Chapter 16 The Citric Acid Cycle
As we saw in Chapter 14, some cells obtain energy (ATP) by fermentation, breaking down glucose in the absence of oxygen. For most eukaryotic cells and many bacteria, which live under aerobic conditions and oxidize their organic fuels to carbon dioxide and water, glycolysis is but the first stage in the complete oxidation of glucose. Rather than being reduced to lactate, ethanol, or some other fermentation product, the pyruvate produced by glycolysis is further oxidized to and through the process of cellular respiration.
Cellular respiration occurs in three major stages (Fig. 16-1), the first two of which we discuss in this chapter. In the first, organic fuel molecules — glucose, fatty acids, and some amino acids — are oxidized to yield two-carbon fragments in the form of the acetyl group of acetyl-coenzyme A (acetyl-CoA). In the second stage, the acetyl groups are oxidized to in the citric acid cycle, also called the tricarboxylic acid (TCA) cycle or the Krebs cycle (after its discoverer, Hans Krebs). Much of the energy of these oxidations is conserved in the reduced electron carriers NADH and . In the third stage of respiration, these reduced coenzymes are themselves oxidized, giving up protons and electrons. The electrons are transferred to via a series of electron-carrying molecules known as the respiratory chain, resulting in the formation of water. In the course of electron transfer, much of the energy available from redox reactions is conserved in the form of ATP, by a process called oxidative phosphorylation. We discuss this third stage in Chapter 19.
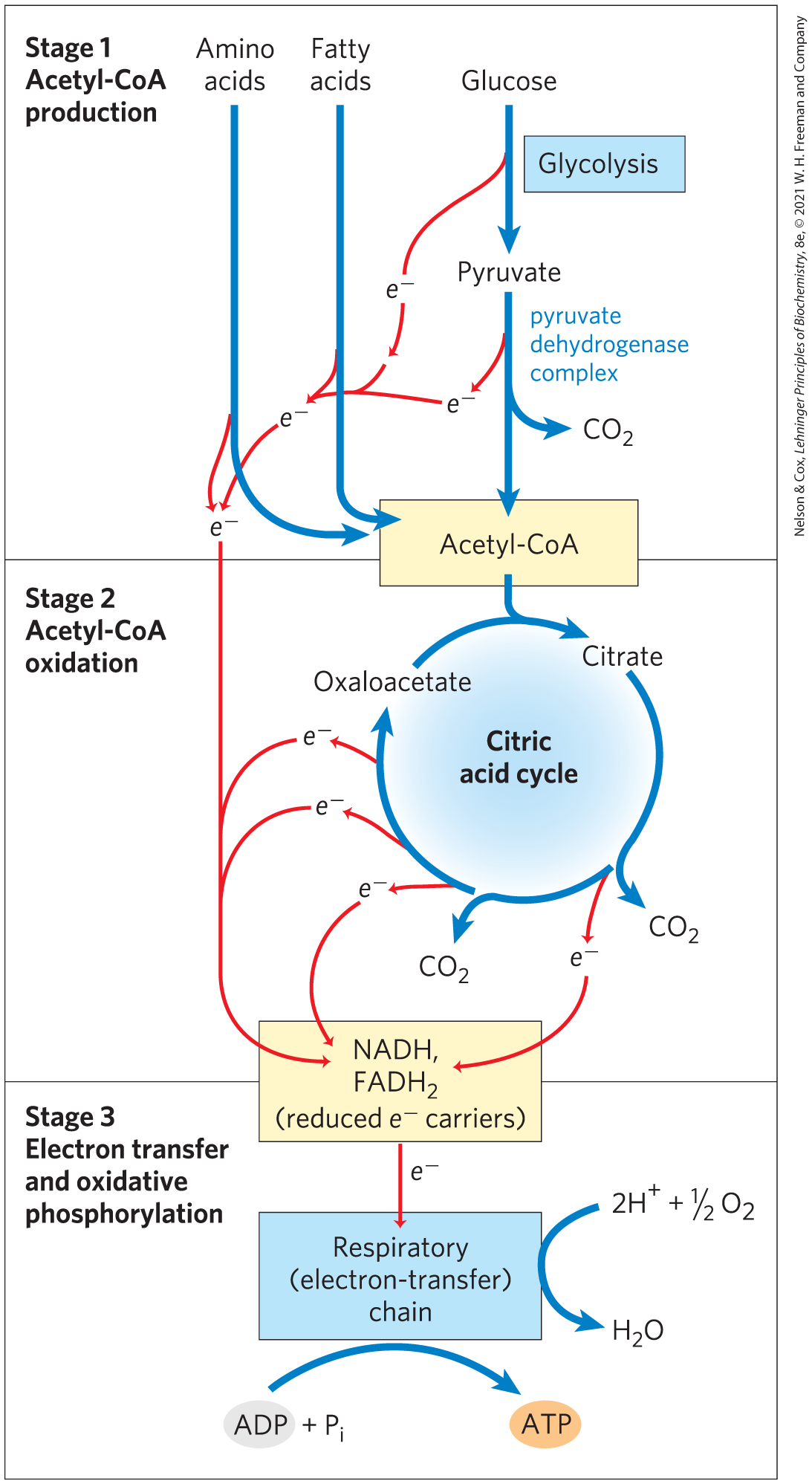
FIGURE 16-1 Catabolism of proteins, fats, and carbohydrates in the three stages of cellular respiration. Stage 1: oxidation of fatty acids, glucose, and some amino acids yields acetyl-CoA. Stage 2: oxidation of acetyl groups in the citric acid cycle includes four steps in which electrons are abstracted. Stage 3: electrons carried by NADH and are funneled into a chain of mitochondrial (or, in bacteria, plasma membrane–bound) electron carriers — the respiratory chain — ultimately reducing to . This electron flow drives the production of ATP.

Hans Krebs, 1900–1981
We use these principles as our guide to this chapter:
Pyruvate is the metabolite that links two central catabolic pathways, glycolysis and the citric acid cycle. It is therefore a logical point for regulation that determines the rate of catabolic activity and the partitioning of pyruvate among its possible uses.
The reactions of the citric acid cycle follow a chemical logic. In its catabolic role the citric acid cycle oxidizes acetyl-CoA to and . Energy from the oxidations in the cycle drives the synthesis of ATP. The chemical strategies for activating groups for oxidation and for conserving energy in the form of reducing power and high-energy compounds are used in many other biochemical pathways.
The citric acid cycle is a hub of metabolism, with catabolic pathways leading in and anabolic pathways leading out. Acetate groups (acetyl-CoA) from the catabolism of various fuels are used in the synthesis of such metabolites as amino acids, fatty acids, and sterols. The breakdown products of many amino acids and nucleotides are intermediates of the cycle, and they can be fed in or siphoned off as needed by the cell.
The central role of the citric acid cycle in metabolism requires that it be regulated in coordination with many other pathways. Regulation occurs by both allosteric and covalent mechanisms that overlap and interact to achieve homeostasis. Some mutations that affect the reactions of the citric acid cycle lead to tumor formation.
Enzymes have evolved to form complexes to efficiently achieve a series of chemical transformations without releasing the intermediates into the bulk solvent. This strategy, seen in the pyruvate dehydrogenase complex and the metabolons of the citric acid cycle, is ubiquitous in other pathways of metabolism, in respiration, and in the many “ — somes” that assemble and disassemble informational macromolecules.
 Pyruvate is the metabolite that links two central catabolic pathways, glycolysis and the citric acid cycle. It is therefore a logical point for regulation that determines the rate of catabolic activity and the partitioning of pyruvate among its possible uses.
Pyruvate is the metabolite that links two central catabolic pathways, glycolysis and the citric acid cycle. It is therefore a logical point for regulation that determines the rate of catabolic activity and the partitioning of pyruvate among its possible uses. The reactions of the citric acid cycle follow a chemical logic. In its catabolic role the citric acid cycle oxidizes acetyl-CoA to and . Energy from the oxidations in the cycle drives the synthesis of ATP. The chemical strategies for activating groups for oxidation and for conserving energy in the form of reducing power and high-energy compounds are used in many other biochemical pathways.
The reactions of the citric acid cycle follow a chemical logic. In its catabolic role the citric acid cycle oxidizes acetyl-CoA to and . Energy from the oxidations in the cycle drives the synthesis of ATP. The chemical strategies for activating groups for oxidation and for conserving energy in the form of reducing power and high-energy compounds are used in many other biochemical pathways. The citric acid cycle is a hub of metabolism, with catabolic pathways leading in and anabolic pathways leading out. Acetate groups (acetyl-CoA) from the catabolism of various fuels are used in the synthesis of such metabolites as amino acids, fatty acids, and sterols. The breakdown products of many amino acids and nucleotides are intermediates of the cycle, and they can be fed in or siphoned off as needed by the cell.
The citric acid cycle is a hub of metabolism, with catabolic pathways leading in and anabolic pathways leading out. Acetate groups (acetyl-CoA) from the catabolism of various fuels are used in the synthesis of such metabolites as amino acids, fatty acids, and sterols. The breakdown products of many amino acids and nucleotides are intermediates of the cycle, and they can be fed in or siphoned off as needed by the cell. The central role of the citric acid cycle in metabolism requires that it be regulated in coordination with many other pathways. Regulation occurs by both allosteric and covalent mechanisms that overlap and interact to achieve homeostasis. Some mutations that affect the reactions of the citric acid cycle lead to tumor formation.
The central role of the citric acid cycle in metabolism requires that it be regulated in coordination with many other pathways. Regulation occurs by both allosteric and covalent mechanisms that overlap and interact to achieve homeostasis. Some mutations that affect the reactions of the citric acid cycle lead to tumor formation. Enzymes have evolved to form complexes to efficiently achieve a series of chemical transformations without releasing the intermediates into the bulk solvent. This strategy, seen in the pyruvate dehydrogenase complex and the metabolons of the citric acid cycle, is ubiquitous in other pathways of metabolism, in respiration, and in the many “ — somes” that assemble and disassemble informational macromolecules.
Enzymes have evolved to form complexes to efficiently achieve a series of chemical transformations without releasing the intermediates into the bulk solvent. This strategy, seen in the pyruvate dehydrogenase complex and the metabolons of the citric acid cycle, is ubiquitous in other pathways of metabolism, in respiration, and in the many “ — somes” that assemble and disassemble informational macromolecules.