20.6 Biosynthesis of Starch, Sucrose, and Cellulose
During active photosynthesis in bright light, a plant leaf produces more carbohydrate (as triose phosphates) than it needs for generating energy or synthesizing precursors. The excess is converted to sucrose and transported to other parts of the plant, to be used as fuel or stored. In most plants, starch is the main storage form of carbohydrate, but in a few plants, such as sugar beet and sugarcane, sucrose is the primary storage form. The synthesis of sucrose and starch occurs in different cellular compartments (cytosol and plastids, respectively), and these processes are coordinated by a variety of regulatory mechanisms that respond to changes in light level and photosynthetic rate. The synthesis of sucrose and starch is important to the plant but also to humans: starch provides more than 80% of human dietary calories worldwide.
ADP-Glucose Is the Substrate for Starch Synthesis in Plant Plastids and for Glycogen Synthesis in Bacteria
Starch, like glycogen, is a high molecular weight polymer of d-glucose in linkage. It is synthesized in chloroplasts for temporary storage as one of the stable end products of photosynthesis, and for long-term storage it is synthesized in amyloplasts of the nonphotosynthetic parts of plants: seeds, roots, and tubers (underground stems).
The mechanism of glucose activation in starch synthesis is similar to that in glycogen synthesis, described in Chapter 15. An activated sugar nucleotide, in this case ADP-glucose, is formed by condensation of glucose 1-phosphate with ATP in a reaction made essentially irreversible by the presence in plastids of inorganic pyrophosphatase. Starch synthase then transfers glucose residues from ADP-glucose to preexisting starch molecules. The monomeric units are almost certainly added to the nonreducing end of the growing polymer, as they are in glycogen synthesis.
The amylose of starch is unbranched, but amylopectin has numerous -linked branches (see Fig. 7-13). Chloroplasts contain a branching enzyme, similar to the glycogen-branching enzyme that introduces the branches of amylopectin. Taking into account the hydrolysis by inorganic pyrophosphatase of the produced during ADP-glucose synthesis, the overall reaction for starch formation from glucose 1-phosphate is
Starch synthesis is regulated at the level of ADP-glucose formation, as discussed below.
UDP-Glucose Is the Substrate for Sucrose Synthesis in the Cytosol of Leaf Cells
Most of the triose phosphate generated by fixation in plants is converted to sucrose (Fig. 20-41) or starch. In the course of evolution, sucrose may have been selected as the transport form of carbon because of its unusual linkage between the anomeric C-1 of glucose and the anomeric C-2 of fructose. This bond is not hydrolyzed by amylases or other common carbohydrate-cleaving enzymes, and the unavailability of the sucrose molecule’s anomeric carbons prevents it from reacting nonenzymatically (as does glucose) with amino acids and proteins.
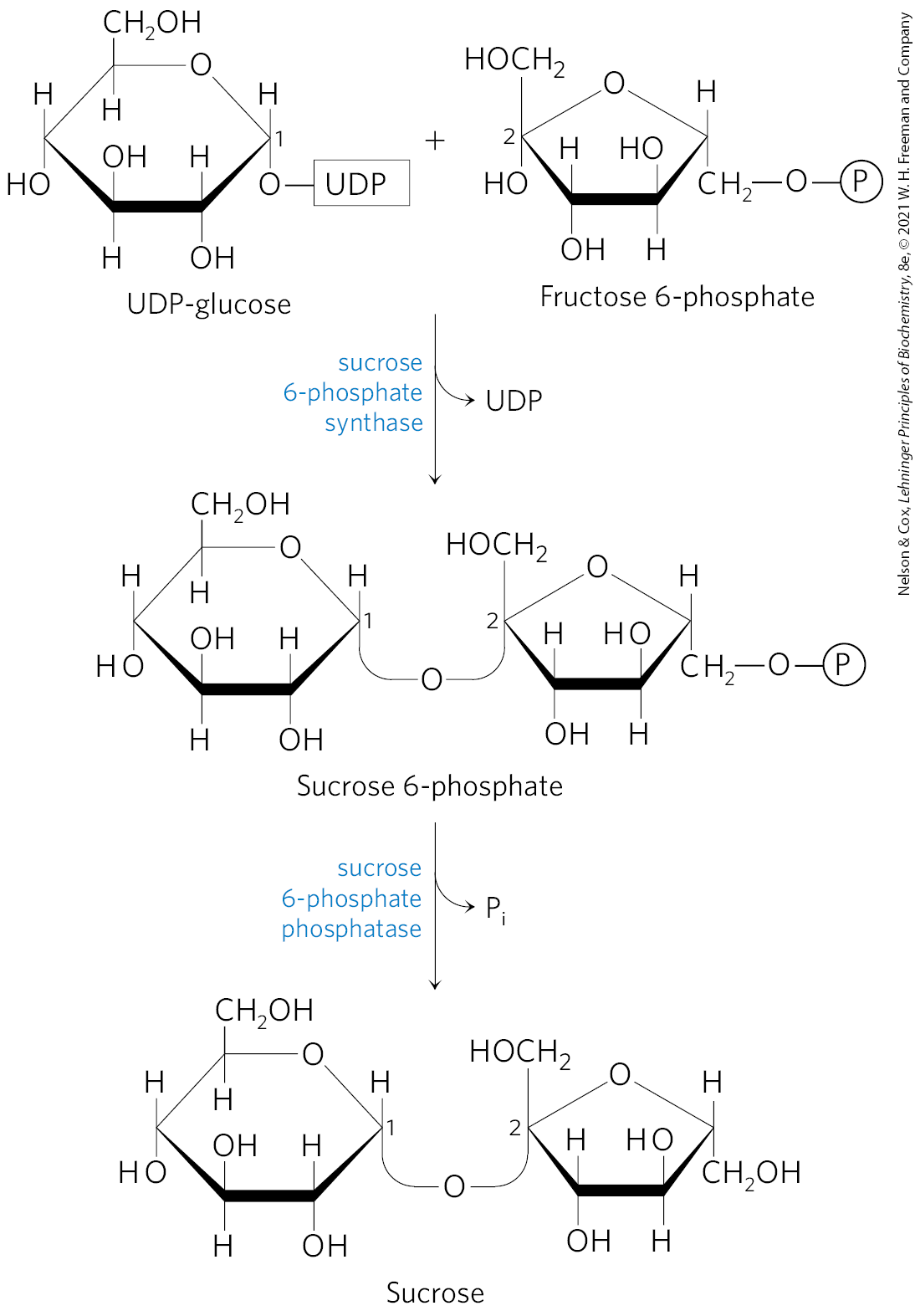
FIGURE 20-41 Sucrose synthesis. Sucrose is synthesized from UDP-glucose and fructose 6-phosphate, which are synthesized from triose phosphates in the plant cell cytosol. The sucrose 6-phosphate synthase of most plant species is allosterically regulated by glucose 6-phosphate and .
Sucrose is synthesized in the cytosol, beginning with dihydroxyacetone phosphate and glyceraldehyde 3-phosphate exported from the chloroplast. After condensation of two triose phosphates to form fructose 1,6-bisphosphate (catalyzed by aldolase), hydrolysis by fructose 1,6-bisphosphatase yields fructose 6-phosphate. Sucrose 6-phosphate synthase then catalyzes the reaction of fructose 6-phosphate with UDP-glucose to form sucrose 6-phosphate (Fig. 20-41). Finally, sucrose 6-phosphate phosphatase removes the phosphate group, making sucrose available for export to other tissues. The reaction catalyzed by sucrose 6-phosphate synthase is a low-energy process , but the hydrolysis of sucrose 6-phosphate to sucrose is sufficiently exergonic to make the overall synthesis of sucrose thermodynamically favorable. Sucrose synthesis is regulated and closely coordinated with starch synthesis, as we shall see.
One remarkable difference between the cells of plants and animals is the absence in the plant cell cytosol of the enzyme inorganic pyrophosphatase, which catalyzes the reaction
For many biosynthetic reactions that liberate , pyrophosphatase activity makes the process more favorable energetically, tending to make these reactions irreversible. In plants, this enzyme is present in plastids but absent from the cytosol. As a result, the cytosol of leaf cells contains a substantial concentration of — enough (~0.3 mm) to make reactions such as that catalyzed by UDP-glucose pyrophosphorylase (see Fig. 15-7) readily reversible.
Conversion of Triose Phosphates to Sucrose and Starch Is Tightly Regulated
Triose phosphates produced by the Calvin cycle in bright sunlight, as we have noted, may be stored temporarily in the chloroplast as starch, or converted to sucrose and exported to nonphotosynthetic parts of the plant, or both. The balance between the two processes is tightly regulated, and both must be coordinated with the rate of fixation. Five-sixths of the triose phosphate formed in the Calvin cycle must be recycled to ribulose 1,5-bisphosphate (Fig. 20-32); if more than one-sixth of the triose phosphate is drawn out of the cycle to make sucrose and starch, the cycle will slow or stop. However, insufficient conversion of triose phosphate to starch or sucrose would tie up phosphate, leaving a chloroplast deficient in , which is also essential for operation of the Calvin cycle.
The flow of triose phosphates into sucrose is regulated by the activity of fructose 1,6-bisphosphatase (FBPase-1) and the enzyme that effectively reverses its action, -dependent phosphofructokinase (PP-PFK-1). These enzymes are therefore critical points for determining the fate of triose phosphates produced by photosynthesis. Both enzymes are regulated by fructose 2,6-bisphosphate (F26BP), which inhibits FBPase-1 and stimulates PP-PFK-1. In vascular plants, the concentration of F26BP varies inversely with the rate of photosynthesis (Fig. 20-42). Phosphofructokinase-2, responsible for F26BP synthesis, is inhibited by dihydroxyacetone phosphate or 3-phosphoglycerate and is stimulated by fructose 6-phosphate and . During active photosynthesis, dihydroxyacetone phosphate is produced and is consumed, resulting in inhibition of PFK-2 and lowered concentrations of F26BP. This favors greater flux of triose phosphate into fructose 6-phosphate formation and sucrose synthesis. With this regulatory system, sucrose synthesis occurs when the level of triose phosphate produced by the Calvin cycle exceeds that needed to maintain operation of the cycle.
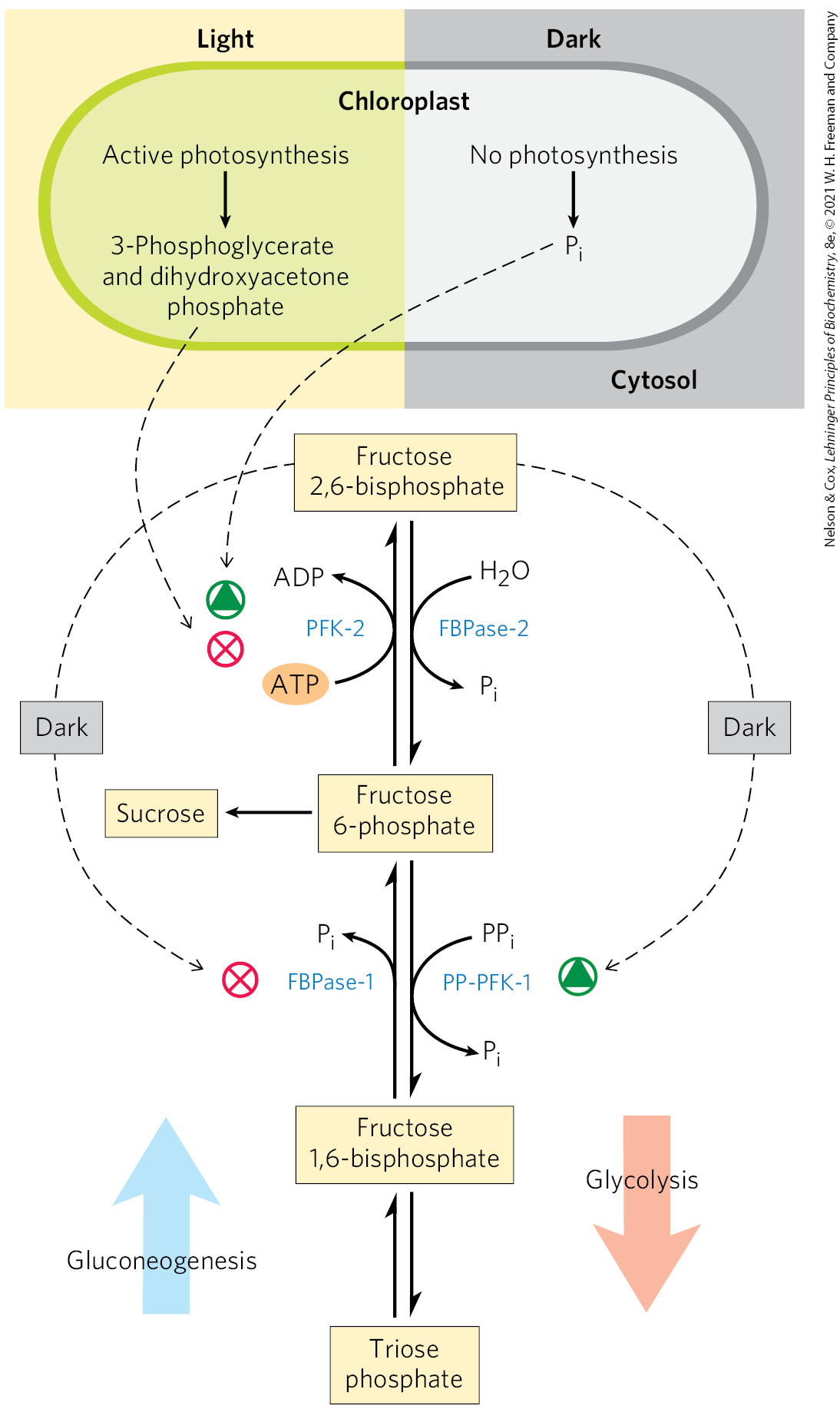
FIGURE 20-42 Fructose 2,6-bisphosphate as regulator of sucrose synthesis. The concentration of the allosteric regulator fructose 2,6-bisphosphate in plant cells is regulated by the products of photosynthetic assimilation and by . Dihydroxyacetone phosphate and 3-phosphoglycerate produced by assimilation inhibit phosphofructokinase-2 (PFK-2), the enzyme that synthesizes the regulator; stimulates PFK-2. The concentration of the regulator is therefore inversely proportional to the rate of photosynthesis. In the dark, the concentration of fructose 2,6-bisphosphate increases and stimulates the glycolytic enzyme -dependent phosphofructokinase-1 (PP-PFK-1), while inhibiting the gluconeogenic enzyme fructose 1,6-bisphosphatase (FBPase-1). When photosynthesis is active (in the light), the concentration of the regulator drops and the synthesis of fructose 6-phosphate and sucrose is favored.
Sucrose synthesis is also regulated at the level of sucrose 6-phosphate synthase, which is allosterically activated by glucose 6-phosphate and inhibited by . This enzyme is further regulated by phosphorylation and dephosphorylation; a protein kinase phosphorylates the enzyme on a specific Ser residue, making it less active, and a phosphatase reverses this inactivation by removing the phosphate (Fig. 20-43). Inhibition of the kinase by glucose 6-phosphate, and of the phosphatase by , enhances the effects of these two compounds on sucrose synthesis. When hexose phosphates are abundant, sucrose 6-phosphate synthase is activated by glucose 6-phosphate; when is elevated (as when photosynthesis is slow), sucrose synthesis is slowed. During active photosynthesis, triose phosphates are converted to fructose 6-phosphate, which is rapidly equilibrated with glucose 6-phosphate by phosphohexose isomerase. Because the equilibrium lies far toward glucose 6-phosphate, as soon as fructose 6-phosphate accumulates, the level of glucose 6-phosphate rises and sucrose synthesis is stimulated.
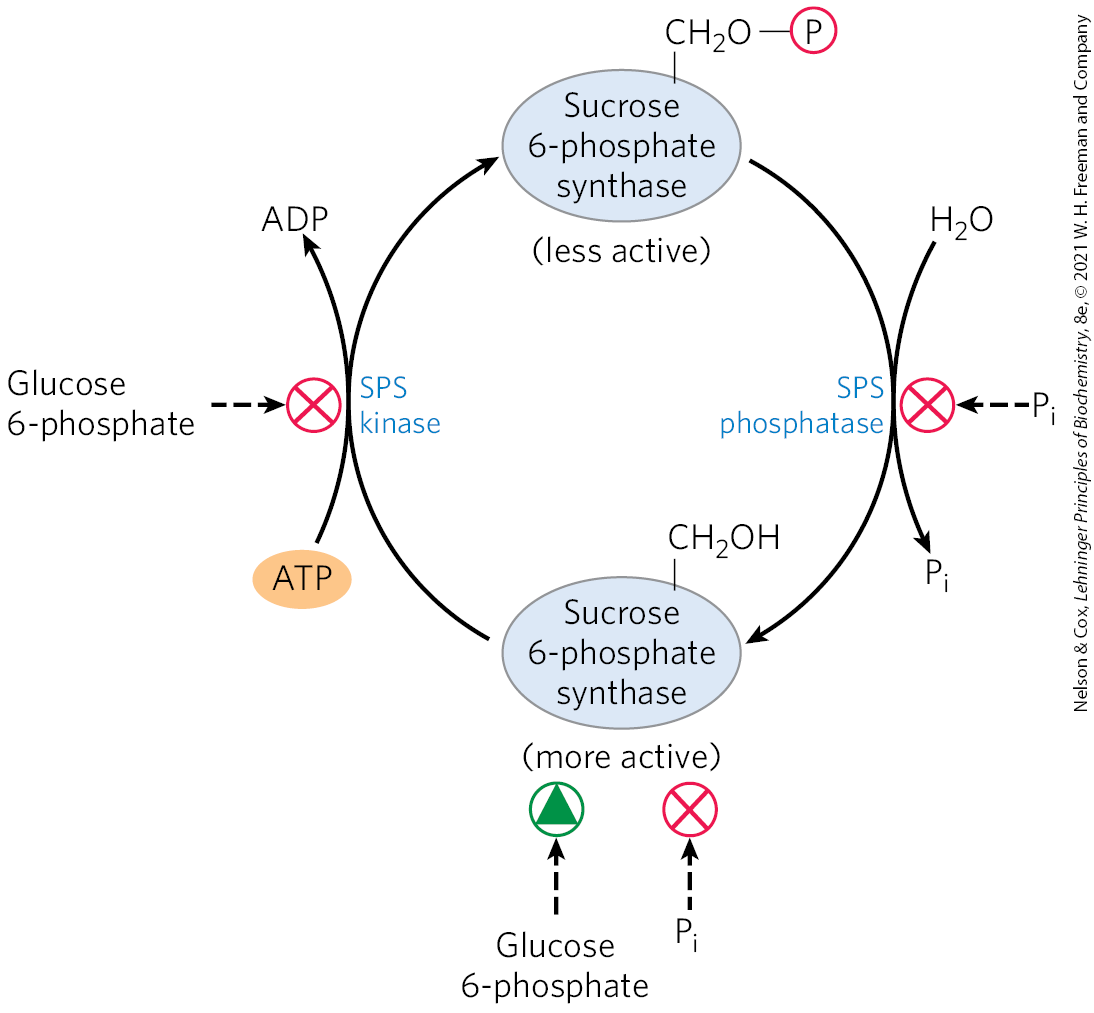
FIGURE 20-43 Regulation of sucrose phosphate synthase by phosphorylation. A protein kinase (SPS kinase) specific for sucrose phosphate synthase (SPS) phosphorylates a Ser residue in SPS, inactivating it; a specific phosphatase (SPS phosphatase) reverses this inhibition. The kinase is inhibited allosterically by glucose 6-phosphate, which also activates SPS allosterically. The phosphatase is inhibited by , which also inhibits SPS directly. Thus, when the concentration of glucose 6-phosphate is high as a result of active photosynthesis, SPS is activated and produces sucrose phosphate. A high concentration, which occurs when photosynthetic conversion of ADP to ATP is slow, inhibits sucrose phosphate synthesis.
The key regulatory enzyme in starch synthesis is ADP-glucose pyrophosphorylase (Fig. 20-44); it is activated by 3-phosphoglycerate, which accumulates during active photosynthesis, and inhibited by , which accumulates when light-driven condensation of ADP and slows. When sucrose synthesis slows, 3-phosphoglycerate formed by fixation accumulates, activating this enzyme and stimulating the synthesis of starch.
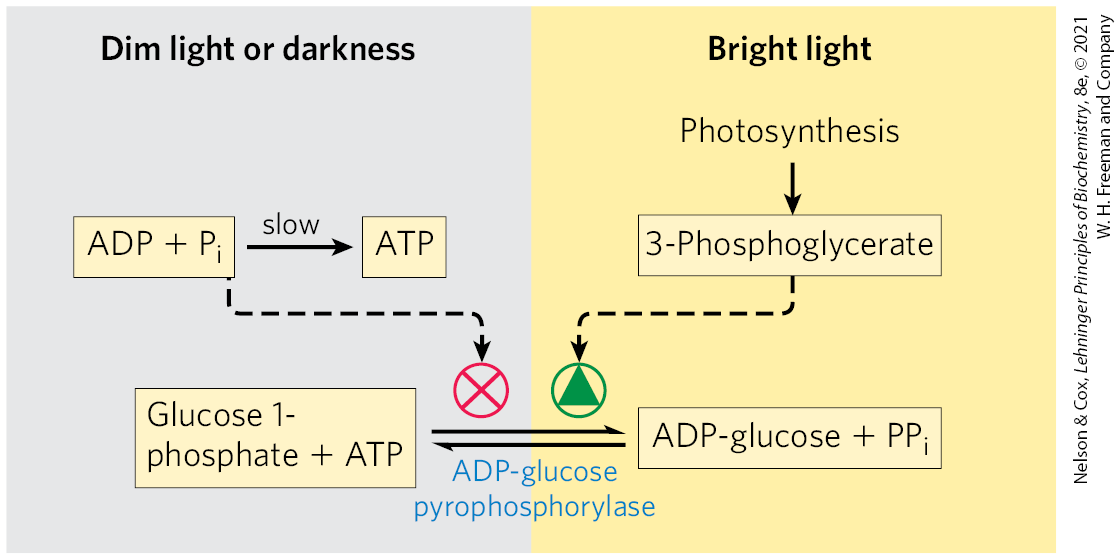
FIGURE 20-44 Regulation of ADP-glucose pyrophosphorylase by 3-phosphoglycerate and . This enzyme, which produces the precursor for starch synthesis, is rate-limiting in starch production. The enzyme is stimulated allosterically by 3-phosphoglycerate (3-PGA) and inhibited by ; in effect, the ratio , which rises with increasing rates of photosynthesis, controls starch synthesis at this step.
The Glyoxylate Cycle and Gluconeogenesis Produce Glucose in Germinating Seeds
Many plants store lipids (oils) and proteins in their seeds, to be used as sources of energy and as biosynthetic precursors during germination, before photosynthetic capacity has developed. These stored components are converted to carbohydrates by the combined action of several pathways. Glucogenic amino acids (see Table 14-4) derived from the breakdown of stored seed proteins are transaminated and oxidized to succinyl-CoA, pyruvate, oxaloacetate, fumarate, and α-ketoglutarate (Chapter 18) — all good starting materials for gluconeogenesis. Active gluconeogenesis in germinating seeds provides glucose for the synthesis of sucrose, polysaccharides, and many metabolites derived from hexoses. In plant seedlings, sucrose provides much of the chemical energy needed for initial growth.
Triacylglycerols stored in seeds also provide fuel for the germinating plants. They are hydrolyzed to free fatty acids, which undergo β oxidation to acetyl-CoA in specialized peroxisomes called glyoxysomes that develop during seed germination (see Fig. 17-14). The acetyl-CoA formed from seed oils enters the glyoxylate cycle (Fig. 20-45), which brings about the net conversion of acetate to succinate or other four-carbon intermediate of the citric acid cycle:
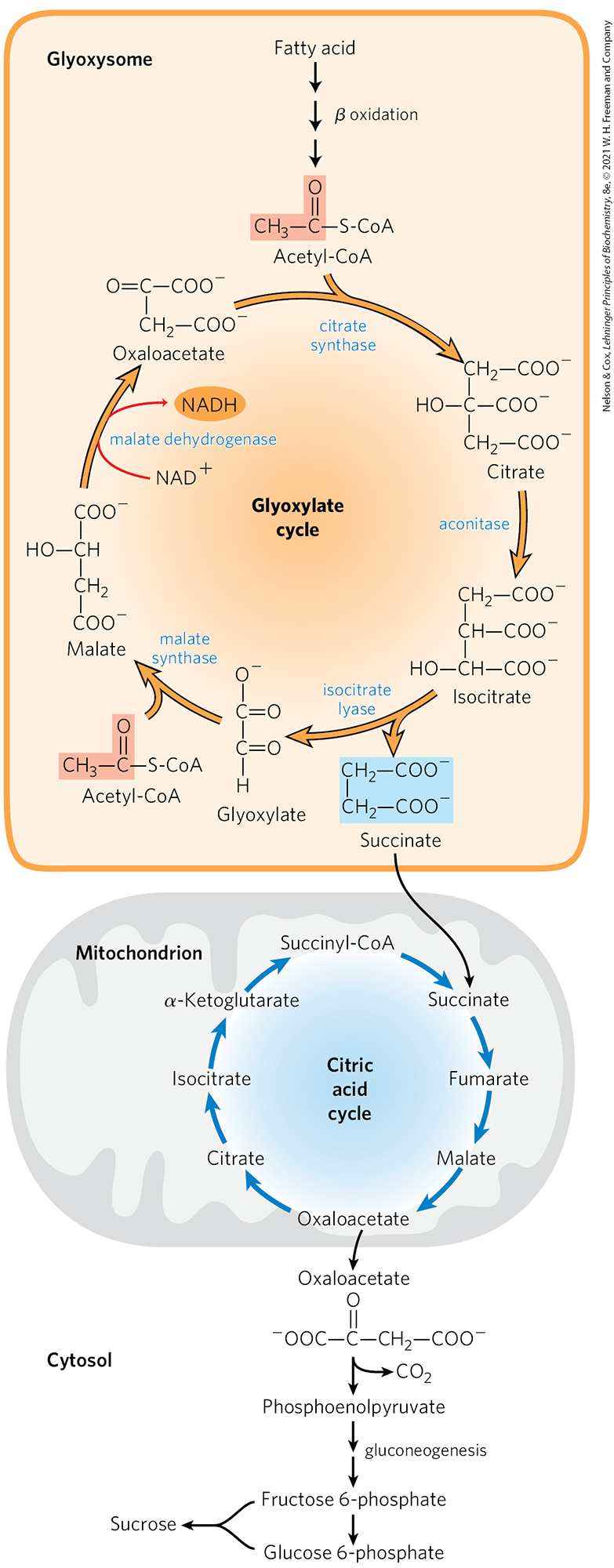
FIGURE 20-45 Conversion of stored fatty acids to sucrose in germinating seeds through the glyoxylate cycle. This pathway begins in specialized peroxisomes called glyoxysomes. The citrate synthase, aconitase, and malate dehydrogenase of the glyoxylate cycle are isozymes of the citric acid cycle enzymes; isocitrate lyase and malate synthase are unique to the glyoxylate cycle. Notice that two acetyl groups enter the cycle and four carbons leave as succinate. Succinate is exported to mitochondria, where it is converted to oxaloacetate by enzymes of the citric acid cycle. Oxaloacetate enters the cytosol and serves as the starting material for gluconeogenesis and for synthesis of sucrose, the transport form of carbon in plants.
In the glyoxylate cycle, acetyl-CoA condenses with oxaloacetate to form citrate, and citrate is converted to isocitrate, exactly as in the citric acid cycle. The next step, however, is not the breakdown of isocitrate by isocitrate dehydrogenase but the cleavage of isocitrate by isocitrate lyase, forming succinate and glyoxylate. The glyoxylate then condenses with a second molecule of acetyl-CoA to yield malate, in a reaction catalyzed by malate synthase. The malate is subsequently oxidized to oxaloacetate, which can condense with another molecule of acetyl-CoA to start another turn of the cycle. The succinate passes into the mitochondrial matrix, where it is converted by citric acid cycle enzymes to oxaloacetate. The oxaloacetate moves into the cytosol and can be converted to phosphoenolpyruvate by PEP carboxykinase, then to fructose 6-phosphate, the precursor of sucrose, by gluconeogenesis. Thus, reaction sequences carried out in three subcellular compartments (glyoxysomes, mitochondria, and cytosol) are integrated for the production of fructose 6-phosphate or sucrose from stored lipids.
Enzymes common to the citric acid and glyoxylate cycles have two isozymes, one specific to mitochondria, the other to glyoxysomes. Physical separation of the glyoxylate cycle and β-oxidation enzymes from the mitochondrial citric acid cycle enzymes prevents further oxidation of acetyl-CoA to . Each turn of the glyoxylate cycle consumes two molecules of acetyl-CoA and produces one molecule of succinate, which is then available for biosynthetic purposes. Hydrolysis of stored triacylglycerols also produces glycerol 3-phosphate, which can enter the gluconeogenic pathway, after its oxidation to dihydroxyacetone phosphate (see Fig. 14-16).
We noted in Chapter 14 that animal cells can carry out gluconeogenesis from three- and four-carbon precursors, but not from the two acetyl carbons of acetyl-CoA. Because the pyruvate dehydrogenase reaction is effectively irreversible (see Section 16.1) and animals do not have the enzymes specific to the glyoxylate cycle (isocitrate lyase and malate synthase), they have no way to convert acetyl-CoA to pyruvate or oxaloacetate. So, unlike vascular plants, animals cannot bring about the net synthesis of glucose from fatty acids.
Cellulose Is Synthesized by Supramolecular Structures in the Plasma Membrane
Cellulose is a major constituent of plant cell walls, providing strength and rigidity and preventing the swelling of the cell and rupture of the plasma membrane that might result when osmotic conditions favor water entry into the cell. Each year, worldwide, plants synthesize more than metric tons of cellulose, making this simple polymer one of the most abundant compounds in the biosphere. The structure of cellulose in the plant cell wall is simple: linear polymers of thousands of -linked d-glucose units, assembled into bundles of at least 18 chains, which co-crystalize to form microfibrils, which may in turn be assembled into larger macrofibrils. (Fig. 20-46).
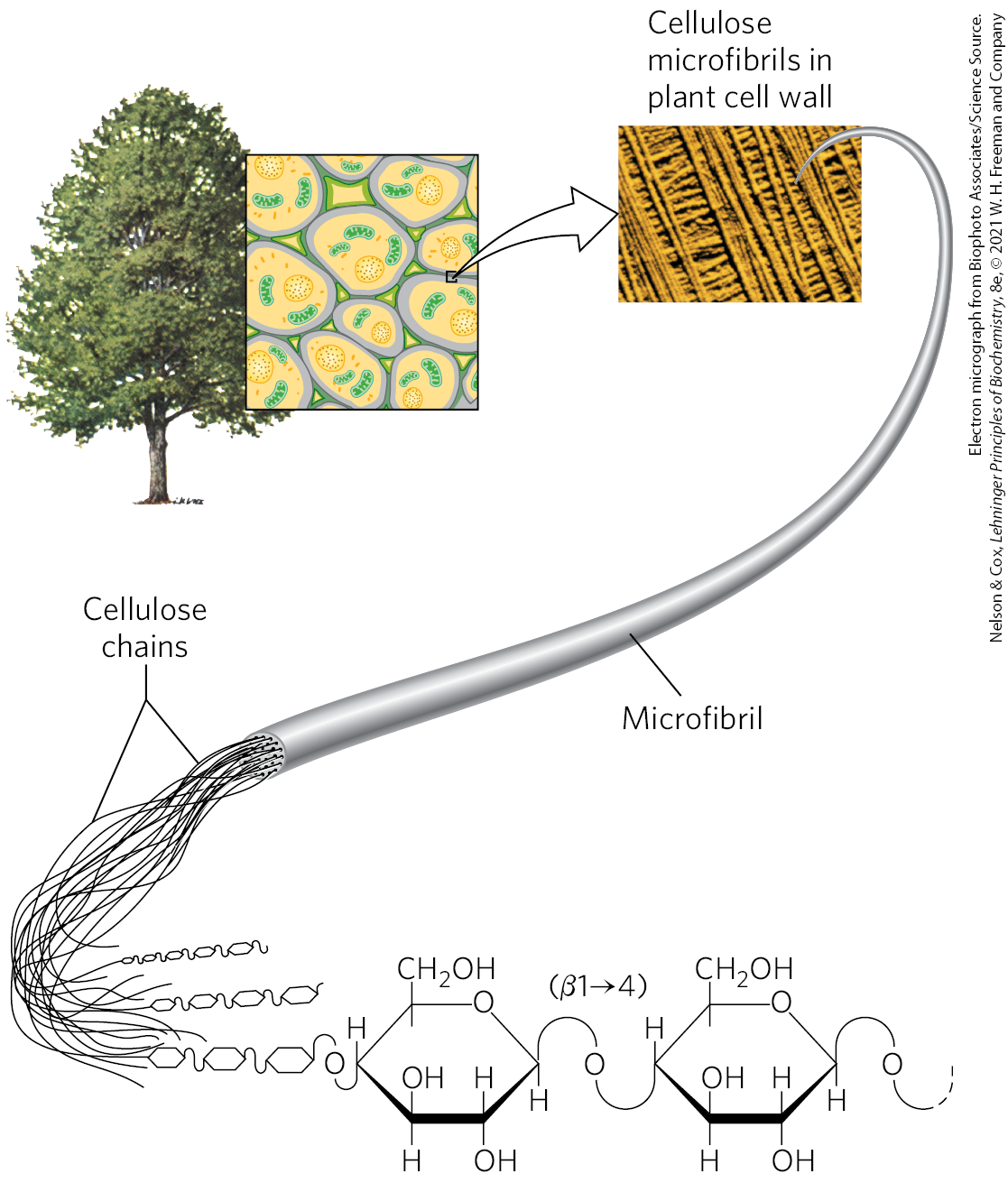
FIGURE 20-46 Cellulose structure. The plant cell wall is made up in part of cellulose molecules arranged side by side to form crystalline arrays — cellulose microfibrils. Several microfibrils may combine to form larger cellulose macrofibrils. The scanning electron microscope shows macrofibrils, 5 to 12 nm in diameter, laid down on the cell surface in several layers distinguishable by the different orientations of the fibrils.
As a major component of the plant cell wall, cellulose must be synthesized from intracellular precursors but deposited and assembled outside the plasma membrane. The enzymatic machinery for initiation, elongation, and export of cellulose chains is therefore more complicated than that used to synthesize starch or glycogen (which are not exported).
The complex enzymatic machinery that assembles cellulose chains spans the plasma membrane, with one part on the cytoplasmic side positioned to bind the substrate, UDP-glucose, and elongate the chains, and another part extending to the outside, responsible for exporting the cellulose molecules to the extracellular space. Freeze-fracture electron microscopy shows a cellulose synthesis complex, or rosette, composed of six large particles arranged in a regular hexagon with a diameter of about 30 nm (Fig. 20-47a). Several proteins, including the catalytic subunit of cellulose synthase, make up this structure. The structure of the plant cellulose synthase is similar to that of the bacterium Rhodobacter sphaeroides, which has been determined by x-ray crystallography (Fig. 20-47b).
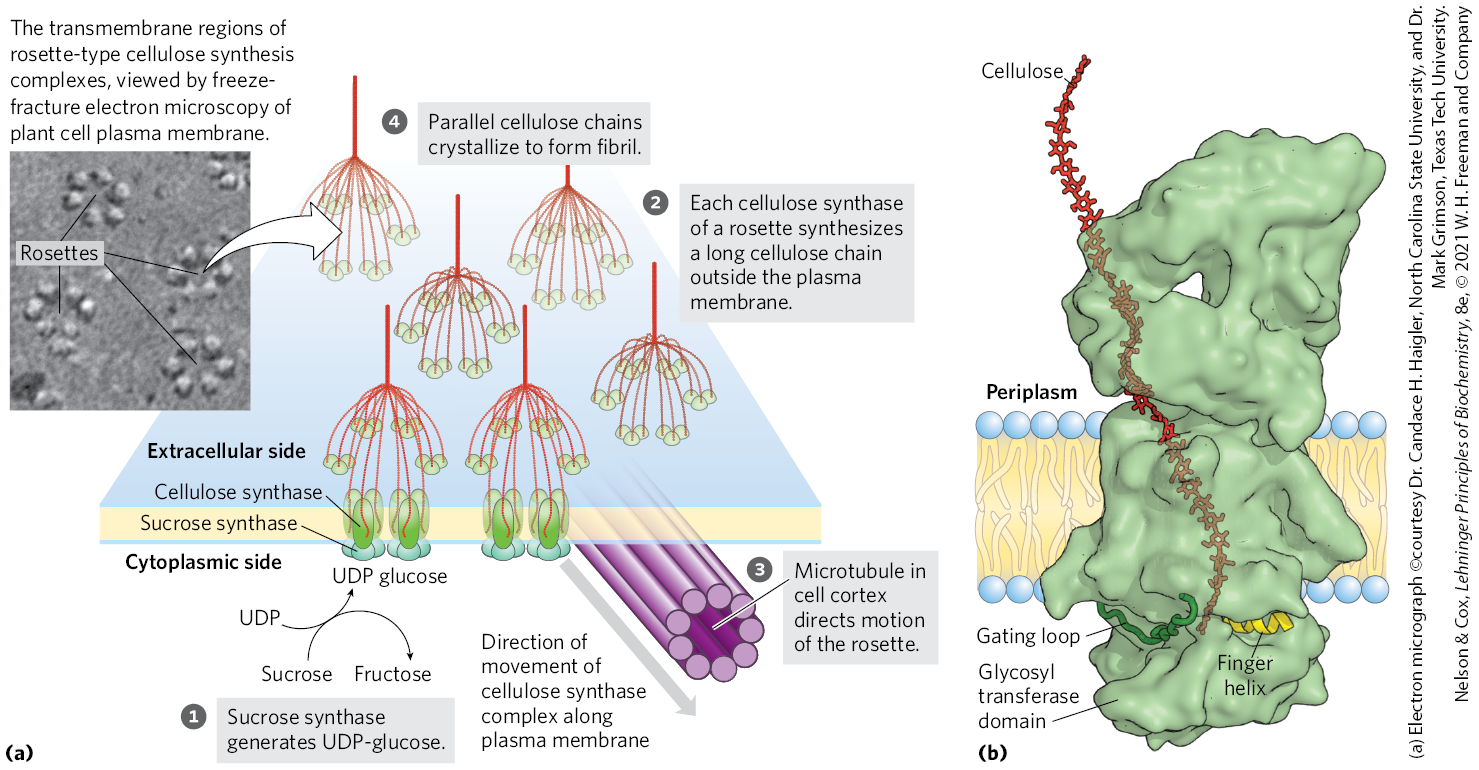
FIGURE 20-47 A model for the synthesis of cellulose. (a) Schematic derived from a combination of genetic, electron microscopic, and biochemical studies of Arabidopsis thaliana and other vascular plants. (b) The structure of cellulose synthase from the bacterium Rhodobacter sphaeroides. The transmembrane part of the protein provides a channel through which the lengthening cellulose polymer (red) is pushed into the periplasm as the chain grows by addition of glucose units on the inside surface of the plasma membrane. Two structures of the enzyme move during the catalytic cycle. The gating loop moves into the substrate-binding site when UDP-glucose binds, then moves out to allow UDP to leave. The finger helix touches the glucose residue at the growing polymer end, then, after a new residue is added, moves so as to touch this new terminal glucose. The glycosyl transferase domain extends into the cytoplasm, where it binds its substrate UDP-glucose. [(b) Data from PDB ID 5EJZ, J. L. W. Morgan et al., Nature 531:329, 2016. An extension of the cellulose chain was modeled in.]
In one working model of cellulose synthesis, cellulose chains are initiated by the transfer of a glucose residue from UDP-glucose to a “primer” glucose already bound to cellulose synthase on the cytoplasmic side of the plasma membrane, to form a disaccharide. As addition of further glucose residues lengthens the chain, it is extruded through a channel formed by the transmembrane helices of cellulose synthase and, on the outer surface of the plasma membrane, joins growing chains from neighboring cellulose synthase molecules to form a cellulose microfibril. Polymers of more than 6 to 8 glucose units are insoluble in water, promoting microfibril crystallization. There is no definite length for a cellulose polymer; synthesis is highly processive, and some polymers are as long as 15,000 glucose units.
The UDP-glucose used for cellulose synthesis (step in Fig. 20-47) is generated from sucrose produced during photosynthesis, in a reaction catalyzed by sucrose synthase (named for the reverse reaction):
A membrane-bound form of sucrose synthase may produce a high local concentration of UDP-glucose for cellulose synthesis.
Each of the six particles of the rosette most likely contains three cellulose synthase molecules, each synthesizing a single cellulose chain (step ). The large enzyme complex that catalyzes this process moves along the plasma membrane with directionality often related to the course of microtubules in the cell cortex, the cytoplasmic layer just below the membrane (step ). When these microtubules lie perpendicular to the axis of the plant’s growth, the cellulose microfibrils are laid down similarly to promote elongation. The motion of the cellulose synthase complexes is believed to be driven by energy released in the polymerization reaction, not by a molecular motor such as kinesin.
The fundamental cellulose microfibril made by one rosette-type cellulose synthesis complex is thought to be composed of 18 chains lying side by side with the same (parallel) orientation of nonreducing and reducing ends. The 18 separate polymers coalesce on the outer surface of the cell and crystallize soon after they are polymerized (step ), just prior to integrating into the cell wall.
In UDP-glucose, the glucose is α-linked to the nucleotide, but in cellulose, the glucose residues are -linked, so there is an inversion of configuration at the anomeric carbon (C-1) as the glycosidic bond forms. Glycosyltransferases that invert configuration are generally assumed to use a single-displacement mechanism, with nucleophilic attack by the acceptor species at the anomeric carbon of the donor sugar (in this case, UDP-glucose).
Pools of Common Intermediates Link Pathways in Different Organelles
Although we have described metabolic transformations in plant cells in terms of individual pathways, these pathways interconnect so completely that we should instead consider pools of metabolic intermediates shared among these pathways and connected by readily reversible reactions (Fig. 20-48). One such metabolite pool includes the hexose phosphates glucose 1-phosphate, glucose 6-phosphate, and fructose 6-phosphate; a second includes the 5-phosphates of the pentoses ribose, ribulose, and xylulose; a third includes the triose phosphates dihydroxyacetone phosphate and glyceraldehyde 3-phosphate. Metabolite fluxes through these pools change in magnitude and direction in response to changes in the circumstances of the plant, and they vary with tissue type. Transporters in the membranes of each organelle move specific compounds in and out, and the regulation of these transporters presumably influences the degree to which the pools mix.
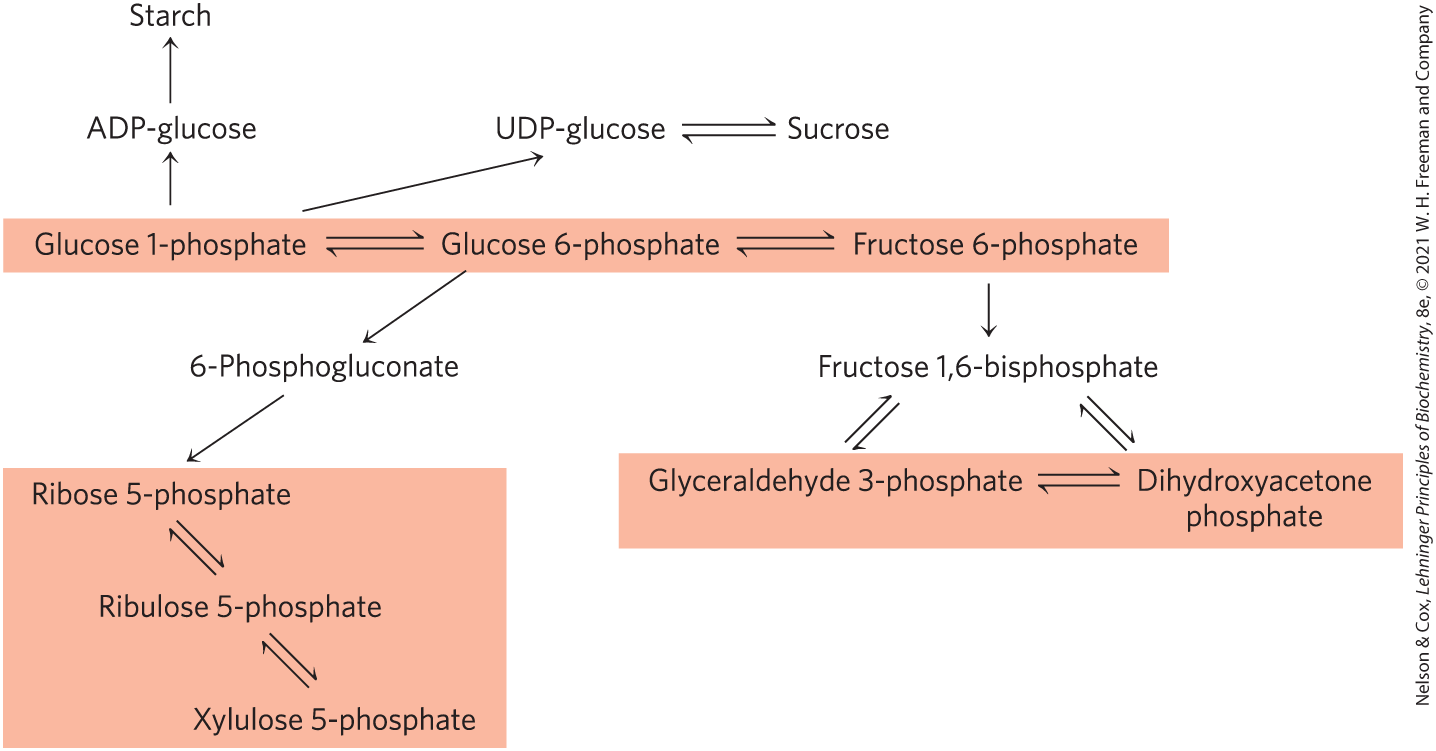
FIGURE 20-48 Pools of hexose phosphates, pentose phosphates, and triose phosphates. The compounds in each pool are readily interconvertible by reactions that have small standard free-energy changes. When one component of the pool is temporarily depleted, a new equilibrium is quickly established to replenish it. Movement of the sugar phosphates between intracellular compartments is limited; specific transporters must be present in an organelle membrane.
During daylight hours, triose phosphates produced in photosynthetic leaf tissue (“source” tissues, in which there is a net fixation of ) move out of the chloroplast and into the cytosolic hexose phosphate pool, where they are converted to sucrose for transport via the plant phloem (sap) to nonphotosynthetic “sink” tissues (Fig. 20-49). In sink tissues such as roots, tubers, and bulbs, sucrose is converted to starch for storage or is used as an energy source via glycolysis. In growing plants, hexose phosphates are also withdrawn from the pool for the synthesis of cell walls. At night, starch is metabolized by glycolysis and oxidative phosphorylation to provide energy for both source and sink tissues.
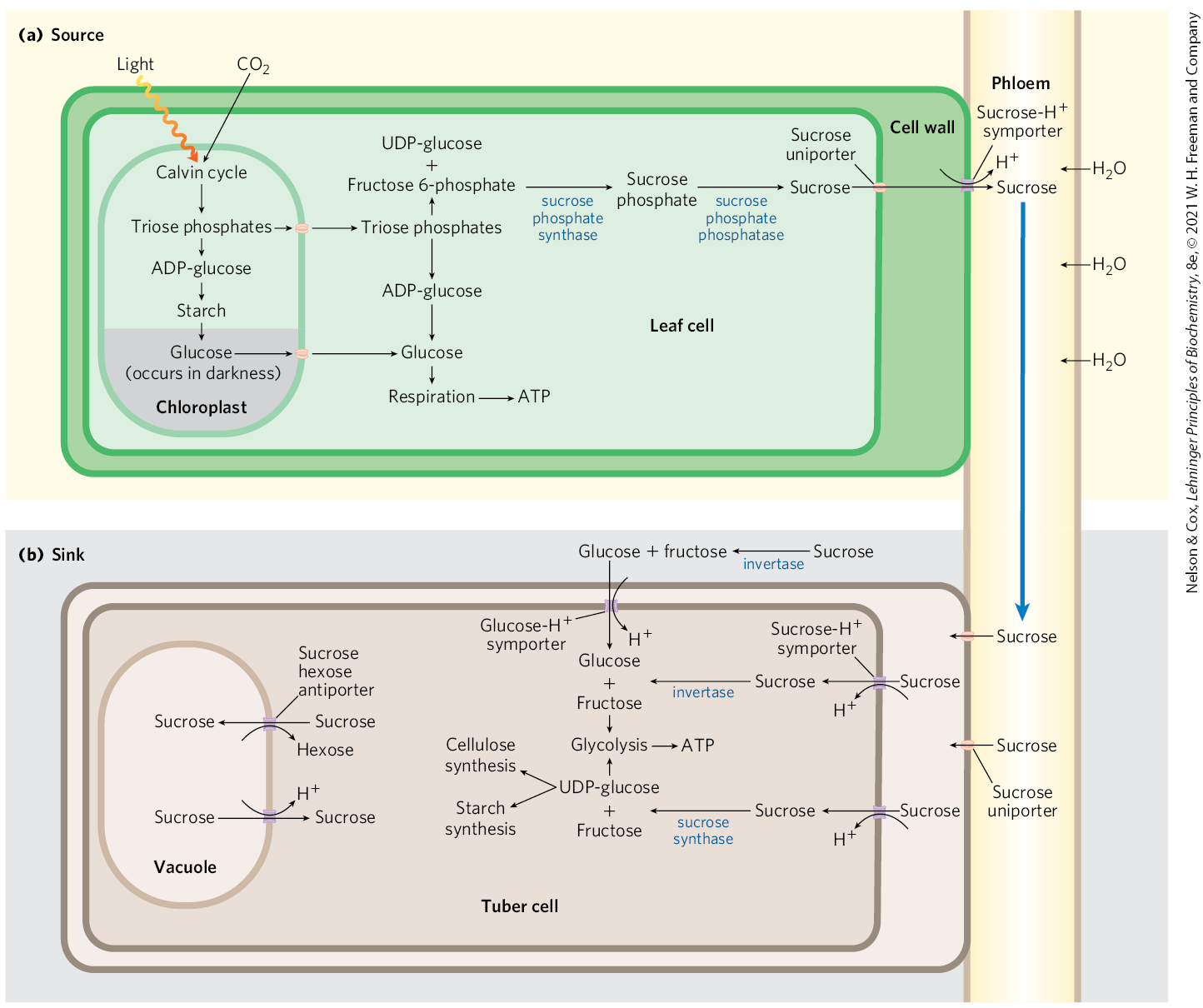
FIGURE 20-49 Movement of sucrose between source and sink tissues. (a) In daylight, photosynthetic leaves (source tissue) fix into triose phosphates via the Calvin cycle in chloroplasts. Some of the triose phosphate is used in the chloroplasts to synthesize starch; the rest is exported to the cytosol, where it can be converted via gluconeogenesis to fructose 6-phosphate and glucose 1-phosphate. Sucrose, synthesized from UDP-glucose and fructose, is exported from leaf mesophyll cells to the plant phloem; the resulting high sucrose content draws water into the phloem by osmosis. The resulting increased turgor pressure (p. 52) pushes the solution in the phloem toward sink tissues. (b) Sucrose moves from the phloem into the sink tissues, where it is converted to starch or cell wall cellulose, or is used as fuel for glycolysis, the citric acid cycle, and oxidative phosphorylation to provide ATP for these nonphotosynthetic tissues. Sugar transport across the plasma membrane and between intracellular compartments is catalyzed by several symporters and antiporters coupled to a proton gradient. [Information from Dr. Gerald Edwards, School of Biological Sciences, Washington State University.]
SUMMARY 20.6 Biosynthesis of Starch, Sucrose, and Cellulose
- Starch synthase in chloroplasts and amyloplasts catalyzes the addition of single glucose residues, donated by ADP-glucose, to the growing polymer chain.
- Sucrose is synthesized in the cytosol from UDP-glucose and fructose 1-phosphate, in two steps.
- The partitioning of triose phosphates between sucrose synthesis and starch synthesis is regulated by fructose 2,6-bisphosphate (F26BP). [F26BP] varies inversely with the rate of photosynthesis, and F26BP inhibits the synthesis of fructose 6-phosphate, the precursor of sucrose.
- The glyoxylate cycle, taking place in the glyoxysomes of germinating seeds of some plants, uses several citric acid cycle enzymes and two additional enzymes: isocitrate lyase and malate synthase. The two decarboxylation steps of the citric acid cycle are bypassed, making possible the net formation of succinate, oxaloacetate, and other cycle intermediates from acetyl-CoA.
- Cellulose synthase has a glycosyl transferase activity in its cytoplasmic domain and forms a transmembrane channel through which the growing cellulose chain is extruded. Glucose units are transferred from UDP-glucose to the nonreducing end of the growing chain.
- The plant cell shares pools of common intermediates, including hexose-, pentose-, and triose-phosphates. Transporters in the membranes of chloroplasts, mitochondria, and amyloplasts mediate the movement of sugar phosphates between organelles. The direction of metabolite flow through the pools within a leaf changes from day to night.
- Sucrose produced in a photosynthetic (source) tissue is exported to nonphotosynthetic (sink) tissue such as roots and tubers via the plant phloem.
 The excess is converted to sucrose and transported to other parts of the plant, to be used as fuel or stored. In most plants, starch is the main storage form of carbohydrate, but in a few plants, such as sugar beet and sugarcane, sucrose is the primary storage form. The synthesis of sucrose and starch occurs in different cellular compartments (cytosol and plastids, respectively), and these processes are coordinated by a variety of regulatory mechanisms that respond to changes in light level and photosynthetic rate. The synthesis of sucrose and starch is important to the plant but also to humans: starch provides more than 80% of human dietary calories worldwide.
The excess is converted to sucrose and transported to other parts of the plant, to be used as fuel or stored. In most plants, starch is the main storage form of carbohydrate, but in a few plants, such as sugar beet and sugarcane, sucrose is the primary storage form. The synthesis of sucrose and starch occurs in different cellular compartments (cytosol and plastids, respectively), and these processes are coordinated by a variety of regulatory mechanisms that respond to changes in light level and photosynthetic rate. The synthesis of sucrose and starch is important to the plant but also to humans: starch provides more than 80% of human dietary calories worldwide. in
in  ). The large enzyme complex that catalyzes this process moves along the plasma membrane with directionality often related to the course of microtubules in the cell cortex, the cytoplasmic layer just below the membrane (step
). The large enzyme complex that catalyzes this process moves along the plasma membrane with directionality often related to the course of microtubules in the cell cortex, the cytoplasmic layer just below the membrane (step  ). When these microtubules lie perpendicular to the axis of the plant’s growth, the cellulose microfibrils are laid down similarly to promote elongation. The motion of the cellulose synthase complexes is believed to be driven by energy released in the polymerization reaction, not by a molecular motor such as kinesin.
). When these microtubules lie perpendicular to the axis of the plant’s growth, the cellulose microfibrils are laid down similarly to promote elongation. The motion of the cellulose synthase complexes is believed to be driven by energy released in the polymerization reaction, not by a molecular motor such as kinesin. ), just prior to integrating into the cell wall.
), just prior to integrating into the cell wall. Starch synthase in chloroplasts and amyloplasts catalyzes the addition of single glucose residues, donated by ADP-glucose, to the growing polymer chain.
Starch synthase in chloroplasts and amyloplasts catalyzes the addition of single glucose residues, donated by ADP-glucose, to the growing polymer chain.