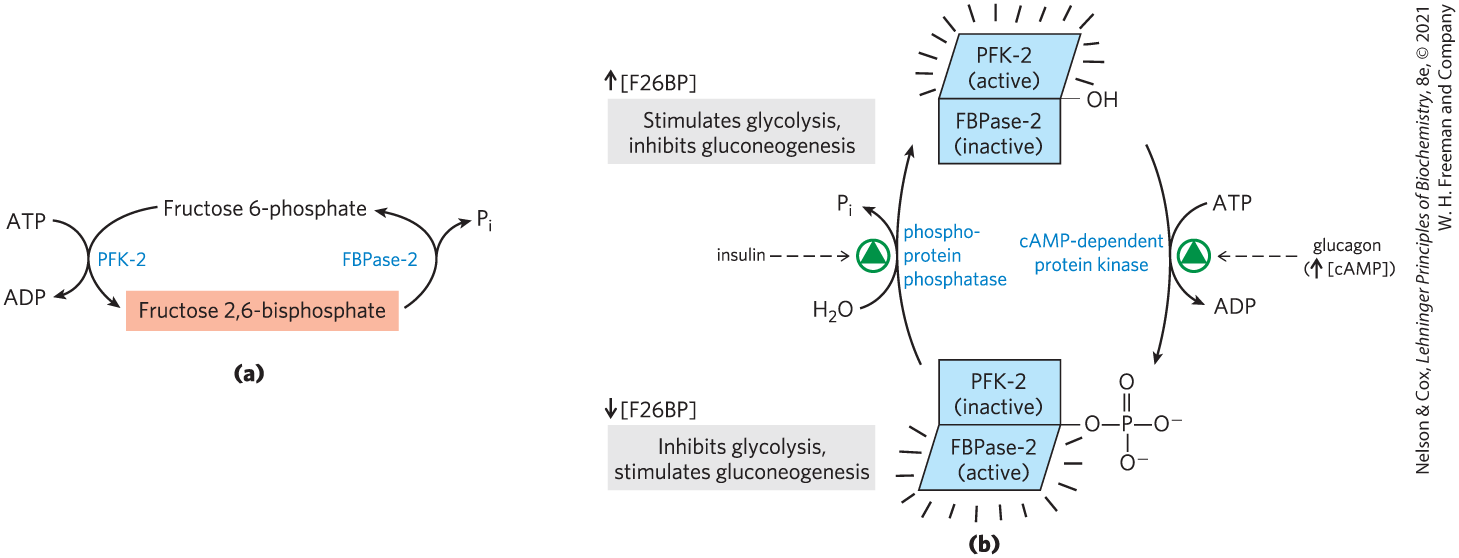
FIGURE 14-25 Regulation of fructose 2,6-bisphosphate level. (a) The cellular concentration of the regulator fructose 2,6-bisphosphate (F26BP) is determined by the rates of its synthesis by phosphofructokinase-2 (PFK-2) and its breakdown by fructose 2,6-bisphosphatase (FBPase-2). (b) Both enzyme activities are part of the same polypeptide chain, and they are reciprocally regulated by insulin and glucagon.
As we saw in Chapter 12, glucagon stimulates the adenylyl cyclase of liver to synthesize -cyclic AMP (cAMP) from ATP. Cyclic AMP then activates cAMP-dependent protein kinase, which transfers a phosphoryl group from ATP to the bifunctional protein PFK-2/FBPase-2. Phosphorylation of this protein enhances its FBPase-2 activity and inhibits its PFK-2 activity. Glucagon thereby lowers the cellular level of fructose 2,6-bisphosphate, inhibiting glycolysis and stimulating gluconeogenesis. The resulting production of more glucose enables the liver to replenish blood glucose in response to glucagon. Insulin has the opposite effect, stimulating the activity of a phosphoprotein phosphatase that catalyzes removal of the phosphoryl group from the bifunctional protein PFK-2/FBPase-2, activating its PFK-2 activity, increasing the level of fructose 2,6-bisphosphate, stimulating glycolysis, and inhibiting gluconeogenesis.
Xylulose 5-Phosphate Is a Key Regulator of Carbohydrate and Fat Metabolism
Another regulatory mechanism also acts by controlling the level of fructose 2,6-bisphosphate. In the mammalian liver, xylulose 5-phosphate, a product of the pentose phosphate pathway, mediates the increase in glycolysis that follows ingestion of a high-carbohydrate meal. The xylulose 5-phosphate concentration rises as glucose entering the liver is converted to glucose 6-phosphate and enters both the glycolytic and pentose phosphate pathways. Xylulose 5-phosphate activates phosphoprotein phosphatase 2A, which dephosphorylates the bifunctional PFK-2/FBPase-2 enzyme (Fig. 14-25). Dephosphorylation activates PFK-2 and inhibits FBPase-2, and the resulting rise in fructose 2,6-bisphosphate concentration stimulates glycolysis and inhibits gluconeogenesis. The increased glycolysis boosts the production of acetyl-CoA, while the increased flow of hexose through the pentose phosphate pathway generates NADPH. Acetyl-CoA and NADPH are the starting materials for fatty acid synthesis, which increases dramatically in response to intake of a high-carbohydrate meal. Xylulose 5-phosphate also increases the synthesis of all the enzymes required for fatty acid synthesis, as we shall see (Fig. 14-28).
The Glycolytic Enzyme Pyruvate Kinase Is Allosterically Inhibited by ATP
At least three isozymes of pyruvate kinase are found in vertebrates, differing in their tissue distribution and their response to modulators. High concentrations of ATP, acetyl-CoA, and long-chain fatty acids (signs of abundant energy supply) allosterically inhibit all isozymes of pyruvate kinase (Fig. 14-26). The liver isozyme (L form), but not the muscle isozyme (M form), is subject to further regulation by phosphorylation. When low blood glucose causes glucagon release, cAMP-dependent protein kinase phosphorylates the L isozyme of pyruvate kinase, inactivating it. This slows the use of glucose as a fuel in liver, sparing it for export to the brain and other organs. In muscle, the effect of increased [cAMP] is quite different. In response to epinephrine, cAMP activates glycogen breakdown and glycolysis and provides the fuel needed for the fight-or-flight response.

FIGURE 14-26 Regulation of pyruvate kinase. The enzyme is allosterically inhibited by ATP, acetyl-CoA, and long-chain fatty acids (all signs of an abundant energy supply), and the accumulation of fructose 1,6-bisphosphate triggers its activation. Accumulation of alanine, which can be synthesized from pyruvate in one step, allosterically inhibits pyruvate kinase, slowing the production of pyruvate by glycolysis. The liver isozyme (L form) is also regulated hormonally. Glucagon activates cAMP-dependent protein kinase (PKA; see Fig. 15-12), which phosphorylates the pyruvate kinase L isozyme, inactivating it. When the glucagon level drops, a protein phosphatase (PP) dephosphorylates pyruvate kinase, activating it. This mechanism prevents the liver from consuming glucose by glycolysis when blood glucose is low; instead, the liver exports glucose. The muscle isozyme (M form) is not affected by this phosphorylation mechanism.
Conversion of Pyruvate to Phosphoenolpyruvate Is Stimulated When Fatty Acids Are Available
In the pathway leading from pyruvate to glucose, the first control point determines the fate of pyruvate in the mitochondrion: its conversion either to acetyl-CoA (by the pyruvate dehydrogenase complex) to fuel the citric acid cycle (Chapter 16) or to oxaloacetate (by pyruvate carboxylase) to start the process of gluconeogenesis (Fig. 14-27). When fatty acids are readily available as fuels, their breakdown in liver mitochondria yields acetyl-CoA, a signal that further oxidation of glucose for fuel is not necessary. Acetyl-CoA is a positive allosteric modulator of pyruvate carboxylase and a negative modulator of pyruvate dehydrogenase, through stimulation of a protein kinase that inactivates the dehydrogenase. When the cell’s energy needs are being met, oxidative phosphorylation slows, [NADH] rises relative to and inhibits the citric acid cycle, and acetyl-CoA accumulates. The increased concentration of acetyl-CoA inhibits the pyruvate dehydrogenase complex, slowing the formation of acetyl-CoA from pyruvate, and stimulates gluconeogenesis by activating pyruvate carboxylase, allowing conversion of excess pyruvate to oxaloacetate (and, eventually, glucose).
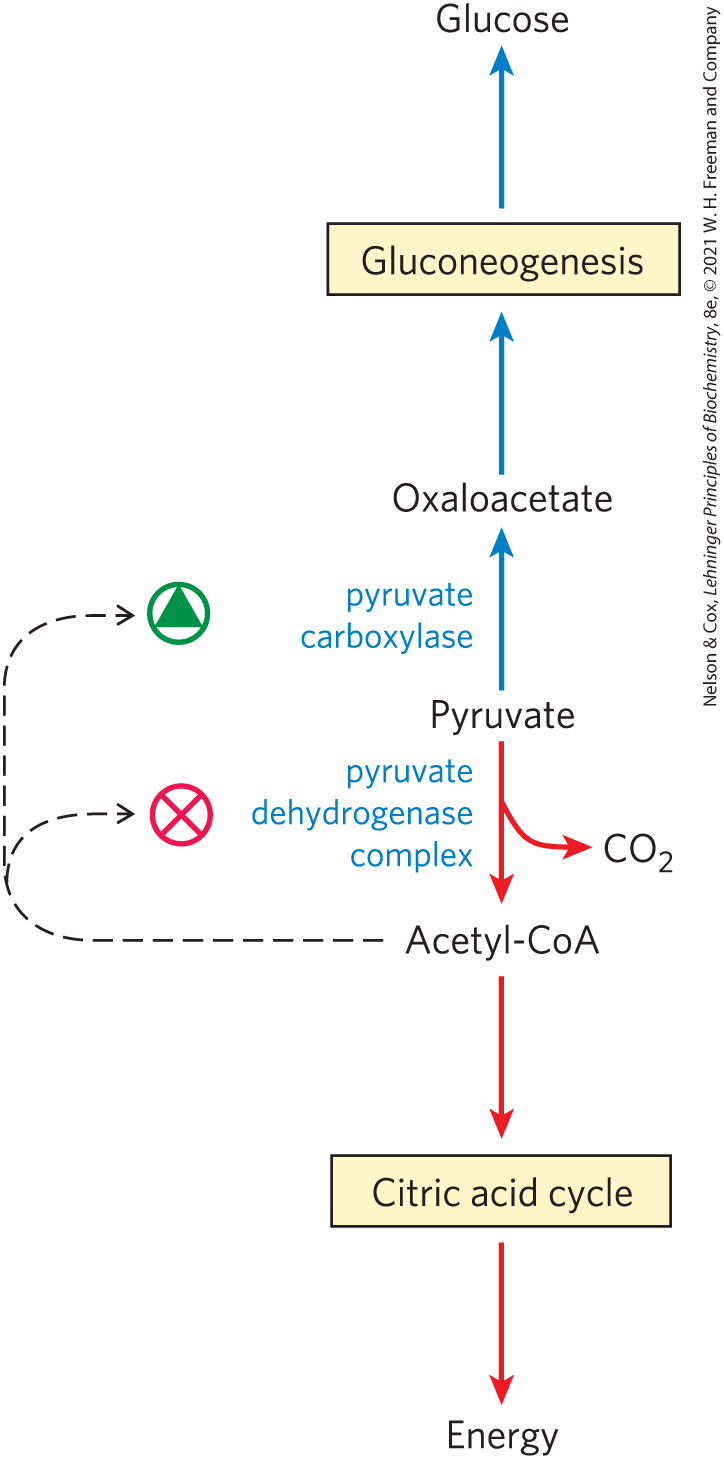
FIGURE 14-27 Two alternative fates for pyruvate. Pyruvate can be converted to glucose and glycogen via gluconeogenesis or oxidized to acetyl-CoA for energy production. The first enzyme in each path is regulated allosterically; acetyl-CoA, produced either by fatty acid oxidation or by the pyruvate dehydrogenase complex, stimulates pyruvate carboxylase and inhibits pyruvate dehydrogenase.
Oxaloacetate formed in this way is converted to phosphoenolpyruvate (PEP) in the reaction catalyzed by PEP carboxykinase (Fig. 14-16). In mammals, the regulation of this key enzyme occurs primarily at the level of its synthesis and breakdown, in response to dietary and hormonal signals. Fasting or high glucagon levels act through cAMP to increase the rate of transcription and to stabilize the mRNA. Insulin, or high blood [glucose], has the opposite effects. We discuss this transcriptional regulation in more detail below. Generally triggered by a signal from outside the cell, these changes take place on a time scale of minutes to days.
Transcriptional Regulation Changes the Number of Enzyme Molecules
Most of the regulatory actions discussed thus far are mediated by fast, reversible mechanisms to change the activity of existing enzyme molecules: allosteric effects, covalent alteration (phosphorylation) of the enzyme, or binding of a regulatory protein. Another set of regulatory processes involves changes in the number of molecules of an enzyme in the cell, through changes in the balance of enzyme synthesis and breakdown. Our discussion now turns briefly to regulation of transcription through signal-activated transcription factors. Transcriptional control is discussed in more detail in Chapter 28.
In Chapter 12 we encountered nuclear receptors and transcription factors in the context of insulin signaling. Insulin acts through its receptor in the plasma membrane to turn on at least two distinct signaling pathways, each involving activation of a protein kinase (MAP kinase and protein kinase B). The kinases phosphorylate transcription factors, which then act in the nucleus to stimulate the synthesis of enzymes needed for cell growth and division. More than 150 genes are transcriptionally regulated by insulin, many of which encode proteins we have described here (Table 14-5).
Change in gene expression |
Role in glucose metabolism |
|---|---|
|
Essential for glycolysis, which consumes glucose for energy |
|
Produce NADPH, which is essential for conversion of glucose to lipids |
|
Produce acetyl-CoA, which is essential for conversion of glucose to lipids |
|
Essential for conversion of glucose to lipids |
|
Essential for glucose production by gluconeogenesis |
One transcription factor important to carbohydrate metabolism is ChREBP (carbohydrate response element binding protein; Fig. 14-28), which is expressed primarily in liver, adipose tissue, and kidney. It coordinates the synthesis of enzymes needed for carbohydrate and fat synthesis. ChREBP in its phosphorylated form is inactive and is located in the cytosol. When the phosphoprotein phosphatase PP2A removes a phosphoryl group from ChREBP, the transcription factor can enter the nucleus. Here, nuclear PP2A removes another phosphoryl group, and ChREBP now joins with a partner protein, Mlx, and turns on the synthesis of several enzymes: pyruvate kinase; fatty acid synthase; and acetyl-CoA carboxylase, the first enzyme in the path to fatty acid synthesis.
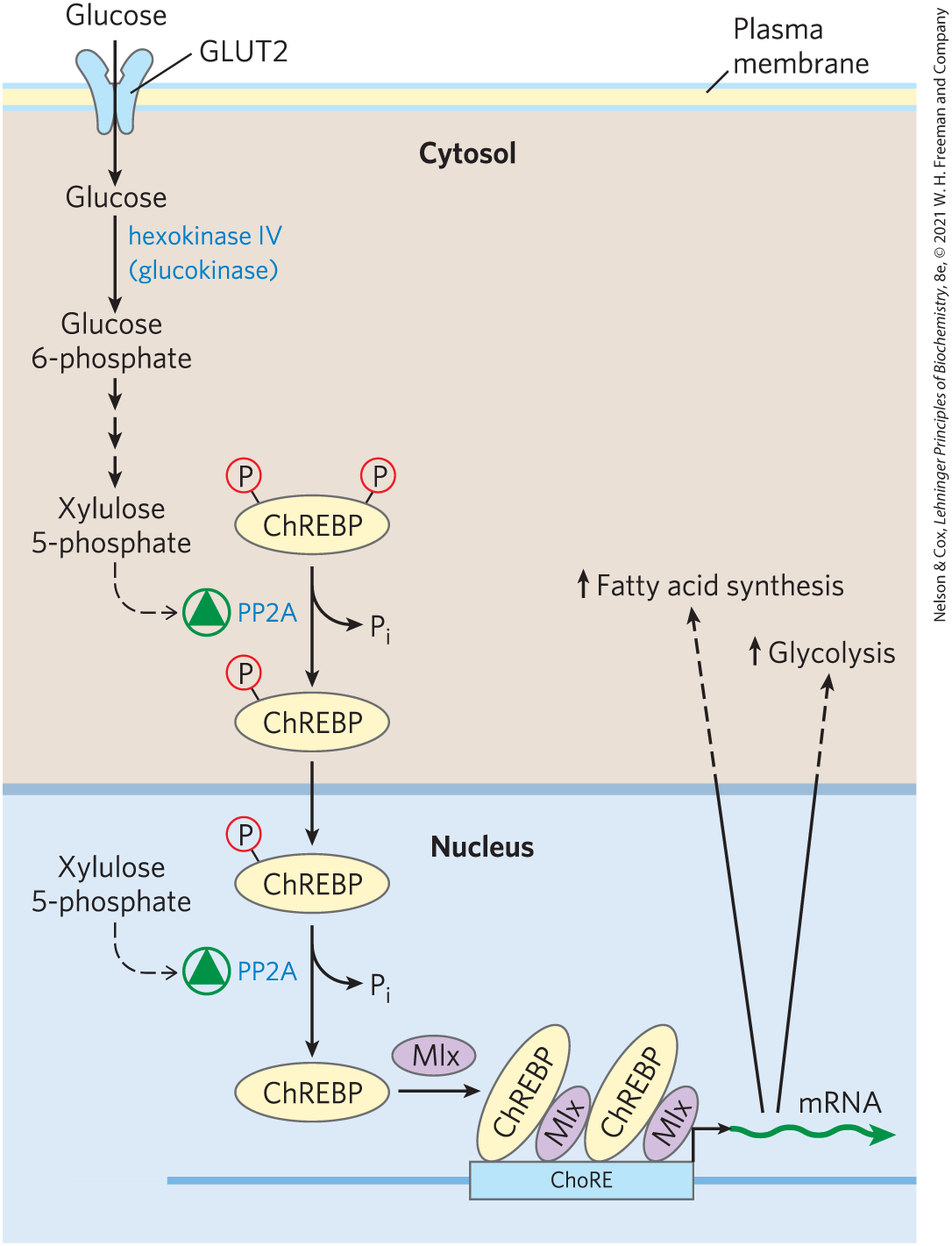
FIGURE 14-28 Mechanism of gene regulation by the transcription factor ChREBP. When ChREBP in the cytosol of a hepatocyte is phosphorylated on a Ser residue and a Thr residue, it cannot enter the nucleus. Dephosphorylation of —Ser by protein phosphatase PP2A allows ChREBP to enter the nucleus, where a second dephosphorylation, of —Thr, activates ChREBP so that it can associate with its partner protein, Mlx. ChREBP-Mlx now binds to the carbohydrate response element (ChoRE) in the promoter and stimulates transcription. PP2A is allosterically activated by xylulose 5-phosphate, an intermediate in the pentose phosphate pathway.
Controlling the activity of PP2A — and thus, ultimately, the synthesis of this group of metabolic enzymes — is xylulose 5-phosphate, an intermediate of the pentose phosphate pathway (see Fig. 14-31). When blood glucose concentration is high, glucose enters the liver and is phosphorylated by hexokinase IV. The glucose 6-phosphate thus formed can enter either the glycolytic pathway or the pentose phosphate pathway. If the latter, two initial oxidations produce xylulose 5-phosphate, which serves as a signal that the glucose-utilizing pathways are well-supplied with substrate. It accomplishes this by allosterically activating PP2A, which then dephosphorylates ChREBP, allowing the transcription factor to turn on the expression of genes for enzymes of glycolysis and fat synthesis (Fig. 14-28).
SUMMARY 14.5 Coordinated Regulation of Glycolysis and Gluconeogenesis
- Glycolysis and gluconeogenesis are reciprocally regulated to prevent wasteful operation of both pathways at the same time.
- Hexokinase IV (glucokinase) has kinetic properties related to its special role in the liver: releasing glucose to the blood when blood [glucose] is low; taking up and metabolizing glucose when blood [glucose] is high. Hexokinases I, II, and III are all inhibited by their product, glucose 6-phosphate.
- PFK-1 is allosterically inhibited by high [ATP]; low [AMP] inhibits FBPase-1. High [ATP] therefore slows glycolysis and speeds gluconeogenesis.
- Reciprocal allosteric control of glycolysis and gluconeogenesis is mainly achieved by the opposing effects of fructose 2,6-bisphosphate on PFK-1 and FBPase-1. Fructose 2,6-bisphosphate formation is stimulated, indirectly, by insulin, and inhibited by epinephrine.
- Xylulose 5-phosphate, an intermediate of the pentose phosphate pathway, activates phosphoprotein phosphatase PP2A. Activated PP2A tips the balance toward glucose uptake, glycogen synthesis, and lipid synthesis in the liver.
- Pyruvate kinase is allosterically inhibited by ATP, and the liver isozyme also is inhibited by cAMP-dependent phosphorylation. When [ATP] is high, glycolysis is slowed.
- When fatty acids are readily available as fuels, their breakdown in liver mitochondria yields acetyl-CoA, a signal that further oxidation of glucose for fuel is not necessary. Acetyl-CoA activates pyruvate carboxylase, thus favoring gluconeogenesis.
- Transcription factors such as ChREBP act in the nucleus to regulate the expression of specific genes coding for enzymes of the glycolytic and gluconeogenic pathways.
 Glycolysis (the conversion of glucose to pyruvate) and gluconeogenesis (the conversion of pyruvate to glucose) generally do not both occur at the same time in the same tissues. In mammals, gluconeogenesis occurs primarily in the liver, where its role is to provide glucose for export to other tissues when glycogen stores are exhausted and when no dietary glucose is available. Glycolysis occurs in most tissues, including, brain, kidney, muscle and liver. Glycolysis provides ATP to support all of the energy-requiring activities of cells: active transport of ions; synthesis of macromolecules and of their precursors; synthesis of lipids and storage compounds like glycogen; and muscle contraction.
Glycolysis (the conversion of glucose to pyruvate) and gluconeogenesis (the conversion of pyruvate to glucose) generally do not both occur at the same time in the same tissues. In mammals, gluconeogenesis occurs primarily in the liver, where its role is to provide glucose for export to other tissues when glycogen stores are exhausted and when no dietary glucose is available. Glycolysis occurs in most tissues, including, brain, kidney, muscle and liver. Glycolysis provides ATP to support all of the energy-requiring activities of cells: active transport of ions; synthesis of macromolecules and of their precursors; synthesis of lipids and storage compounds like glycogen; and muscle contraction. —Ser by protein phosphatase PP2A allows ChREBP to enter the nucleus, where a second dephosphorylation, of
—Ser by protein phosphatase PP2A allows ChREBP to enter the nucleus, where a second dephosphorylation, of  Glycolysis and gluconeogenesis are reciprocally regulated to prevent wasteful operation of both pathways at the same time.
Glycolysis and gluconeogenesis are reciprocally regulated to prevent wasteful operation of both pathways at the same time.