15.1 The Structure and Function of Glycogen
Structure–function relationships are key to understanding biomolecules and biochemical systems, and glycogen is no exception. The compact structure of glycogen granules allows cells to store glucose when it is available in excess, and to make it available on short notice when needed. Subtle tissue-specific differences in the enzymes (isozymes) that act on glycogen determine the dynamics of glycogen metabolism in each tissue.
Vertebrate Animals Require a Ready Fuel Source for Brain and Muscle
For all vertebrate animals, maintaining a ready supply of glucose for the brain and muscles is a top metabolic priority. The challenge for cells is to be able to store glucose in a form that rapidly sequesters it when the glucose concentration in the blood is high (say, after a meal) but allows it to be accessed quickly for use particularly by the brain and skeletal muscle. Recall from our discussion in Chapter 7 (p. 242) that a typical hepatocyte in the fed state stores an amount of glucose, polymerized as glycogen, that in monomeric form would be equivalent to about . At this concentration, the osmolarity of the cell would be so high relative to the surrounding fluid that water would enter the cell and likely rupture it.
When the diet temporarily provides more carbohydrate than is needed immediately as fuel, excess glucose is polymerized into glycogen. Small amounts of glycogen are present in all animal cells, but it is stored primarily in liver and muscle, where it is a significant portion of the wet weight of the organ (5% to 10% of liver and 1% to 2% of muscle). A 70 kg human stores about 100 g of glycogen in the liver and up to 400 g in skeletal muscle. The total amount of energy stored in the body as glycogen is far less (about 1%) than the amount stored as fat (triacylglycerol), but fats cannot be converted to glucose in vertebrates and cannot be catabolized anaerobically through glycolysis, as is often required in skeletal muscle.
When a sudden burst of physical activity demands a quick source of energy in muscle, the rapid breakdown of glycogen stored there provides glucose for glycolysis within seconds. Between meals or during a fast, the release of glucose from glycogen stored in the liver provides a steady supply of glucose in the blood. This is especially important for the brain, a major consumer of metabolic energy, which, unlike muscle, cannot use fatty acids as fuel; long-chain fatty acids do not cross the blood-brain barrier. The brain therefore depends on a constant supply of glucose, from the diet or from the liver.
Glycogen Granules Have Many Tiers of Branched Chains of d-Glucose
Glycogen is stored as cytosolic granules, called β-granules, that vary in size, structure, and subcellular location depending on the tissue or cell type. (In this chapter we focus on liver and muscle.) The size of β-granules also varies with the state of activity and feeding of the animal. In muscle, β-granules are 20–30 nm in diameter and have an of . They consist of up to 55,000 glucose residues with about 2,000 nonreducing ends available for degradative enzymes to work on. In liver, 20 to 40 β-granules cluster together to form protein-rich α-granules as large as 300 nm in diameter and of greater than . They are visible with the electron microscope in tissue samples from well-fed animals (Fig. 15-1), but they are essentially absent after a 24-hour fast. The β-granules of muscle release glucose more quickly than the α-granules of liver, consistent with the different needs of these tissues for glucose.
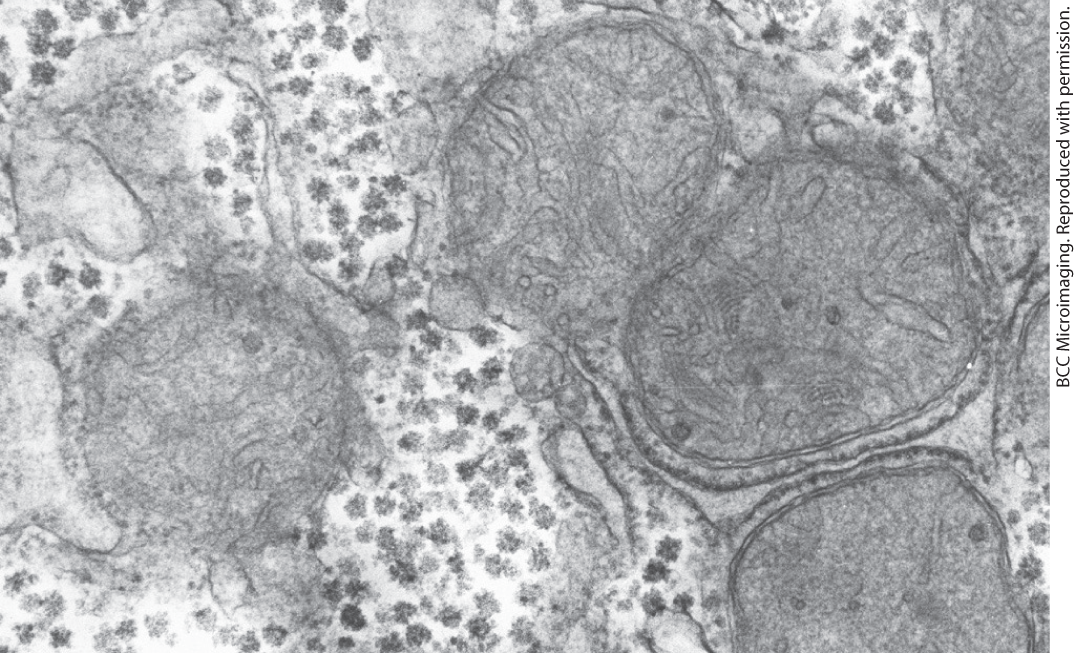
FIGURE 15-1 Glycogen granules in a hepatocyte. Glycogen β-granules appear as electron-dense particles. In liver they form larger clusters called α-granules and are often associated with tubules of the smooth endoplasmic reticulum. Four mitochondria are also evident in this micrograph.
All glycogen granules have at their core a dimer of the protein glycogenin, which serves as a primer for the synthesis of polymers of d-glucose. In the tiered β-granule model, the central glycogenin dimer is surrounded by tier upon tier of chains of about 13 glucose residues in linkage, with -linked branches. Inner B-chains contain two branch points, and outer A-chains are unbranched (Fig. 15-2). Granules typically have 6 or 7 tiers, with the outermost tier of unbranched A-chains making up the majority of the granule. Associated with each β-granule are patches of electron-dense, protein-rich material, called γ-particles. Among the associated proteins are the enzymes that synthesize and break down glycogen.
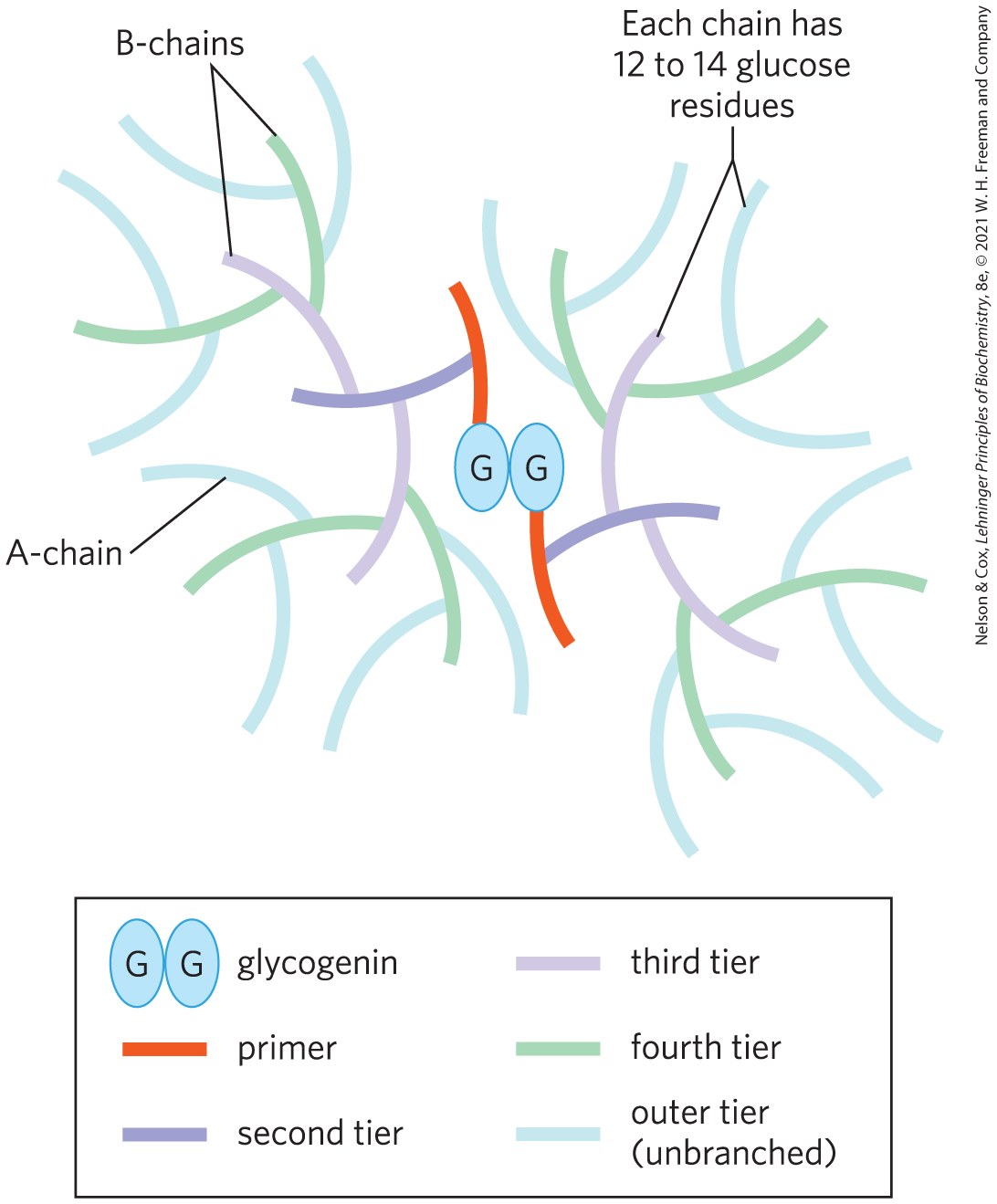
FIGURE 15-2 Structure of a glycogen β-granule. Starting at a central glycogenin homodimer, glycogen chains (12 to 14 residues) extend in tiers. Inner chains (B-chains) have two branches each. A-chains in the outer tier are unbranched. There are in theory a maximum of 12 tiers in a mature glycogen β-granule (only 5 are shown here), consisting of about 55,000 glucose residues in a molecule of about 21 nm diameter and .
The general mechanisms for storing and mobilizing glycogen are the same in muscle and liver, but the enzymes involved differ in subtle yet important ways that reflect the different roles of glycogen in the two tissues. In the next two sections we will look at the enzymatic basis for glycogen synthesis and breakdown, and at the regulation of these processes.
SUMMARY 15.1 The Structure and Function of Glycogen
- All cells need ready access to glucose, either from the diet or from supplies stored in cells. Glycogen is a polymeric storage form of glucose in animals that is found primarily in muscle and liver. Glycogen breakdown in muscle delivers glucose needed for muscle contraction. Glycogen stored in the liver provides a reservoir that maintains homeostasis of blood glucose throughout the body.
- Glycogen β-granules have tiers of glucose residues in linkage, with -linked branches, providing many free nonreducing ends for synthetic and degradative enzymes to access. In liver, β-granules cluster into larger α-granules, which release glucose more slowly.
 For all vertebrate animals, maintaining a ready supply of glucose for the brain and muscles is a top metabolic priority. The challenge for cells is to be able to store glucose in a form that rapidly sequesters it when the glucose concentration in the blood is high (say, after a meal) but allows it to be accessed quickly for use particularly by the brain and skeletal muscle. Recall from our discussion in
For all vertebrate animals, maintaining a ready supply of glucose for the brain and muscles is a top metabolic priority. The challenge for cells is to be able to store glucose in a form that rapidly sequesters it when the glucose concentration in the blood is high (say, after a meal) but allows it to be accessed quickly for use particularly by the brain and skeletal muscle. Recall from our discussion in  All cells need ready access to glucose, either from the diet or from supplies stored in cells. Glycogen is a polymeric storage form of glucose in animals that is found primarily in muscle and liver. Glycogen breakdown in muscle delivers glucose needed for muscle contraction. Glycogen stored in the liver provides a reservoir that maintains homeostasis of blood glucose throughout the body.
All cells need ready access to glucose, either from the diet or from supplies stored in cells. Glycogen is a polymeric storage form of glucose in animals that is found primarily in muscle and liver. Glycogen breakdown in muscle delivers glucose needed for muscle contraction. Glycogen stored in the liver provides a reservoir that maintains homeostasis of blood glucose throughout the body.