7.2 Polysaccharides
Most carbohydrates found in nature occur as polysaccharides, polymers of medium to high molecular weight . Polysaccharides, also called glycans, differ from each other in the identity of their recurring monosaccharide units, in the length of their chains, in the types of bonds linking the units, and in the degree of branching. Homopolysaccharides contain only a single monomeric sugar species; heteropolysaccharides contain two or more kinds of monomers (Fig. 7-12). Some homopolysaccharides serve as storage forms of monosaccharides that are used as fuels; starch and glycogen are homopolysaccharides of this type. Other homopolysaccharides (cellulose and chitin, for example) serve as structural elements in plant cell walls and animal exoskeletons. Heteropolysaccharides provide extracellular support for organisms of all kingdoms. For example, the rigid layer of the bacterial cell envelope (the peptidoglycan) is composed in part of a heteropolysaccharide built from two alternating monosaccharide units (see Fig. 6-32). In animal tissues, the extracellular space is occupied by several types of heteropolysaccharides, which form a matrix that holds individual cells together and provides protection, shape, and support to cells, tissues, and organs.

FIGURE 7-12 Homopolysaccharides and heteropolysaccharides. Polysaccharides may be composed of one, two, or several different monosaccharides, in straight or branched chains of varying length.
Unlike proteins, polysaccharides generally do not have defined lengths or molecular weights. This difference is a consequence of the mechanisms of assembly of the two types of polymer. As we shall see in Chapter 27, proteins are synthesized on a template (messenger RNA) of defined sequence and length, by enzymes that follow the template exactly. For polysaccharide synthesis there is no template; rather, the program for polysaccharide synthesis is intrinsic to the enzymes that catalyze the polymerization of the monomeric units, and there is no specific stopping point in the synthetic process; the products thus vary in length.
Some Homopolysaccharides Are Storage Forms of Fuel
The most important storage polysaccharides are starch in plant cells and glycogen in animal cells. Both polysaccharides occur intracellularly as large clusters or granules. Starch and glycogen molecules are heavily hydrated, because they have many exposed hydroxyl groups available to hydrogen-bond with water. Most plant cells have the ability to form starch, and starch storage is especially abundant in tubers (underground stems), such as potatoes, and in seeds.
Starch contains two types of glucose polymer, amylose and amylopectin (Fig. 7-13). Amylose consists of long, unbranched chains of d-glucose residues connected by linkages (as in maltose). Such chains vary in molecular weight from a few thousand to more than a million. Amylopectin is even larger ( up to 200 million) but unlike amylose is highly branched. The glycosidic linkages joining successive glucose residues in amylopectin chains are ; the branch points (occurring every 24 to 30 residues) are linkages.
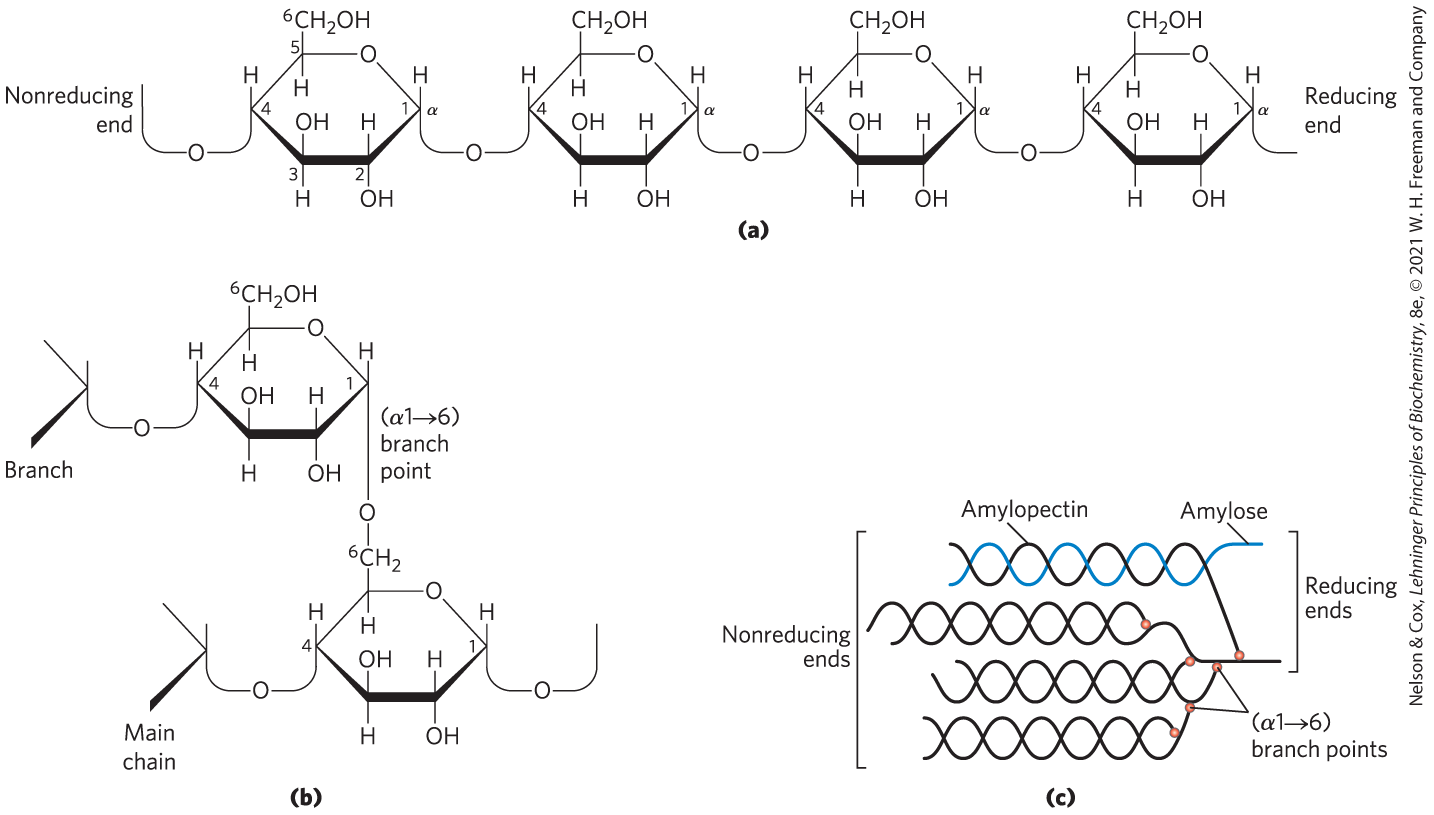
FIGURE 7-13 Starch and glycogen. (a) A short segment of a linear polymer of d-glucose residues in linkage, shown here as Haworth perspectives. This is the basic structure of amylose of starch (in plants) and glycogen (in animals). A single chain of amylose can contain several thousand glucose residues. (b) An branch point of amylopectin or glycogen. (c) A cluster of amylose and amylopectin like that believed to occur in starch granules. Strands of amylopectin (black) form double-helical structures with each other or with amylose strands (blue). Amylopectin has frequent branch points (red). Glucose residues at the nonreducing ends of the outer branches are removed enzymatically during the mobilization of starch for energy production. Glycogen has a similar structure but is more highly branched and more compact.
Glycogen is the main storage polysaccharide of animal cells. Like amylopectin, glycogen is a polymer of -linked glucose subunits, with -linked branches, but glycogen is more extensively branched (on average, a branch every 8 to 12 residues) and more compact than starch. Glycogen is especially abundant in the liver, where it may constitute as much as 7% of the wet weight; it is also present in skeletal muscle. In hepatocytes, glycogen is found in large α granules (see Fig. 15-1), which are clusters of smaller β granules, each of which is a single, highly branched glycogen molecule with an average molecular weight of several million. The large α glycogen granules also contain, in tightly bound form, the enzymes responsible for the synthesis and degradation of glycogen (see Fig. 15-16).
Because each branch of glycogen ends with a nonreducing sugar unit, a glycogen molecule with n branches has nonreducing ends, but only one reducing end. When glycogen is used as an energy source, glucose units are removed one at a time from the nonreducing ends. Degradative enzymes that act only at nonreducing ends can work simultaneously on the many branches, speeding the conversion of the polymer to monosaccharides.
Why not store glucose in its monomeric form? Hepatocytes in the fed state store glycogen equivalent to a glucose concentration of 0.4 m. The actual concentration of glycogen, which contributes little to the osmolarity of the cytosol, is about 0.01 μm. If the cytosol contained 0.4 m glucose, the osmolarity would be threateningly elevated, leading to osmotic entry of water that might rupture the cell (see Fig. 2-12).
Some Homopolysaccharides Serve Structural Roles
Cellulose, a tough, fibrous, water-insoluble substance, is found in the cell walls of plants, particularly in stalks, stems, trunks, and all the woody portions of the plant body. Cellulose constitutes much of the mass of wood, and cotton is almost pure cellulose. Like amylose, the cellulose molecule is a linear, unbranched homopolysaccharide, consisting of 10,000 to 15,000 d-glucose units. But there is a very important difference: in cellulose the glucose residues have the β configuration (Fig. 7-14), whereas in amylose the glucose is in the α configuration. The glucose residues in cellulose are linked by glycosidic bonds, in contrast to the bonds of amylose. This difference causes individual molecules of cellulose and amylose to fold differently in space, giving them very different macroscopic structures and physical properties.
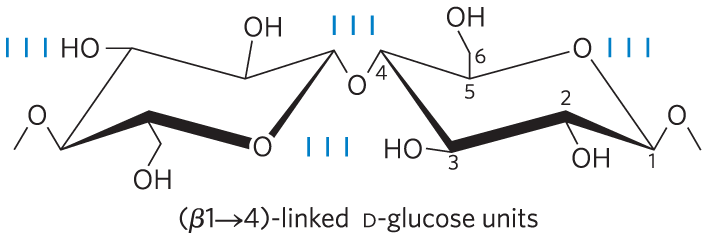
FIGURE 7-14 Cellulose. Two units of a cellulose chain; the d-glucose residues are in linkage. The rigid chair structures can rotate relative to one another.
The tough, fibrous nature of cellulose makes it useful in such commercial products as cardboard and insulation material, and it is a major constituent of cotton and linen fabrics. Cellulose is also the starting material for the commercial production of cellophane, rayon, and lyocell.
Chitin is a linear homopolysaccharide composed of N-acetylglucosamine residues in linkage (Fig. 7-15). The only chemical difference from cellulose is the replacement of the hydroxyl group at C-2 with an acetylated amino group, which makes chitin more hydrophobic and water-resistant than cellulose. Chitin forms extended fibers similar to those of cellulose, and like cellulose cannot be digested by vertebrates. Chitin is the principal component of the hard exoskeletons of nearly a million species of arthropods — insects, lobsters, and crabs, for example — and is probably the second most abundant polysaccharide, next to cellulose, in nature; an estimated 1 billion tons of chitin are produced in the biosphere each year.
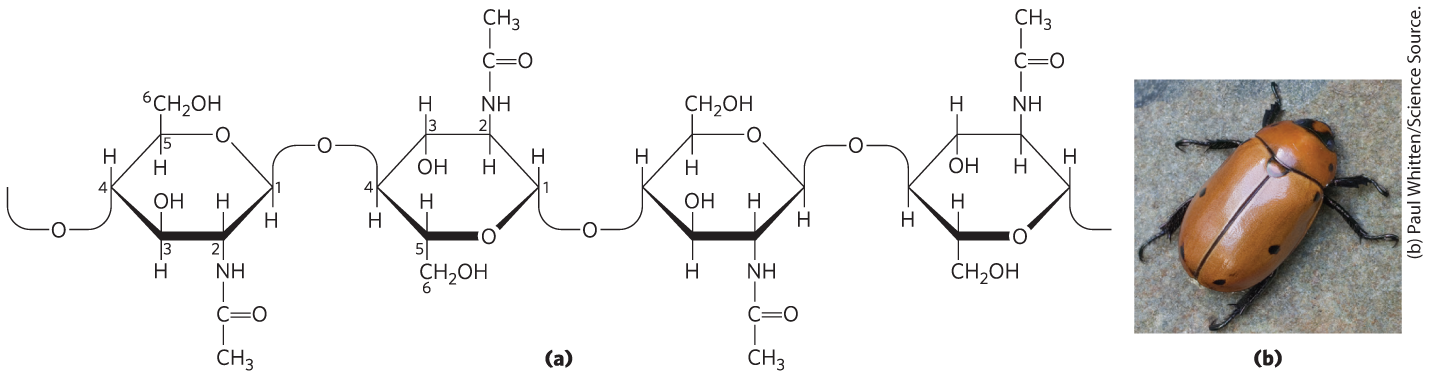
FIGURE 7-15 Chitin. (a) A short segment of chitin, a homopolymer of N-acetyl-d-glucosamine units in linkage. (b) A spotted June beetle (Pelidnota punctata), showing its surface armor (exoskeleton) of chitin.
Steric Factors and Hydrogen Bonding Influence Homopolysaccharide Folding
The folding of polysaccharides in three dimensions follows the same principles as those governing polypeptide structure: subunits with a more-or-less rigid structure dictated by covalent bonds form three-dimensional macromolecular structures that are stabilized by weak interactions within or between molecules, such as hydrogen bonds, interactions due to the hydrophobic effect, van der Waals interactions, and, for polymers with charged subunits, electrostatic interactions. Because polysaccharides have so many hydroxyl groups, hydrogen bonding has an especially important influence on their structure. Glycogen, starch, cellulose, and chitin are composed of pyranoside (six-membered ring) subunits, as are the oligosaccharides of glycoproteins and glycolipids, to be discussed later. Such molecules can be represented as a series of rigid pyranose rings connected by an oxygen atom bridging two carbon atoms (the glycosidic bond). There is, in principle, free rotation about both COO bonds linking the residues, but as in polypeptides (see Figs 4-2, 4-8), rotation about each bond is limited by steric hindrance by substituents. The three-dimensional structures of these molecules can be described in terms of the dihedral angles, ϕ and ψ, about the glycosidic bond (Fig. 7-16). The bulkiness of the pyranose ring and its substituents, along with electronic effects at the anomeric carbon, place constraints on the angles ϕ and ψ; thus, certain conformations are much more stable than others.

FIGURE 7-16 Different energetic conformations of a disaccharide. The torsion angles ϕ (phi) and ψ (psi), which define the spatial relationship between adjacent rings, can in principle have any value from to . In fact, some torsion angles give conformations that maximize hydrogen bonding and minimize energy, whereas others give conformations that are sterically hindered, as we can see in two conformers of the disaccharide GalGal at opposite energetic extremes.
The most stable three-dimensional structure for the -linked chains of starch and glycogen is a tightly coiled helix (Fig. 7-17), stabilized by interchain hydrogen bonds. The average plane of each residue along the amylose chain forms a angle with the average plane of the preceding residue, so the helical structure has six residues per turn. For amylose, the core of the helix is of precisely the right dimensions to accommodate iodine as complex ions . This interaction gives an intensely blue product, making it a common qualitative test for amylose.
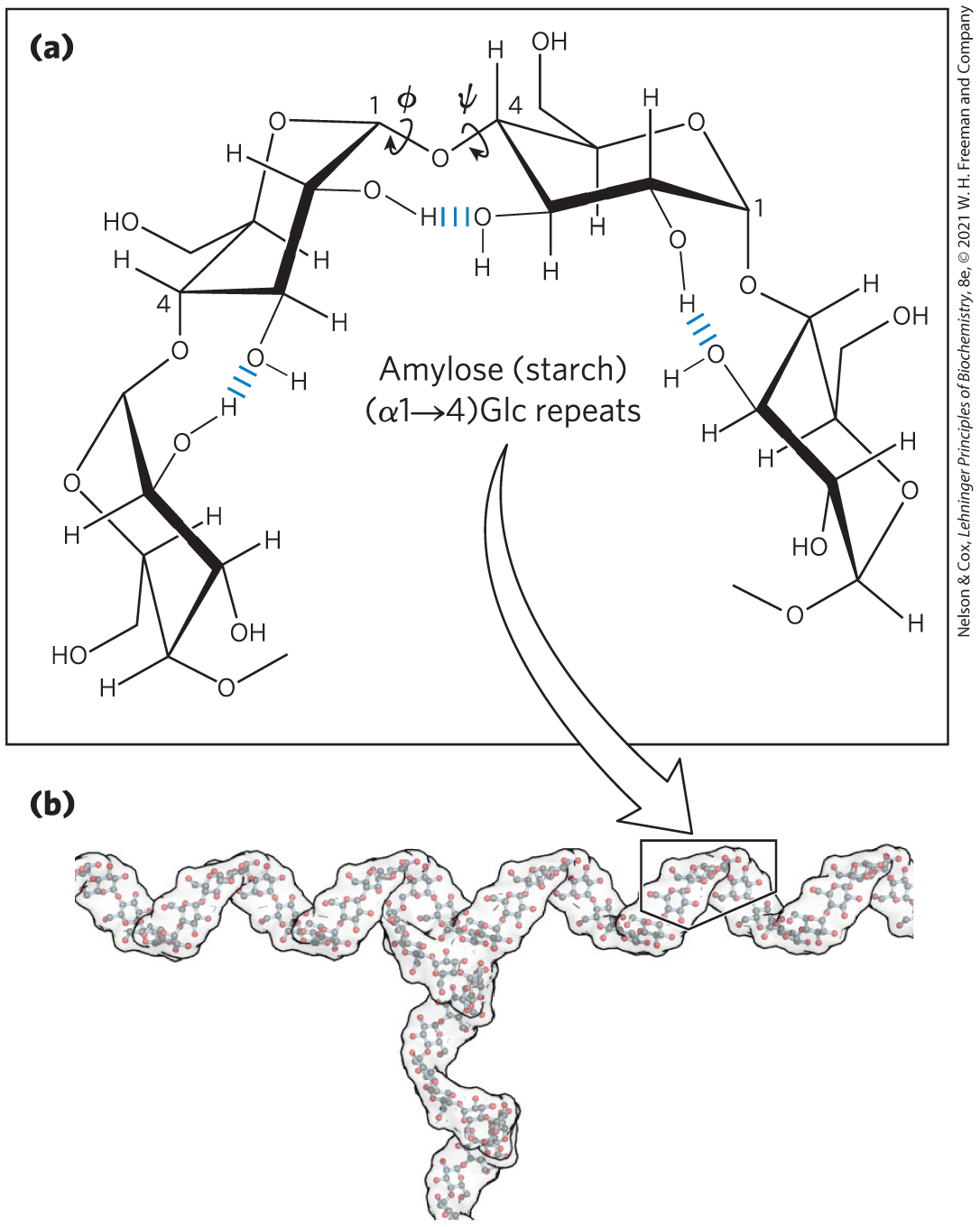
FIGURE 7-17 Helical structure of starch (amylose). (a) Four glucose units of starch (amylose), showing both the free rotation possible around the ψ and ϕ angles of the glycosidic bonds and the hydrogen bonding possible between adjacent rings. In this most stable conformation, with adjacent rigid chairs at to one another, the polysaccharide chain is curved. (b) A model of a segment of amylopectin (branched amylose). The conformation of linkages in amylose, amylopectin, and glycogen causes these polymers to assume tightly coiled helical structures. [(b) Data from www.biotopics.co.uk/jsmol/amylopectin.html.]
For cellulose, the most stable conformation is that in which each chair is turned relative to its neighbors, yielding a straight, extended chain. All —OH groups are available for hydrogen bonding with neighboring chains. With several chains lying side by side, a stabilizing network of interchain and intrachain hydrogen bonds produces straight, stable supramolecular fibers of great tensile strength (Fig. 7-18). This property of cellulose has made it useful to civilizations for millennia. Many manufactured products, including papyrus, paper, cardboard, rayon, insulating tiles, and a variety of other useful materials, are derived from cellulose. The water content of these materials is low because extensive interchain hydrogen bonding between cellulose molecules satisfies their capacity for hydrogen-bond formation.
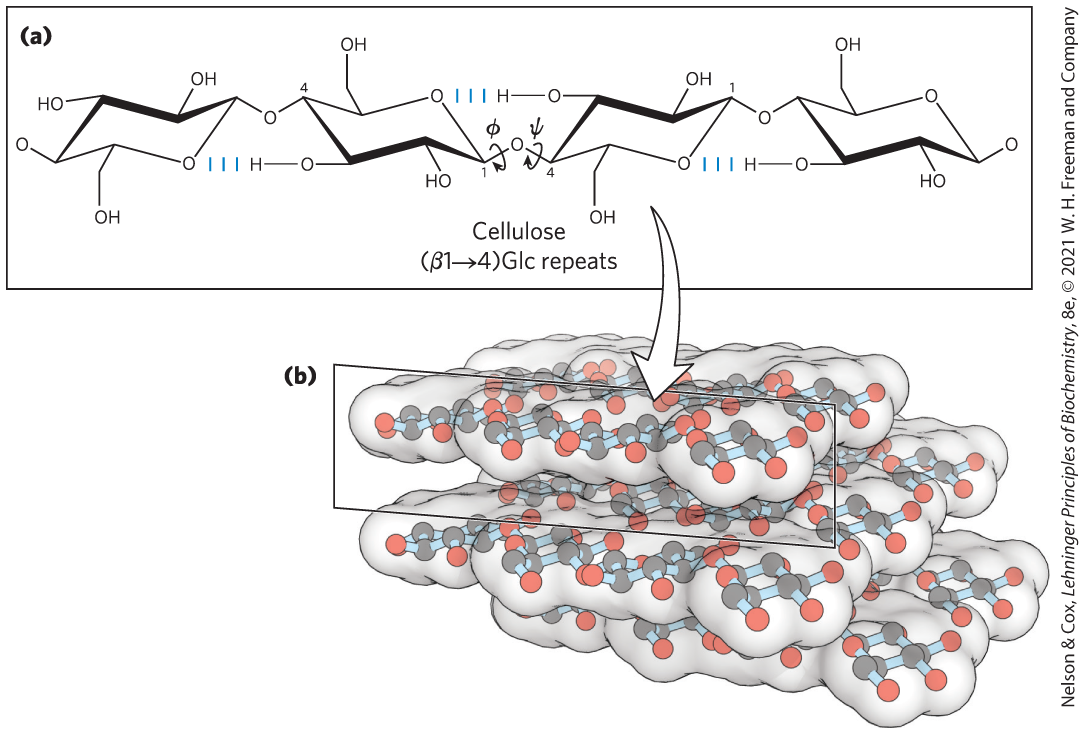
FIGURE 7-18 The linear structure of cellulose chains. (a) Four glucose units of cellulose. In the most stable conformation of cellulose, the glycosidic linkages put the adjacent rigid chairs at relative to each other. (b) A model of straight, unbranched cellulose segments, held together in layers stabilized by interchain hydrogen bonds. [(b) Data from Cornell, B. 2016. Sugar Polymers. Available at: http://ib.bioninja.com.au. (Accessed 11 March 2020).]
Peptidoglycan Reinforces the Bacterial Cell Wall
The rigid component of bacterial cell walls (peptidoglycan) is a heteropolymer of alternating -linked N-acetylglucosamine and N-acetylmuramic acid residues (see Fig. 6-32). The linear polymers lie side by side in the cell wall, cross-linked by short peptides, the exact structure of which depends on the bacterial species. The peptide cross-links weld the polysaccharide chains into a strong sheath (peptidoglycan) that envelops the entire cell and prevents cellular swelling and lysis due to the osmotic entry of water.
The enzyme lysozyme kills bacteria by hydrolyzing the glycosidic bond between N-acetylglucosamine and N-acetylmuramic acid. The enzyme is found in human tears, where it is presumably a defense against bacterial infections of the eye, and in hen’s eggs, where it prevents bacterial infection of the developing embryo. Penicillin and related antibiotics kill bacteria by preventing synthesis of the peptidoglycan cross-links, leaving the cell wall too weak to resist osmotic lysis (p. 211).
Glycosaminoglycans Are Heteropolysaccharides of the Extracellular Matrix
The extracellular space in the tissues of multicellular animals is filled with a gel-like material, the extracellular matrix (ECM), which holds the cells together and provides a porous pathway for the diffusion of nutrients and oxygen to individual cells. The ECM is composed of an interlocking meshwork of heteropolysaccharides (also called ground substance) and fibrous proteins such as fibrillar collagens, elastins, and fibronectins. The basement membrane is a specialized ECM that underlies epithelial cells; it consists of specialized collagens, laminins, and heteropolysaccharides.
These heteropolysaccharides, the glycosaminoglycans, are a family of linear polymers composed of repeating disaccharide units (Fig. 7-19). They are unique to animals and bacteria and are not found in plants. One of the two monosaccharides is always either N-acetylglucosamine or N-acetylgalactosamine; the other is in most cases a uronic acid, usually d-glucuronic or l-iduronic acid. Some glycosaminoglycans contain esterified sulfate groups. The combination of sulfate groups and the carboxylate groups of the uronic acid residues gives glycosaminoglycans a very high density of negative charge. To minimize the repulsive forces among neighboring charged groups, these molecules assume an extended conformation in solution, forming a rodlike helix in which the negatively charged carboxylate groups occur on alternate sides of the helix (as shown for heparin in Fig. 7-19). The extended rod form also provides maximum separation between the negatively charged sulfate groups. The specific patterns of sulfated and nonsulfated sugar residues in glycosaminoglycans allow specific recognition by a variety of protein ligands that bind electrostatically to these molecules. The sulfated glycosaminoglycans are attached to extracellular proteins to form proteoglycans (Section 7.3).
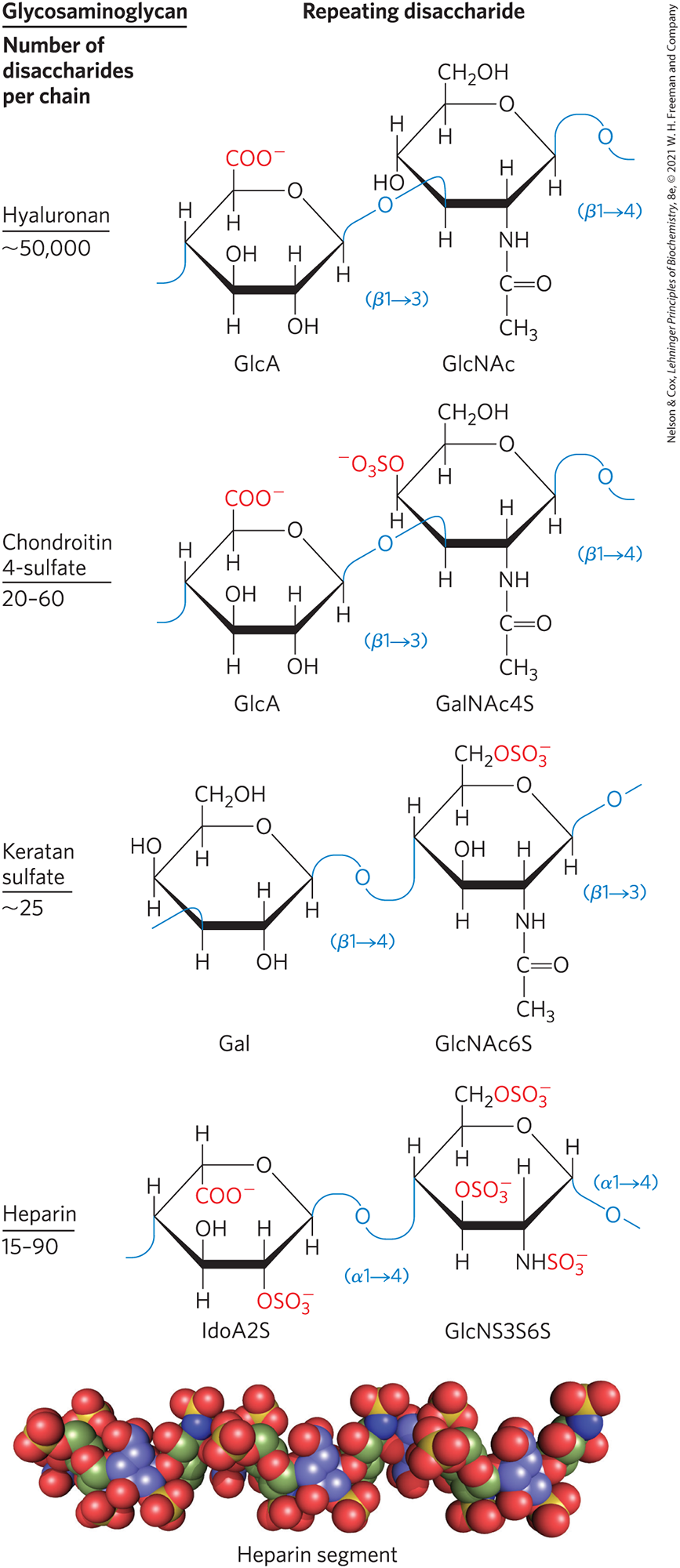
FIGURE 7-19 Repeating units of some common glycosaminoglycans of extracellular matrix. The molecules are copolymers of alternating uronic acid and amino sugar residues (keratan sulfate is the exception), with sulfate esters in any of several positions, except in hyaluronan. The ionized carboxylate and sulfate groups give these polymers their characteristic high negative charge. Therapeutic heparin contains primarily iduronic acid (IdoA) and a smaller proportion of glucuronic acid (not shown) and is generally highly sulfated and heterogeneous in length. The space-filling model shows a heparin segment as its structure in solution, as determined by NMR spectroscopy. Carbons in iduronic acid sulfate are colored blue; those in glucosamine sulfate are green. Oxygen and sulfur are shown in red and yellow, respectively. Hydrogen atoms are not shown (for clarity). [Data for molecular model from PDB ID 1HPN, B. Mulloy et al., Biochem. J. 293:849, 1993.]
The glycosaminoglycan hyaluronan (hyaluronic acid) contains alternating residues of d-glucuronic acid and N-acetylglucosamine (Fig. 7-19). With up to 50,000 repeats of the basic disaccharide unit, hyaluronan has a molecular weight of several million; it forms clear, highly viscous, noncompressible solutions that serve as lubricants in the synovial fluid of joints and give the vitreous humor of the vertebrate eye its jellylike consistency (the Greek hyalos means “glass”; hyaluronan can have a glassy or translucent appearance). Hyaluronan is also a component of the ECM of cartilage and tendons, to which it contributes tensile strength and elasticity as a result of its strong noncovalent interactions with other components of the matrix. Hyaluronidase, an enzyme secreted by some pathogenic bacteria, can hydrolyze the glycosidic linkages of hyaluronan, rendering tissues more susceptible to bacterial invasion. In many animal species, a similar enzyme in sperm hydrolyzes the outer glycosaminoglycan coat around an ovum, allowing sperm penetration.
Other glycosaminoglycans differ from hyaluronan in three respects: they are generally much shorter polymers, they are covalently linked to specific proteins (proteoglycans), and one or both monomeric units differ from those of hyaluronan. Chondroitin sulfate (Greek chondros, “cartilage”) contributes to the tensile strength of cartilage, tendons, ligaments, heart valves, and the walls of the aorta. Dermatan sulfate (Greek derma, “skin”) contributes to the pliability of skin and is also present in blood vessels and heart valves. In this polymer, many of the glucuronate residues present in chondroitin sulfate are replaced by their C-5 epimer, l-iduronate (IdoA).
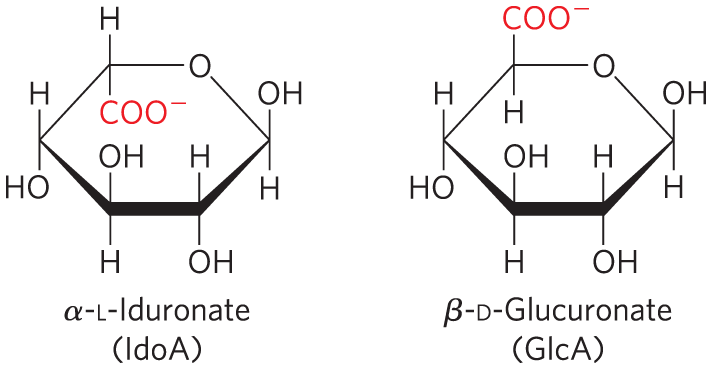
Keratan sulfates (Greek keras, “horn”) have no uronic acid, and their sulfate content is variable. They are present in cornea, cartilage, bone, and a variety of horny structures formed from dead cells: horn, hair, hoofs, nails, and claws. Heparan sulfate (Greek hēpar, “liver”; it was originally isolated from dog liver) contains variable, nonrandom arrangements of sulfated and nonsulfated sugars. The exact sequence of sulfated residues gives the molecule the ability to interact specifically with a large number of proteins, including growth factors and ECM components, as well as various enzymes and factors present in plasma. Heparin is a highly sulfated, intracellular form of heparan sulfate produced primarily by mast cells (a type of leukocyte, or immune cell). Its physiological role is not yet clear, but purified heparin is used as a therapeutic agent to inhibit coagulation of blood through its capacity to bind the protease inhibitor antithrombin (see Fig. 7-24).
Table 7-2 summarizes the composition, properties, roles, and occurrence of the polysaccharides described in Section 7.2.
| Polymer | Typea | Repeating unitb | Size (number of monosaccharide units) | Roles/significance |
|---|---|---|---|---|
| Starch | Energy storage: in plants | |||
| Amylose | Homo- | , linear | 50–5,000 | |
| Amylopectin | Homo- | , with branches every 24–30 residues | Up to | |
| Glycogen | Homo- | , with branches every 8–12 residues | Up to 50,000 | Energy storage: in bacteria and animal cells |
| Cellulose | Homo- | Up to 15,000 | Structural: in plants, gives rigidity and strength to cell walls | |
| Chitin | Homo- | NAc | Very large | Structural: in insects, spiders, crustaceans, gives rigidity and strength to exoskeletons |
| Dextran | Homo- | , with branches | Wide range | Structural: in bacteria, extracellular adhesive |
| Peptidoglycan | Hetero-; peptides attached | 4)Mur2Ac GlcNAc | Very large | Structural: in bacteria, gives rigidity and strength to cell envelope |
| Hyaluronan (a glycosaminoglycan) | Hetero-; acidic | 4)GlcA GlcNAc | Up to 100,000 | Structural: in vertebrates, extracellular matrix of skin and connective tissue; viscosity and lubrication in joints |
|
||||
SUMMARY 7.2 Polysaccharides
- Homopolysaccharides contain only a single monomeric sugar species; heteropolysaccharides contain two or more kinds of monomers.
- The homopolysaccharides starch and glycogen are storage fuels in plant, animal, and bacterial cells. They consist of d-glucose units with linkages, and both contain some branches.
- The homopolysaccharides cellulose, chitin, and dextran serve structural roles. Cellulose, composed of -linked d-glucose residues, lends strength and rigidity to plant cell walls. Chitin, a polymer of -linked N-acetylglucosamine, strengthens the exoskeletons of arthropods.
- Homopolysaccharides assume stable conformations dictated by weak interactions. The chair form of the pyranose ring is essentially rigid, so the conformation of the polymers is determined by rotation about the C—O bonds of the glycosidic linkage. Starch and glycogen form helical structures with intrachain hydrogen bonding; cellulose and chitin form long, straight strands that interact with neighboring strands.
- Bacterial cell walls are strengthened by peptidoglycan in which the repeating disaccharide is GlcNAc Mur2Ac.
- Glycosaminoglycans are extracellular heteropolysaccharides in which one of the two monosaccharide units is a uronic acid (keratan sulfate is an exception) and the other is an N-acetylated amino sugar. The high density of negative charge on these molecules forces them to assume extended conformations. These polymers (hyaluronan, chondroitin sulfate, dermatan sulfate, and keratan sulfate) provide viscosity, adhesiveness, and tensile strength to the extracellular matrix.
 Polysaccharides, also called
Polysaccharides, also called  Unlike proteins, polysaccharides generally do not have defined lengths or molecular weights. This difference is a consequence of the mechanisms of assembly of the two types of polymer. As we shall see in
Unlike proteins, polysaccharides generally do not have defined lengths or molecular weights. This difference is a consequence of the mechanisms of assembly of the two types of polymer. As we shall see in  Hepatocytes in the fed state store glycogen equivalent to a glucose concentration of 0.4
Hepatocytes in the fed state store glycogen equivalent to a glucose concentration of 0.4  in cellulose the glucose residues have the β configuration (
in cellulose the glucose residues have the β configuration ( Homopolysaccharides contain only a single monomeric sugar species; heteropolysaccharides contain two or more kinds of monomers.
Homopolysaccharides contain only a single monomeric sugar species; heteropolysaccharides contain two or more kinds of monomers.