16.4 Regulation of the Citric Acid Cycle
The central role of the citric acid cycle in metabolism requires stringent regulation to balance the supply of key intermediates with the demands of energy production and biosynthetic processes. Regulation occurs at several levels, including the oxidation of pyruvate to acetyl-CoA (catalyzed by the PDH complex) and the entry of acetyl-CoA into the cycle (the citrate synthase reaction). The transport of pyruvate into mitochondria by the mitochondrial pyruvate carrier (MPC) determines to some degree the fate of pyruvate produced by glycolysis. Most cells also produce acetyl-CoA from the oxidation of fatty acids (Chapter 17) and certain amino acids (Chapter 18), and the availability of intermediates from those other pathways is important in the regulation of pyruvate oxidation and of the citric acid cycle. There are therefore multiple points at which the metabolism of pyruvate and the citric acid cycle may be regulated.
Production of Acetyl-CoA by the PDH Complex Is Regulated by Allosteric and Covalent Mechanisms
The PDH complex of mammals is strongly inhibited allosterically by ATP and by acetyl-CoA and NADH, the products of the reaction catalyzed by the complex (Fig. 16-18). Long-chain fatty acids, which can be broken down to acetyl-CoA, are also inhibitory. AMP, CoA, and , all of which accumulate when too little acetate flows into the citric acid cycle, allosterically activate the PDH complex. Thus, PDH complex activity is turned off when ample fuel is available in the form of fatty acids and acetyl-CoA and when the cell’s and ratios are high; it is turned on again when energy demands are high and the cell requires greater flux of acetyl-CoA into the citric acid cycle.
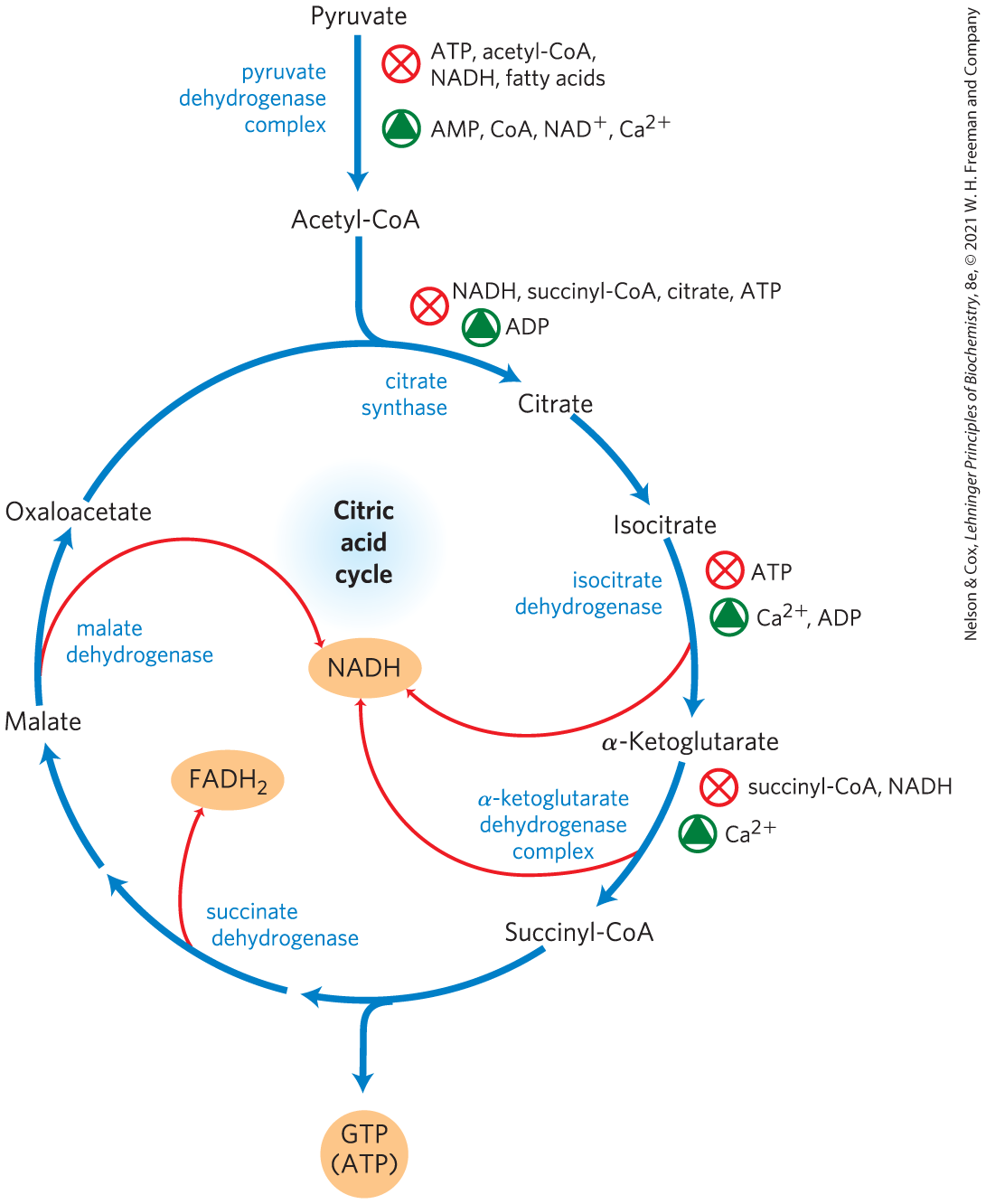
FIGURE 16-18 Regulation of metabolite flow from the PDH complex through the citric acid cycle in mammals. The PDH complex is allosterically inhibited when , , and ratios are high, all of which indicate an energy-sufficient metabolic state. When these ratios decrease, allosteric activation of pyruvate oxidation results. The rate of flow through the citric acid cycle can be limited by the availability of the citrate synthase substrates, oxaloacetate and acetyl-CoA, or of , which is depleted by its conversion to NADH, slowing the three -dependent oxidation steps. Feedback inhibition by succinyl-CoA, citrate, and ATP also slows the cycle by inhibiting early steps. In muscle tissue, stimulates contraction and, as shown here, stimulates energy-yielding metabolism to replace the ATP consumed by contraction.
In mammals, these allosteric regulatory mechanisms are complemented by a second level of regulation: phosphorylation/dephosphorylation. The PDH complex is inhibited by reversible phosphorylation of Ser residues on by PDH kinase, which is an intrinsic part of the PDH complex. PDH kinase is allosterically activated by the products of the PDH complex (ATP, NADH, and acetyl-CoA), and it is inhibited by the substrates for the PDH complex (ADP, , and pyruvate) (Fig. 16-19). The complex also contains PDH phosphatase, which reverses the inhibition by PDH kinase. Together, the kinase and phosphatase exert strong control over the entry of acetyl-CoA from pyruvate into the citric acid cycle. Higher concentrations of ATP, NADH, or acetyl-CoA lead to inactivation of the PDH complex by phosphorylation of PDH. When the concentrations of ADP, , or pyruvate rise, kinase activity decreases and pyruvate dehydrogenase phosphatase removes the phosphoryl group, reactivating the PDH complex and thereby stimulating the citric acid cycle.

FIGURE 16-19 Pyruvate dehydrogenase is inactivated by phosphorylation catalyzed by pyruvate dehydrogenase kinase. The kinase is regulated by metabolites that signal the energetic state of the cell. Metabolites that accumulate in an energy-sufficient state activate PDH kinase, which phosphorylates and inactivates PDH. Pyruvate is then diverted away from the energy-yielding citric acid cycle (CAC). Metabolites indicating energy need or pyruvate accumulation have the opposite effect, keeping PDH active and sending acetyl-CoA into the CAC.
The simple compound dichloroacetate (DCA), a structural analog of acetate, inhibits PDH kinase in the laboratory and so relieves the inhibition of the PDH complex. This stimulates pyruvate oxidation via the citric acid cycle (Fig. 16-19) and thus may have a use in directing metabolism in tumor cells away from aerobic glycolysis (see Box 14-1). The increase in mitochondrial oxidation also stimulates apoptosis (Chapter 19), thereby suppressing tumor growth. Phase III clinical trials of DCA began in 2019.
The Citric Acid Cycle Is Also Regulated at Three Exergonic Steps
Each of the three strongly exergonic steps in the cycle — those catalyzed by citrate synthase, isocitrate dehydrogenase, and α-ketoglutarate dehydrogenase (Fig. 16-18) — can become the rate-limiting step under some circumstances. The availability of the substrates for citrate synthase (acetyl-CoA and oxaloacetate) varies with the metabolic state of the cell and sometimes limits the rate of citrate formation. NADH, a product of isocitrate and α-ketoglutarate oxidation, accumulates under some conditions, and at high both dehydrogenase reactions are severely inhibited by mass action. Similarly, the malate dehydrogenase reaction is essentially at equilibrium in the cell (that is, it is substrate-limited), and when is high the concentration of oxaloacetate is low, slowing the first step in the cycle. Product accumulation inhibits all three limiting steps of the cycle: succinyl-CoA inhibits α-ketoglutarate dehydrogenase (and also citrate synthase); citrate blocks citrate synthase; and the end product, ATP, inhibits both citrate synthase and isocitrate dehydrogenase. The inhibition of citrate synthase by ATP is relieved by ADP, an allosteric activator of this enzyme. In vertebrate muscle, , the signal for contraction and for a concomitant increase in demand for ATP, activates both isocitrate dehydrogenase and α-ketoglutarate dehydrogenase, as well as the PDH complex. In short, the concentrations of substrates and intermediates in the citric acid cycle set the flux through this pathway at a rate that provides optimal concentrations of ATP and NADH.
Under normal conditions, the rates of glycolysis and of the citric acid cycle are integrated so that only as much glucose is metabolized to pyruvate as is needed to supply the citric acid cycle with fuel (acetyl-CoA). Both pathways are inhibited by high levels of ATP and NADH, but also by the concentration of citrate, the product of the first step of the citric acid cycle, and an important allosteric inhibitor of phosphofructokinase-1 of the glycolytic pathway (see Fig. 14-23).
Citric Acid Cycle Activity Changes in Tumors
The mitochondrial pyruvate carrier (MPC) is down-regulated in tumor cells, leading to pyruvate accumulation in the cytosol. Several other mitochondrial enzymes are also inactivated in tumor cells, including the PDH complex and succinate dehydrogenase. As a result, tumor cells accumulate lactate (from the pyruvate produced by glycolysis) and succinate. Both of these intermediates are oncometabolites; they stimulate tumor growth, acting through specific G protein–coupled receptors (GPCRs; see Chapter 12) in the plasma membrane. The membrane receptor for lactate is upregulated in most cancers. l-Lactate acting through its membrane receptor, lowers [cAMP] and raises , with downstream effects currently under investigation.
Mutations in citric acid cycle enzymes are very rare in humans and other mammals, but those that do occur are devastating. Genetic defects in succinate dehydrogenase lead to tumors of the adrenal gland (pheochromocytomas), and mutations in the fumarase gene lead to tumors of smooth muscle (leiomyomas) and kidney. Their activities thus define both enzymes as tumor suppressors (p. 451).
Another remarkable connection between citric acid cycle intermediates and cancer is the finding that in many glial cell tumors (gliomas), the NADPH-dependent isocitrate dehydrogenase has an unusual genetic defect. The mutant enzyme loses its normal activity (converting isocitrate to α-ketoglutarate) but gains a new activity: it converts α-ketoglutarate to 2-hydroxyglutarate (Fig. 16-20), which accumulates in the tumor cells. α-Ketoglutarate and are essential cofactors for a family of histone demethylases that alter gene expression. They do so by removing methyl groups from Arg and Lys residues in the histones that organize nuclear DNA. By competing with α-ketoglutarate for binding to the histone demethylases, 2-hydroxyglutarate inhibits their activity. Inhibition of the histone demethylases interferes with normal gene regulation, leading to unrestricted glial cell growth. The family of more than 60 dioxygenases that use α-ketoglutarate and as cofactors are also competitively inhibited by 2-hydroxyglutarate. Inhibition of one or more of these enzymes could interfere with normal regulation of cell division and thus produce a tumor.
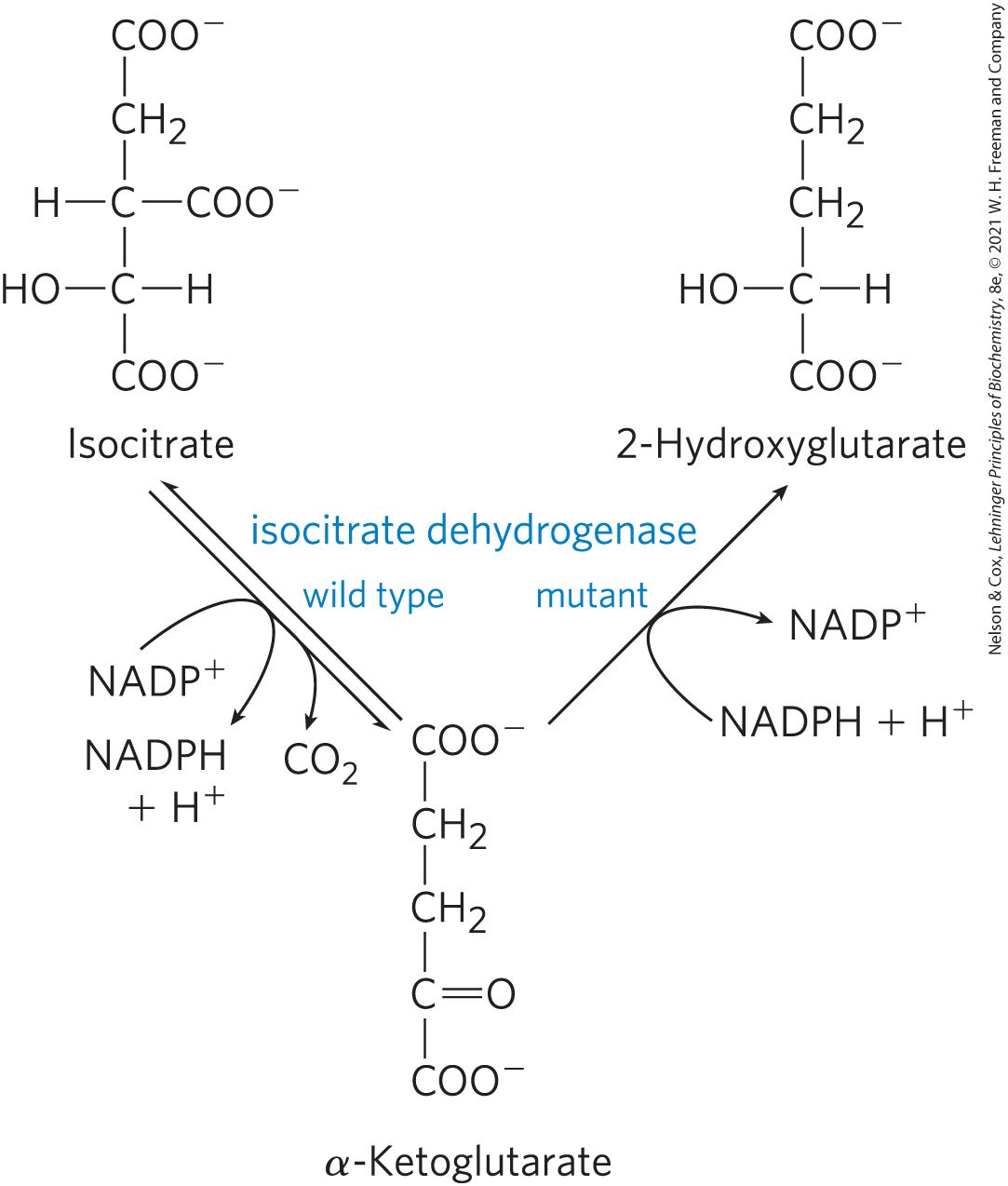
FIGURE 16-20 A mutant isocitrate dehydrogenase acquires a new activity. Wild-type isocitrate dehydrogenase catalyzes the conversion of isocitrate to α-ketoglutarate, but mutations that alter the binding site for isocitrate cause loss of the normal enzymatic activity and gain of a new activity: conversion of α-ketoglutarate to 2-hydroxyglutarate. Accumulation of this product inhibits histone demethylase, altering gene regulation and leading to glial cell tumors in the brain.
Certain Intermediates Are Channeled through Metabolons
We saw an example of substrate channeling in the five-reaction sequence of the PDH complex. Many other reactions occur in similar multienzyme complexes that ensure efficient passage of the product of one enzyme reaction to the next enzyme in the pathway. Such integrated multienzyme complexes, metabolons, are held together by noncovalent interactions and are not easily extracted intact from the cell. In the classic approach of enzymology — purification of individual proteins from extracts of broken cells — once cells are broken open, their contents, including enzymes, are diluted 100- or 1,000-fold (Fig. 16-21), which favors dissociation of noncovalent complexes such as metabolons.

FIGURE 16-21 Effect of protein concentration on complex stability. Dilution of a solution containing a noncovalently bound protein complex — such as a metabolon consisting of three enzymes (illustrated here in red, blue, and green) — favors dissociation of the complex into its constituents.
The enzymes of the citric acid cycle are usually described as soluble components of the mitochondrial matrix (except for succinate dehydrogenase, which is membrane-bound), but there is evidence that at least three sequential enzymes of the citric acid cycle (malate dehydrogenase, citrate synthase, and aconitase) constitute a metabolon (Fig. 16-22). We will see more examples of substrate channeling in some amino acid biosynthesis pathways in Chapter 22. As cryo-EM increases our understanding of larger complexes and “ — somes” (apoptosomes, respirasomes, and replisomes, for example), it seems likely that many more enzymes once thought to function as individual soluble proteins will be found to act in multienzyme complexes that facilitate the channeling of substrates.
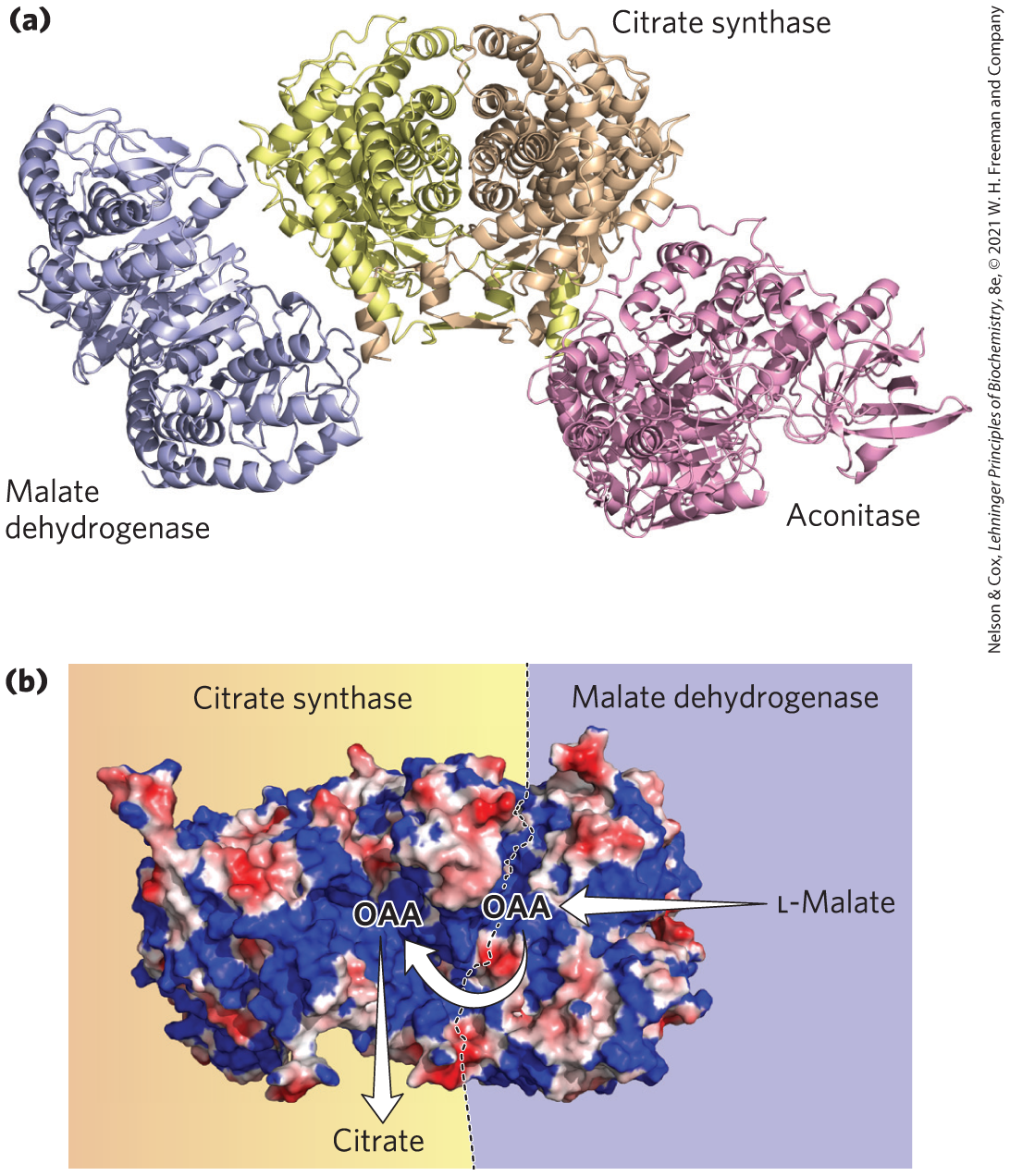
FIGURE 16-22 A three-enzyme metabolon of the citric acid cycle. (a) Purified porcine enzymes malate dehydrogenase (MDH), citrate synthase (CS), and aconitase form a metabolon when combined in vitro. (b) Electrostatic modeling shows that a broad path of positive potential along the surface of a MDH-CS complex connects the active sites of MDH and CS. This path provides a channel for the passage of the negatively charged oxaloacetate (OAA) from the active site of MDH, where it is formed from l-malate, to the active site of CS, where it condenses with acetyl-CoA to form citrate. Engineered mutations that replaced a positively charged Arg residue along this path with a negatively charged Asp residue greatly reduced the rate of substrate channeling through the complex, providing evidence that the functional unit is a metabolon. [Information from B. Bulutoglu et al., ACS Chem. Biol. 11:2847, 2016. Data from PDB ID 1MLD, W. B. Gleason et al., Biochemistry 33:2078, 1994; PDB ID 1CTS, S. Remington et al., J. Mol. Biol. 158:111, 1982; PDB ID 7ACN, H. Lauble et al., Biochemistry 31:2735, 1992.]
SUMMARY 16.4 Regulation of the Citric Acid Cycle
- The production of acetyl-CoA for the citric acid cycle by the PDH complex is inhibited allosterically by metabolites that signal a sufficiency of metabolic energy (ATP, acetyl-CoA, NADH, and fatty acids) and is stimulated by metabolites that indicate a reduced energy supply (AMP, CoA-SH, ).
- The PDH complex is regulated by allosteric mechanisms and covalent modification (phosphorylation). The overall rate of the citric acid cycle is controlled by the rate of conversion of pyruvate to acetyl-CoA and by the flux through citrate synthase, isocitrate dehydrogenase, and α-ketoglutarate dehydrogenase. These fluxes are affected by the concentrations of substrates and products: the end products ATP and NADH are inhibitory, and the substrates and ADP are stimulatory. Long-chain fatty acids, which can break down to acetyl-CoA, are also inhibitory.
- Some mutations that affect the PDH complex or citric acid cycle enzymes are oncogenic: they occur very commonly in certain types of cancer.
- Complexes of consecutive enzymes in a pathway (metabolons) allow substrate channeling and more efficient passage of substrates through the reaction sequences.
 The central role of the citric acid cycle in metabolism requires stringent regulation to balance the supply of key intermediates with the demands of energy production and biosynthetic processes. Regulation occurs at several levels, including the oxidation of pyruvate to acetyl-CoA (catalyzed by the PDH complex) and the entry of acetyl-CoA into the cycle (the citrate synthase reaction). The transport of pyruvate into mitochondria by the mitochondrial pyruvate carrier (MPC) determines to some degree the fate of pyruvate produced by glycolysis. Most cells also produce acetyl-CoA from the oxidation of fatty acids (
The central role of the citric acid cycle in metabolism requires stringent regulation to balance the supply of key intermediates with the demands of energy production and biosynthetic processes. Regulation occurs at several levels, including the oxidation of pyruvate to acetyl-CoA (catalyzed by the PDH complex) and the entry of acetyl-CoA into the cycle (the citrate synthase reaction). The transport of pyruvate into mitochondria by the mitochondrial pyruvate carrier (MPC) determines to some degree the fate of pyruvate produced by glycolysis. Most cells also produce acetyl-CoA from the oxidation of fatty acids ( Thus, PDH complex activity is turned off when ample fuel is available in the form of fatty acids and acetyl-CoA and when the cell’s and ratios are high; it is turned on again when energy demands are high and the cell requires greater flux of acetyl-CoA into the citric acid cycle.
Thus, PDH complex activity is turned off when ample fuel is available in the form of fatty acids and acetyl-CoA and when the cell’s and ratios are high; it is turned on again when energy demands are high and the cell requires greater flux of acetyl-CoA into the citric acid cycle. The simple compound dichloroacetate (DCA), a structural analog of acetate, inhibits PDH kinase in the laboratory and so relieves the inhibition of the PDH complex. This stimulates pyruvate oxidation via the citric acid cycle (
The simple compound dichloroacetate (DCA), a structural analog of acetate, inhibits PDH kinase in the laboratory and so relieves the inhibition of the PDH complex. This stimulates pyruvate oxidation via the citric acid cycle (
 The production of acetyl-CoA for the citric acid cycle by the PDH complex is inhibited allosterically by metabolites that signal a sufficiency of metabolic energy (ATP, acetyl-CoA, NADH, and fatty acids) and is stimulated by metabolites that indicate a reduced energy supply (AMP, CoA-SH, ).
The production of acetyl-CoA for the citric acid cycle by the PDH complex is inhibited allosterically by metabolites that signal a sufficiency of metabolic energy (ATP, acetyl-CoA, NADH, and fatty acids) and is stimulated by metabolites that indicate a reduced energy supply (AMP, CoA-SH, ).