16.1 Production of Acetyl-CoA (Activated Acetate)
Coenzyme A, or CoA (Fig. 16-2), has a reactive thiol group that is critical to its role as an acyl carrier in many metabolic processes, including the citric acid cycle. Acyl groups are covalently linked to the thiol group, forming thioesters. Because of their relatively high standard free energies of hydrolysis (see Figs. 13-16, 13-17), thioesters have a high acyl group transfer potential — that is, donation of their acyl groups to a variety of acceptor molecules is a favorable reaction. The acyl group attached to coenzyme A may thus be thought of as “activated” for group transfer.
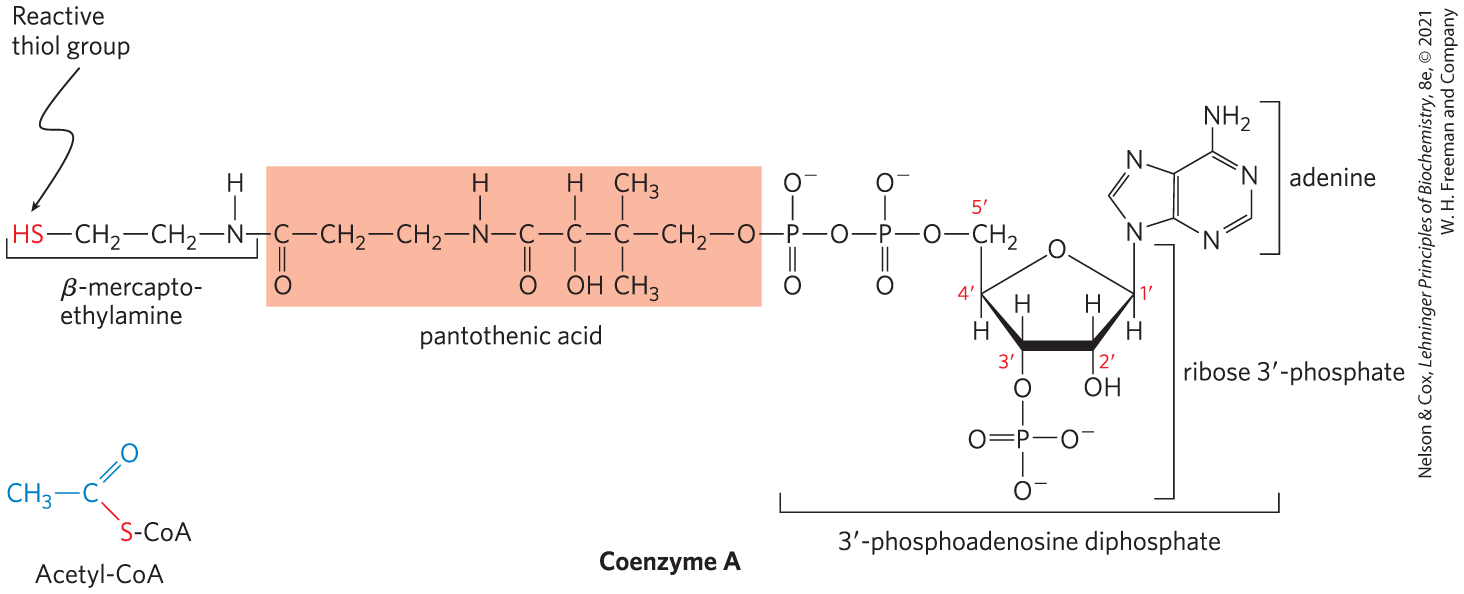
FIGURE 16-2 Coenzyme A (CoA-SH). A hydroxyl group of pantothenic acid is joined to a modified ADP moiety by a phosphate ester bond, and its carboxyl group is attached to β-mercaptoethylamine in amide linkage. The hydroxyl group at the position of the ADP moiety has a phosphoryl group not present in free ADP. The group of the mercaptoethylamine moiety forms a thioester with acetate in acetyl-coenzyme A (acetyl-CoA) (lower left).
In aerobic organisms, glucose and other sugars, fatty acids, and most amino acids are ultimately oxidized to and via the citric acid cycle and the respiratory chain. Before entering the citric acid cycle, the carbon skeletons of monosaccharides and fatty acids are degraded to the acetyl group of acetyl-CoA, the form in which the cycle accepts much of its fuel input. Many amino acid carbons also enter the cycle as acetate, although several amino acids are degraded to other cycle intermediates such as succinate and malate, which then enter the cycle.
Pyruvate Is Oxidized to Acetyl-CoA and
Pyruvate generated in the cytosol by glycolysis represents a node in the metabolism of carbohydrates, proteins, and fats. Under anaerobic conditions, pyruvate may simply be reduced to lactate in the cytosol, regenerating for continued ATP production by glycolysis. Pyruvate may serve as a precursor for the synthesis of amino acids (Chapter 22). In eukaryotes, pyruvate may diffuse into mitochondria, first through large openings in the outer mitochondrial membrane and then into the matrix via an -coupled pyruvate-specific symporter in the inner mitochondrial membrane, the mitochondrial pyruvate carrier (MPC). Pyruvate that enters mitochondria may be oxidized by the citric acid cycle to generate energy or, after conversion to acetyl-CoA, may be used as the starting material for synthesis of fatty acids and sterols.
Pyruvate in the mitochondrial matrix is oxidized to acetyl-CoA and by the pyruvate dehydrogenase (PDH) complex, a highly ordered cluster of enzymes and cofactors. In the PDH complex, a series of chemical intermediates remain bound to the enzyme subunits as a substrate (pyruvate) is transformed into the final product (acetyl-CoA). Five cofactors, four of which are derived from vitamins, participate in the reaction mechanism. The regulation of this enzyme complex illustrates how a combination of covalent modification and allosteric mechanism results in precisely regulated flux through a metabolic step.
The overall reaction catalyzed by the pyruvate dehydrogenase complex is an oxidative decarboxylation, an irreversible oxidation process in which the carboxyl group is removed from pyruvate as a molecule of and the two remaining carbons become the acetyl group of acetyl-CoA (Fig. 16-3). The NADH formed in this reaction gives up a hydride ion to the respiratory chain (Fig. 16-1), which carries the two electrons to oxygen or, in anaerobic microorganisms, to an alternative electron acceptor such as nitrate or sulfate. The transfer of electrons from NADH to oxygen ultimately generates 2.5 molecules of ATP per pair of electrons. The irreversibility of the PDH complex reaction has been demonstrated by isotopic labeling experiments: the complex cannot reattach radioactively labeled to acetyl-CoA to yield carboxyl-labeled pyruvate.
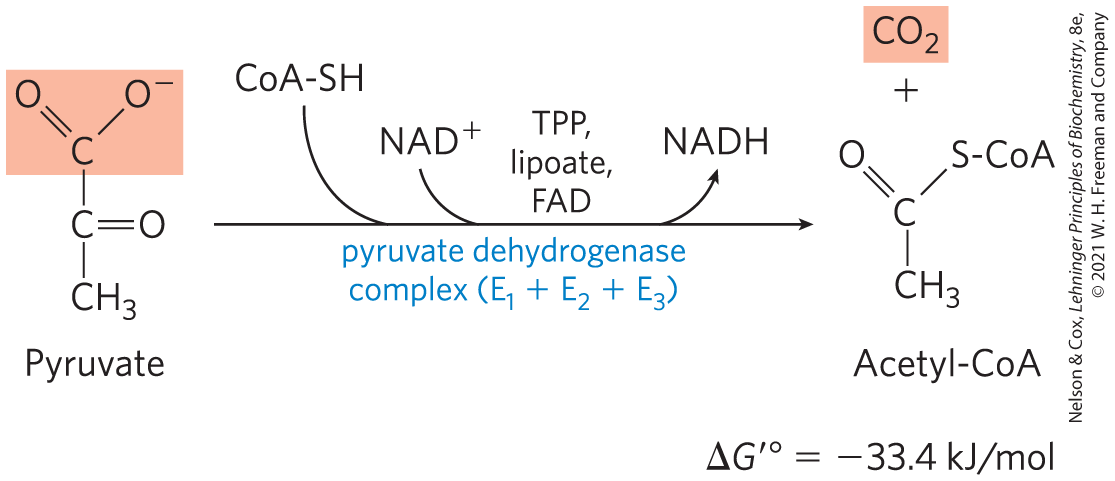
FIGURE 16-3 Overall reaction catalyzed by the pyruvate dehydrogenase complex. The five coenzymes participating in this reaction, and the three enzymes that make up the enzyme complex, are discussed in the text.
The PDH Complex Employs Three Enzymes and Five Coenzymes to Oxidize Pyruvate
The combined dehydrogenation and decarboxylation of pyruvate to the acetyl group of acetyl-CoA (Fig. 16-3) requires the sequential action of three different enzymes and five different coenzymes or prosthetic groups — thiamine pyrophosphate (TPP), lipoate, coenzyme A (CoA, sometimes denoted CoA-SH, to emphasize the role of the group), flavin adenine dinucleotide (FAD), and nicotinamide adenine dinucleotide (NAD). We encountered TPP as the coenzyme of pyruvate decarboxylase (see Fig. 14-13). Lipoate (Fig. 16-4), has two thiol groups that can undergo reversible oxidation to a disulfide bond , similar to that between two Cys residues in a protein. Because of its capacity to undergo oxidation-reduction reactions, lipoate can serve both as an electron (hydrogen) carrier and as an acyl carrier, as we shall see. CoA serves as the carrier of the activated acyl group. We described the roles of FAD and NAD as electron carriers in Chapter 13.
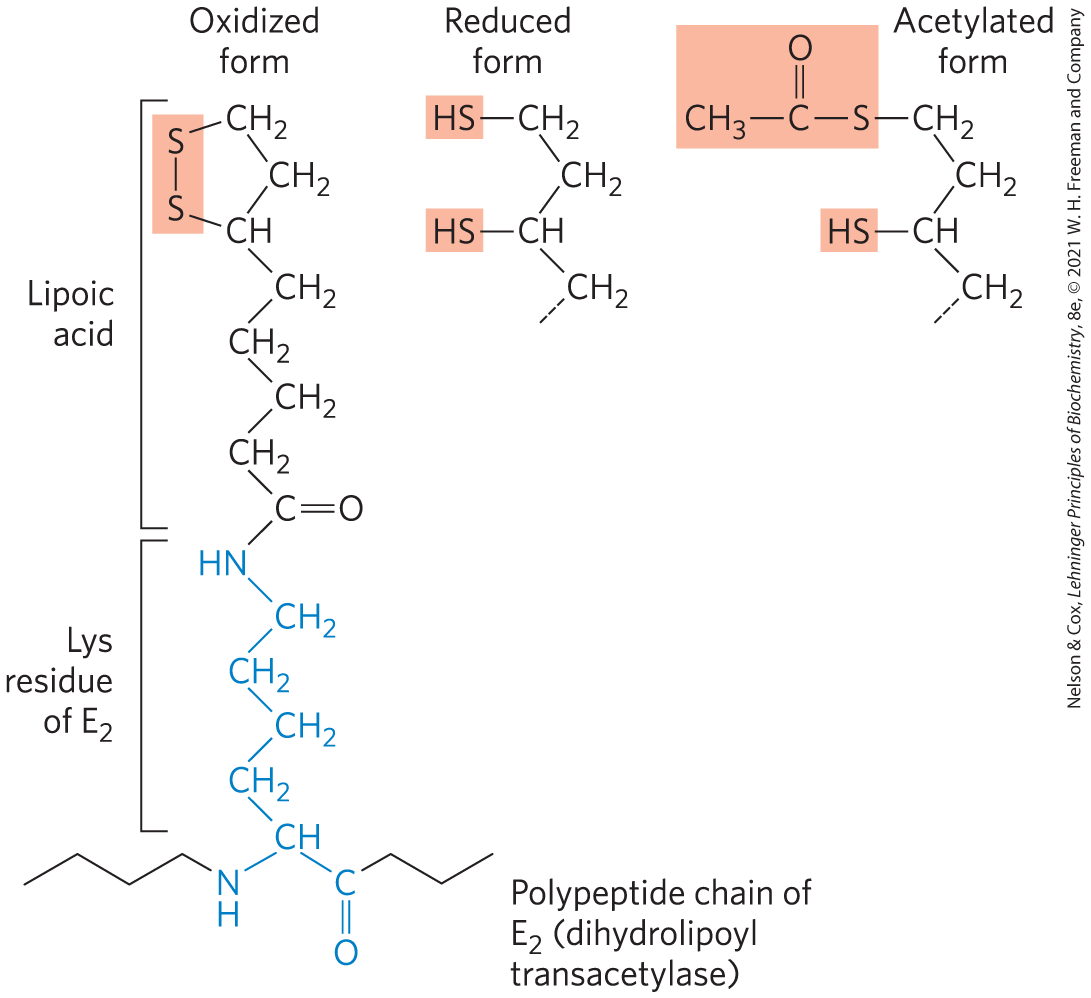
FIGURE 16-4 Lipoic acid (lipoate) in amide linkage with a Lys residue. The lipoyllysyl moiety is the prosthetic group of dihydrolipoyl transacetylase ( of the PDH complex). The lipoyl group occurs in oxidized (disulfide) and reduced (dithiol) forms and acts as a carrier of both hydrogen and an acetyl (or other acyl) group.
The PDH complex contains multiple copies of three enzymes — pyruvate dehydrogenase , dihydrolipoyl transacetylase , and dihydrolipoyl dehydrogenase that catalyze the oxidation of pyruvate. The number of copies of each enzyme and therefore the size of the complex varies among species. A central core is formed of many copies (24 to 60) of surrounded by multiple and variable numbers of copies of and (Fig. 16-5). Two regulatory proteins are also part of the complex: a protein kinase and a phosphoprotein phosphatase, discussed below.
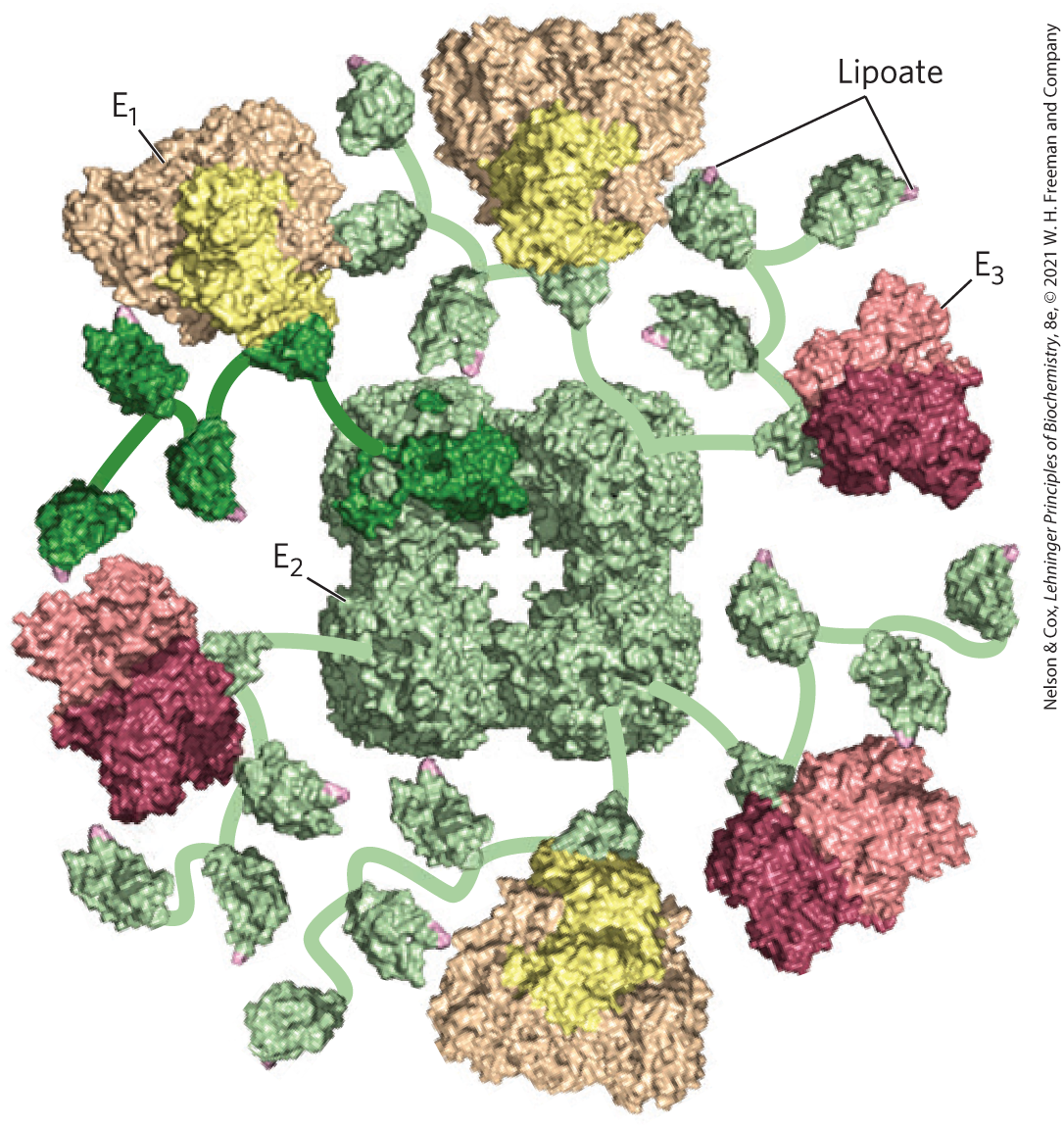
FIGURE 16-5 Structure of the pyruvate dehydrogenase complex. The complex is so big and so flexible that solving its structure required a combination of methods, including x-ray crystallography and NMR spectroscopy; once the individual pieces were solved, cryo-EM of the whole structure was used to assemble the pieces from several organisms to get this view. The core is from the gram-negative bacterium Azotobacter vinelandii. and are from the thermophilic gram-positive bacterium Geobacillus stearothermophilus. , pyruvate dehydrogenase (yellow); , dihydrolipoyl transacetylase (green); and , dihydrolipoyl dehydrogenase (red). The central core of the Azotobacter PDH complex consists of 24 copies of , but to simplify the structure, only six are shown here. Multiple copies of and surround the central core, and flexible arms (shown schematically) reach out from to and , carrying the lipoyl moiety (pink) from the active site of one enzyme to that of the next. The amino acid sequences and three-dimensional structures of individual domains show that both catalytic mechanism and structure have been conserved in evolution. [Information from D. Goodsell, doi:10.2210/rcsb_pdb/mom_2012_9. Data from PDB ID 1LAC F. Dardel et al., J. Mol. Biol. 229:1037, 1993; PDB ID 1EAA A. Mattevi et al., Biochemistry 32:3887, 1993; PDB ID 1W85 R. A. Frank et al., Science 306:872, 2004; PDB ID 1EBD S. S. Mande et al., Structure 4:277, 1996.]
The active site of has noncovalently bound TPP. has the prosthetic group lipoate, attached through an amide bond to the ε-amino group of a Lys residue (Fig. 16-4). has three functionally distinct domains: one or more (depending on the species) amino-terminal lipoyl domains, containing the lipoyl-Lys residue(s); the central - and -binding domain; and the inner-core acyltransferase domain, which contains the acyltransferase active site. The domains of are separated by linkers, sequences of 20 to 30 amino acid residues, rich in Ala and Pro and interspersed with charged residues; these linkers tend to assume their extended forms, holding the three domains apart. The attachment of lipoate to the end of a Lys side chain in produces a long, flexible arm that can move from the active site of to the active sites of and , a distance of perhaps 5 nm or more, while holding the intermediate captive throughout the reaction sequence. has tightly bound FAD.
This basic structure of the PDH complex is seen in two other enzyme complexes that catalyze similar reactions: α-ketoglutarate dehydrogenase, which oxidizes α-ketoglutarate in the citric acid cycle (described below), and the branched-chain α-keto acid dehydrogenase, which oxidizes α-keto acids derived from the breakdown of the branched-chain amino acids valine, isoleucine, and leucine (see Fig. 18-28). Within a given species, is identical in all three complexes. The remarkable similarity in protein structure, cofactor requirements, and reaction mechanisms of these three complexes doubtless reflects a common evolutionary origin; they are paralogs.
The PDH Complex Channels Its Intermediates through Five Reactions
Figure 16-6 shows schematically how the pyruvate dehydrogenase complex carries out the five consecutive reactions in the decarboxylation and dehydrogenation of pyruvate without allowing the intermediates to leave the surface of the complex. Step is essentially identical to the reaction catalyzed by pyruvate decarboxylase (see Fig. 14-13c); C-1 of pyruvate is released as , and C-2, which in pyruvate has the oxidation state of an aldehyde, is attached to TPP as a hydroxyethyl group. This first step is the slowest and therefore limits the rate of the overall reaction. In step the hydroxyethyl group is oxidized to the level of a carboxylic acid (acetate). The two electrons removed in this reaction reduce the of a lipoyl group on to two thiol groups. The acetyl moiety produced in this oxidation-reduction reaction is first esterified to one of the lipoyl groups, then transesterified to CoA to form acetyl-CoA (step ). Thus the energy of oxidation drives the formation of a high-energy thioester of acetate. The remaining reactions catalyzed by the PDH complex (by , in steps and ) are electron transfers necessary to regenerate the oxidized (disulfide) form of the lipoyl group of , to prepare the enzyme complex for another round of oxidation. The electrons removed from the hydroxyethyl group derived from pyruvate pass through FAD to , forming NADH, which can enter the respiratory chain.
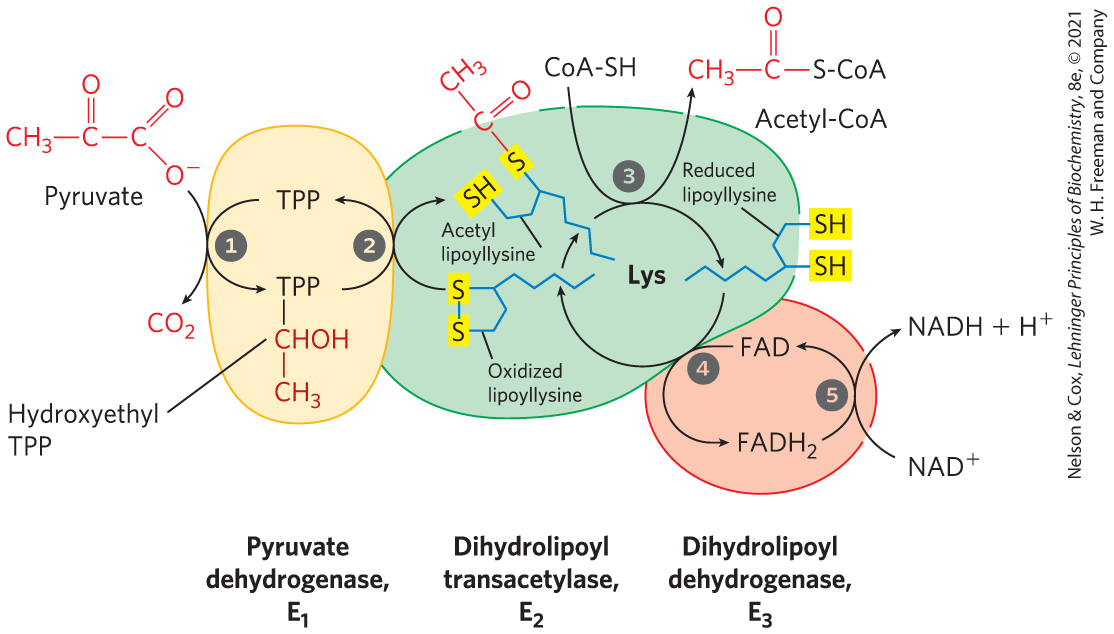
FIGURE 16-6 Oxidative decarboxylation of pyruvate to acetyl-CoA by the PDH complex. The fate of pyruvate is traced in red. In step pyruvate reacts with the bound thiamine pyrophosphate (TPP) of pyruvate dehydrogenase and is decarboxylated to the hydroxyethyl derivative. Pyruvate dehydrogenase also carries out step , the transfer of two electrons and the acetyl group from TPP to the oxidized form of the lipoyllysyl group of the core enzyme, dihydrolipoyl transacetylase , to form the acetyl thioester of the reduced lipoyl group. Step is a transesterification in which the group of CoA replaces the group of to yield acetyl-CoA and the fully reduced (dithiol) form of the lipoyl group. In step dihydrolipoyl dehydrogenase promotes transfer of two hydrogen atoms from the reduced lipoyl groups of to the FAD prosthetic group of , restoring the oxidized form of the lipoyllysyl group of . In step the reduced of transfers a hydride ion to , forming NADH. The enzyme complex is now ready for another catalytic cycle. (Subunit colors correspond to those in Fig. 16-5.)
The five-reaction sequence shown in Figure 16-6 is an example of substrate channeling. The swinging lipoyllysyl arms of accept from the two electrons and the acetyl group that it derived from pyruvate, and pass them to . The intermediates of the multistep sequence never leave the complex, and the local concentration of the substrate of is kept very high. This substrate channeling also prevents theft of the activated acetyl group by other enzymes that use this group as substrate. We will encounter other enzymes that use a similar tethering mechanism to channel substrate between active sites, with lipoate, biotin, or a CoA-like moiety serving as cofactor.
Four different vitamins required in human nutrition are vital components of the pyruvate dehydrogenase complex: thiamine (TPP), pantothenate (CoA), riboflavin (FAD), and niacin (NAD). As one might predict, mutations in the genes for the subunits of the PDH complex, or a dietary vitamin deficiency, can have severe consequences. Thiamine-deficient animals are unable to oxidize pyruvate normally. This is of particular importance to the brain, which usually obtains all its energy from the aerobic oxidation of glucose in a pathway that necessarily includes the oxidation of pyruvate. Beriberi, a disease that results from thiamine deficiency, is characterized by loss of neural function. This disease occurs primarily in populations that rely on a diet consisting mainly of white (polished) rice, which lacks the hulls that contain most of the thiamine found in rice. People who habitually consume large amounts of alcohol can also develop thiamine deficiency, because much of their dietary intake consists of the vitamin-free “empty calories” of distilled spirits. An elevated level of pyruvate in the blood is often an indicator of defects in pyruvate oxidation due to one of these causes.
SUMMARY 16.1 Production of Acetyl-CoA (Activated Acetate)
- Pyruvate, the end product of glycolysis, enters the mitochondrial matrix, where the pyruvate dehydrogenase (PDH) complex oxidizes it to , acetyl-CoA — the starting material for the citric acid cycle — and NADH.
- The supramolecular PDH complex includes multiple copies of three enzymes. Pyruvate dehydrogenase, (with bound TPP), decarboxylates pyruvate, producing hydroxyethyl-TPP, which is then oxidized to an acetyl group. The electrons from this oxidation reduce the disulfide of lipoate bound to , the dihydrolipoyl transacetylase, and the acetyl group is transferred to one of the groups of the reduced lipoate through a thioester bond. catalyzes the transfer of the acetyl group to coenzyme A, forming acetyl-CoA. Dihydrolipoyl dehydrogenase, , catalyzes the regeneration of the disulfide (oxidized) form of lipoate, passing electrons first to FAD, then to .
- In the PDH complex we see examples of two strategies that we will see repeated in other metabolic enzyme systems. Its organization is very similar to that of the enzyme complexes that catalyze the oxidation of α-ketoglutarate and the branched-chain α-keto acids. And the long lipoyllysyl arm of is used to channel the substrate from the active site of to to , tethering the intermediates to the enzyme complex and increasing the efficiency of the overall reaction.
 Pyruvate generated in the cytosol by glycolysis represents a node in the metabolism of carbohydrates, proteins, and fats. Under anaerobic conditions, pyruvate may simply be reduced to lactate in the cytosol, regenerating for continued ATP production by glycolysis. Pyruvate may serve as a precursor for the synthesis of amino acids (
Pyruvate generated in the cytosol by glycolysis represents a node in the metabolism of carbohydrates, proteins, and fats. Under anaerobic conditions, pyruvate may simply be reduced to lactate in the cytosol, regenerating for continued ATP production by glycolysis. Pyruvate may serve as a precursor for the synthesis of amino acids ( In the PDH complex, a series of chemical intermediates remain bound to the enzyme subunits as a substrate (pyruvate) is transformed into the final product (acetyl-CoA). Five cofactors, four of which are derived from vitamins, participate in the reaction mechanism.
In the PDH complex, a series of chemical intermediates remain bound to the enzyme subunits as a substrate (pyruvate) is transformed into the final product (acetyl-CoA). Five cofactors, four of which are derived from vitamins, participate in the reaction mechanism.  The regulation of this enzyme complex illustrates how a combination of covalent modification and allosteric mechanism results in precisely regulated flux through a metabolic step.
The regulation of this enzyme complex illustrates how a combination of covalent modification and allosteric mechanism results in precisely regulated flux through a metabolic step. is essentially identical to the reaction catalyzed by pyruvate decarboxylase (see
is essentially identical to the reaction catalyzed by pyruvate decarboxylase (see  the hydroxyethyl group is oxidized to the level of a carboxylic acid (acetate). The two electrons removed in this reaction reduce the of a lipoyl group on to two thiol groups. The acetyl moiety produced in this oxidation-reduction reaction is first esterified to one of the lipoyl groups, then transesterified to CoA to form acetyl-CoA (step
the hydroxyethyl group is oxidized to the level of a carboxylic acid (acetate). The two electrons removed in this reaction reduce the of a lipoyl group on to two thiol groups. The acetyl moiety produced in this oxidation-reduction reaction is first esterified to one of the lipoyl groups, then transesterified to CoA to form acetyl-CoA (step  ). Thus the energy of oxidation drives the formation of a high-energy thioester of acetate. The remaining reactions catalyzed by the PDH complex (by , in steps
). Thus the energy of oxidation drives the formation of a high-energy thioester of acetate. The remaining reactions catalyzed by the PDH complex (by , in steps  and
and  ) are electron transfers necessary to regenerate the oxidized (disulfide) form of the lipoyl group of , to prepare the enzyme complex for another round of oxidation. The electrons removed from the hydroxyethyl group derived from pyruvate pass through FAD to , forming NADH, which can enter the respiratory chain.
) are electron transfers necessary to regenerate the oxidized (disulfide) form of the lipoyl group of , to prepare the enzyme complex for another round of oxidation. The electrons removed from the hydroxyethyl group derived from pyruvate pass through FAD to , forming NADH, which can enter the respiratory chain. Four different vitamins required in human nutrition are vital components of the pyruvate dehydrogenase complex: thiamine (TPP), pantothenate (CoA), riboflavin (FAD), and niacin (NAD). As one might predict, mutations in the genes for the subunits of the PDH complex, or a dietary vitamin deficiency, can have severe consequences. Thiamine-deficient animals are unable to oxidize pyruvate normally. This is of particular importance to the brain, which usually obtains all its energy from the aerobic oxidation of glucose in a pathway that necessarily includes the oxidation of pyruvate. Beriberi, a disease that results from thiamine deficiency, is characterized by loss of neural function. This disease occurs primarily in populations that rely on a diet consisting mainly of white (polished) rice, which lacks the hulls that contain most of the thiamine found in rice. People who habitually consume large amounts of alcohol can also develop thiamine deficiency, because much of their dietary intake consists of the vitamin-free “empty calories” of distilled spirits. An elevated level of pyruvate in the blood is often an indicator of defects in pyruvate oxidation due to one of these causes.
Four different vitamins required in human nutrition are vital components of the pyruvate dehydrogenase complex: thiamine (TPP), pantothenate (CoA), riboflavin (FAD), and niacin (NAD). As one might predict, mutations in the genes for the subunits of the PDH complex, or a dietary vitamin deficiency, can have severe consequences. Thiamine-deficient animals are unable to oxidize pyruvate normally. This is of particular importance to the brain, which usually obtains all its energy from the aerobic oxidation of glucose in a pathway that necessarily includes the oxidation of pyruvate. Beriberi, a disease that results from thiamine deficiency, is characterized by loss of neural function. This disease occurs primarily in populations that rely on a diet consisting mainly of white (polished) rice, which lacks the hulls that contain most of the thiamine found in rice. People who habitually consume large amounts of alcohol can also develop thiamine deficiency, because much of their dietary intake consists of the vitamin-free “empty calories” of distilled spirits. An elevated level of pyruvate in the blood is often an indicator of defects in pyruvate oxidation due to one of these causes. 
 Pyruvate, the end product of glycolysis, enters the mitochondrial matrix, where the pyruvate dehydrogenase (PDH) complex oxidizes it to , acetyl-CoA — the starting material for the citric acid cycle — and NADH.
Pyruvate, the end product of glycolysis, enters the mitochondrial matrix, where the pyruvate dehydrogenase (PDH) complex oxidizes it to , acetyl-CoA — the starting material for the citric acid cycle — and NADH.