Chapter 19 OXIDATIVE PHOSPHORYLATION
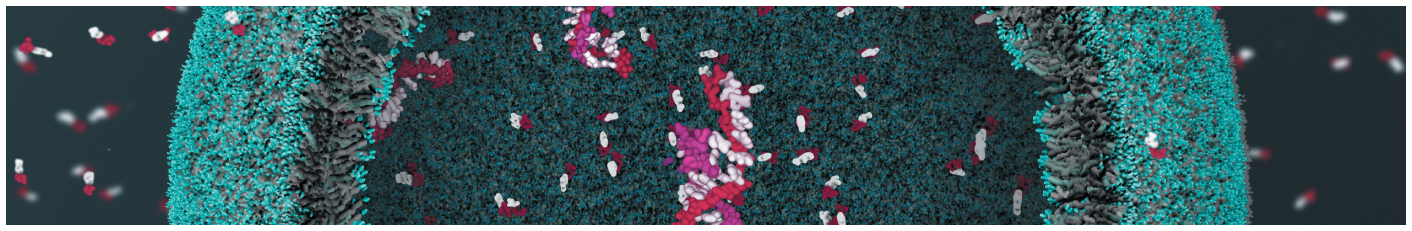
Oxidative phosphorylation is the culmination of energy-yielding metabolism (catabolism) in aerobic organisms. All oxidative steps in the degradation of carbohydrates, fats, and amino acids converge at this final stage of cellular respiration, in which the energy of oxidation drives the synthesis of ATP. Oxidative phosphorylation accounts for most of the ATP synthesized by nonphotosynthetic organisms under most circumstances. In eukaryotes, oxidative phosphorylation occurs in mitochondria and involves huge protein complexes embedded in the mitochondrial membranes. The pathway to ATP synthesis in mitochondria challenged and fascinated biochemists for much of the twentieth century. Our current exploration of the topic is guided by five principles:
Mitochondria play a central role in eukaryotic aerobic metabolism. ATP production is not the only important mitochondrial function. Mitochondria play host to the citric acid cycle, the fatty acid β-oxidation pathway, and the pathways of amino acid oxidation. The mitochondria also act in thermogenesis, steroid synthesis, and apoptosis (programmed cell death). The discovery of these diverse and important roles of mitochondria has stimulated much current research on the biochemistry of this organelle.
Mitochondria trace their evolutionary origin to bacteria. Over 1.45 billion years ago, an endosymbiotic relationship arose between bacteria and a primitive eukaryote or eukaryotic progenitor. Mitochondria are ubiquitous in modern eukaryotes, and their bacterial origin is evident in almost every aspect of their structure and function.
Electrons flow from electron donors (oxidizable substrates) through a chain of membrane-bound carriers to a final electron acceptor with a large reduction potential. The final acceptor is molecular oxygen, . The appearance of oxygen in the atmosphere some 2.3 billion years ago, and its harnessing in living systems via the evolution of oxidative phosphorylation, made more complex life forms possible.
The free energy made available by “downhill” (exergonic) electron flow is coupled to the “uphill” transport of protons across a proton-impermeable membrane. The free energy of fuel oxidation is thus conserved as a transmembrane electrochemical potential.
The transmembrane flow of protons back down their electrochemical gradient through specific protein channels provides the free energy for synthesis of ATP. This process is catalyzed by a membrane protein complex (ATP synthase) that couples proton flow to phosphorylation of ADP.
Principles 3 through 5, illustrated in Figure 19-1, encapsulate the theory, introduced by Peter Mitchell in 1961, that transmembrane differences in proton concentration are the reservoir for the energy extracted from biological oxidation reactions. This chemiosmotic theory has been accepted as one of the great unifying ideas of twentieth-century biology. It provides insight into the processes of oxidative phosphorylation and photophosphorylation in plants, and into such apparently disparate energy transductions as active transport across membranes and the motion of bacterial flagella.
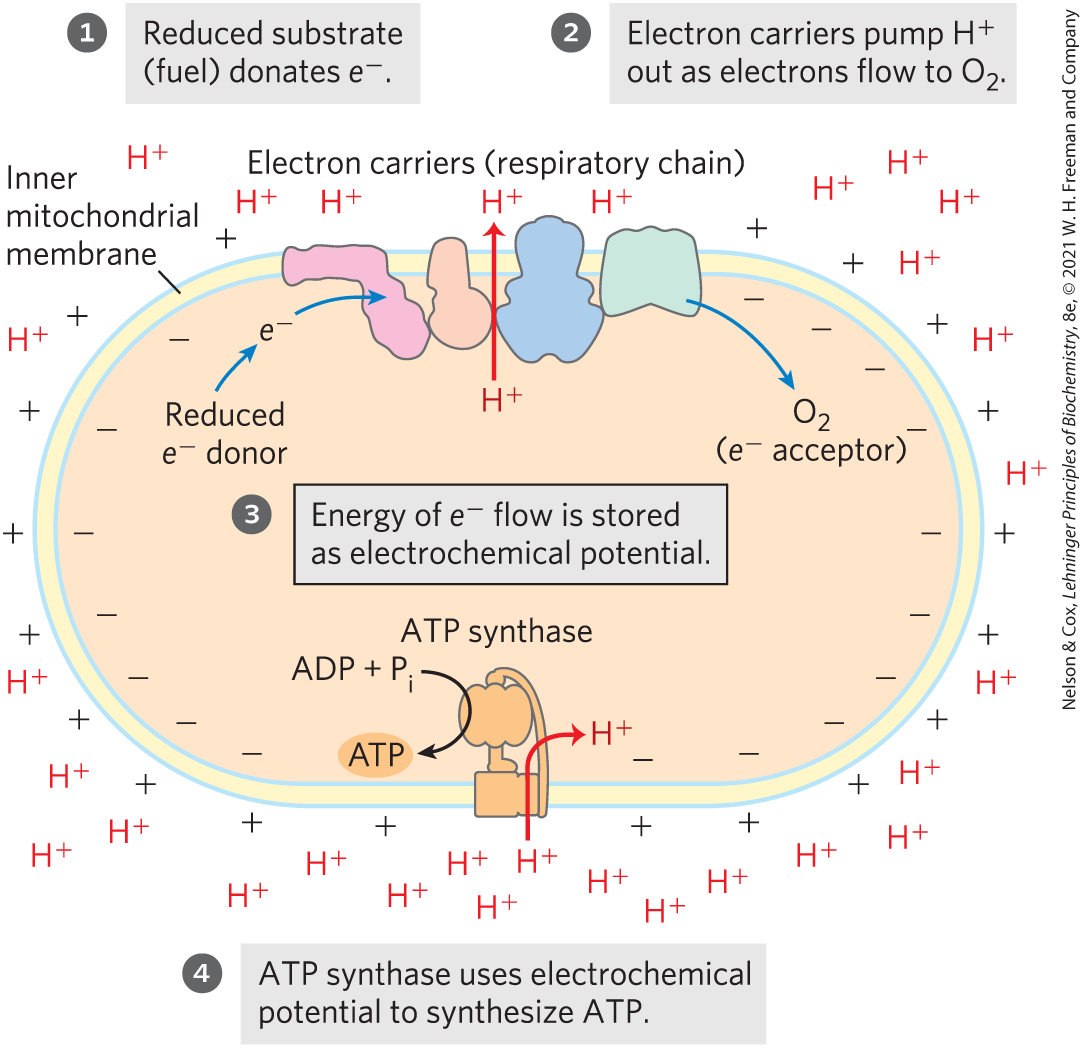
FIGURE 19-1 The chemiosmotic mechanism for ATP synthesis in mitochondria. Electrons move spontaneously through a chain of membrane-bound carriers, the respiratory chain, driven by the high reduction potential of oxygen and the relatively low reduction potentials of the various reduced substrates (fuels) that undergo oxidation in the mitochondrion. Electron flow creates an electrochemical potential by the transmembrane movement of protons and positive charge. This electrochemical potential drives ATP synthesis by a membrane-bound enzyme (ATP synthase) that is fundamentally similar in mitochondria and chloroplasts, and in bacteria and archaea as well.
This chapter begins with a description of the components of the mitochondrial electron-transfer chain — the respiratory chain — and their organization into large functional complexes in the inner mitochondrial membrane, the path of electron flow through these complexes, and the proton movements that accompany this flow. We then consider the remarkable enzyme complex that captures, by “rotational catalysis,” the energy of proton flow in ATP, and the regulatory mechanisms that coordinate oxidative phosphorylation with the many catabolic pathways by which fuels are oxidized.
The metabolic role of mitochondria is so critical to cellular and organismal function that defects in mitochondrial function have serious medical consequences. Mitochondria are central to neuronal and muscular function, and to the regulation of whole-body energy metabolism and body weight. Human neurodegenerative diseases, as well as cancer, diabetes, and obesity, are recognized as possible results of compromised mitochondrial function, and one theory of aging is based on gradual loss of mitochondrial integrity. We consider the diverse functions of mitochondria, and the consequences of defective mitochondrial function in humans.
 Mitochondria play a central role in eukaryotic aerobic metabolism. ATP production is not the only important mitochondrial function. Mitochondria play host to the citric acid cycle, the fatty acid β-oxidation pathway, and the pathways of amino acid oxidation. The mitochondria also act in thermogenesis, steroid synthesis, and apoptosis (programmed cell death). The discovery of these diverse and important roles of mitochondria has stimulated much current research on the biochemistry of this organelle.
Mitochondria play a central role in eukaryotic aerobic metabolism. ATP production is not the only important mitochondrial function. Mitochondria play host to the citric acid cycle, the fatty acid β-oxidation pathway, and the pathways of amino acid oxidation. The mitochondria also act in thermogenesis, steroid synthesis, and apoptosis (programmed cell death). The discovery of these diverse and important roles of mitochondria has stimulated much current research on the biochemistry of this organelle. Mitochondria trace their evolutionary origin to bacteria. Over 1.45 billion years ago, an endosymbiotic relationship arose between bacteria and a primitive eukaryote or eukaryotic progenitor. Mitochondria are ubiquitous in modern eukaryotes, and their bacterial origin is evident in almost every aspect of their structure and function.
Mitochondria trace their evolutionary origin to bacteria. Over 1.45 billion years ago, an endosymbiotic relationship arose between bacteria and a primitive eukaryote or eukaryotic progenitor. Mitochondria are ubiquitous in modern eukaryotes, and their bacterial origin is evident in almost every aspect of their structure and function. Electrons flow from electron donors (oxidizable substrates) through a chain of membrane-bound carriers to a final electron acceptor with a large reduction potential. The final acceptor is molecular oxygen, . The appearance of oxygen in the atmosphere some 2.3 billion years ago, and its harnessing in living systems via the evolution of oxidative phosphorylation, made more complex life forms possible.
Electrons flow from electron donors (oxidizable substrates) through a chain of membrane-bound carriers to a final electron acceptor with a large reduction potential. The final acceptor is molecular oxygen, . The appearance of oxygen in the atmosphere some 2.3 billion years ago, and its harnessing in living systems via the evolution of oxidative phosphorylation, made more complex life forms possible. The free energy made available by “downhill” (exergonic) electron flow is coupled to the “uphill” transport of protons across a proton-impermeable membrane. The free energy of fuel oxidation is thus conserved as a transmembrane electrochemical potential.
The free energy made available by “downhill” (exergonic) electron flow is coupled to the “uphill” transport of protons across a proton-impermeable membrane. The free energy of fuel oxidation is thus conserved as a transmembrane electrochemical potential. The transmembrane flow of protons back down their electrochemical gradient through specific protein channels provides the free energy for synthesis of ATP. This process is catalyzed by a membrane protein complex (ATP synthase) that couples proton flow to phosphorylation of ADP.
The transmembrane flow of protons back down their electrochemical gradient through specific protein channels provides the free energy for synthesis of ATP. This process is catalyzed by a membrane protein complex (ATP synthase) that couples proton flow to phosphorylation of ADP. The metabolic role of mitochondria is so critical to cellular and organismal function that defects in mitochondrial function have serious medical consequences. Mitochondria are central to neuronal and muscular function, and to the regulation of whole-body energy metabolism and body weight. Human neurodegenerative diseases, as well as cancer, diabetes, and obesity, are recognized as possible results of compromised mitochondrial function, and one theory of aging is based on gradual loss of mitochondrial integrity. We consider the diverse functions of mitochondria, and the consequences of defective mitochondrial function in humans.
The metabolic role of mitochondria is so critical to cellular and organismal function that defects in mitochondrial function have serious medical consequences. Mitochondria are central to neuronal and muscular function, and to the regulation of whole-body energy metabolism and body weight. Human neurodegenerative diseases, as well as cancer, diabetes, and obesity, are recognized as possible results of compromised mitochondrial function, and one theory of aging is based on gradual loss of mitochondrial integrity. We consider the diverse functions of mitochondria, and the consequences of defective mitochondrial function in humans. 