14.3 Fates of Pyruvate
With the exception of some interesting variations in the bacterial realm, the pyruvate formed by glycolysis is further metabolized via one of three catabolic routes (Fig. 14-11). Under aerobic conditions, glycolysis is only the first stage in the complete degradation of glucose. The pyruvate formed in the final step of glycolysis is oxidized to acetate (acetyl-CoA), which enters the citric acid cycle and is oxidized completely to and (Chapter 16). The electrons from these oxidations are carried by NADH and , which are ultimately reoxidized to and FAD by passage of electrons to through a chain of carriers in mitochondrial respiration, to form . The energy from the electron-transfer reactions drives the synthesis of ATP (Chapter 19).
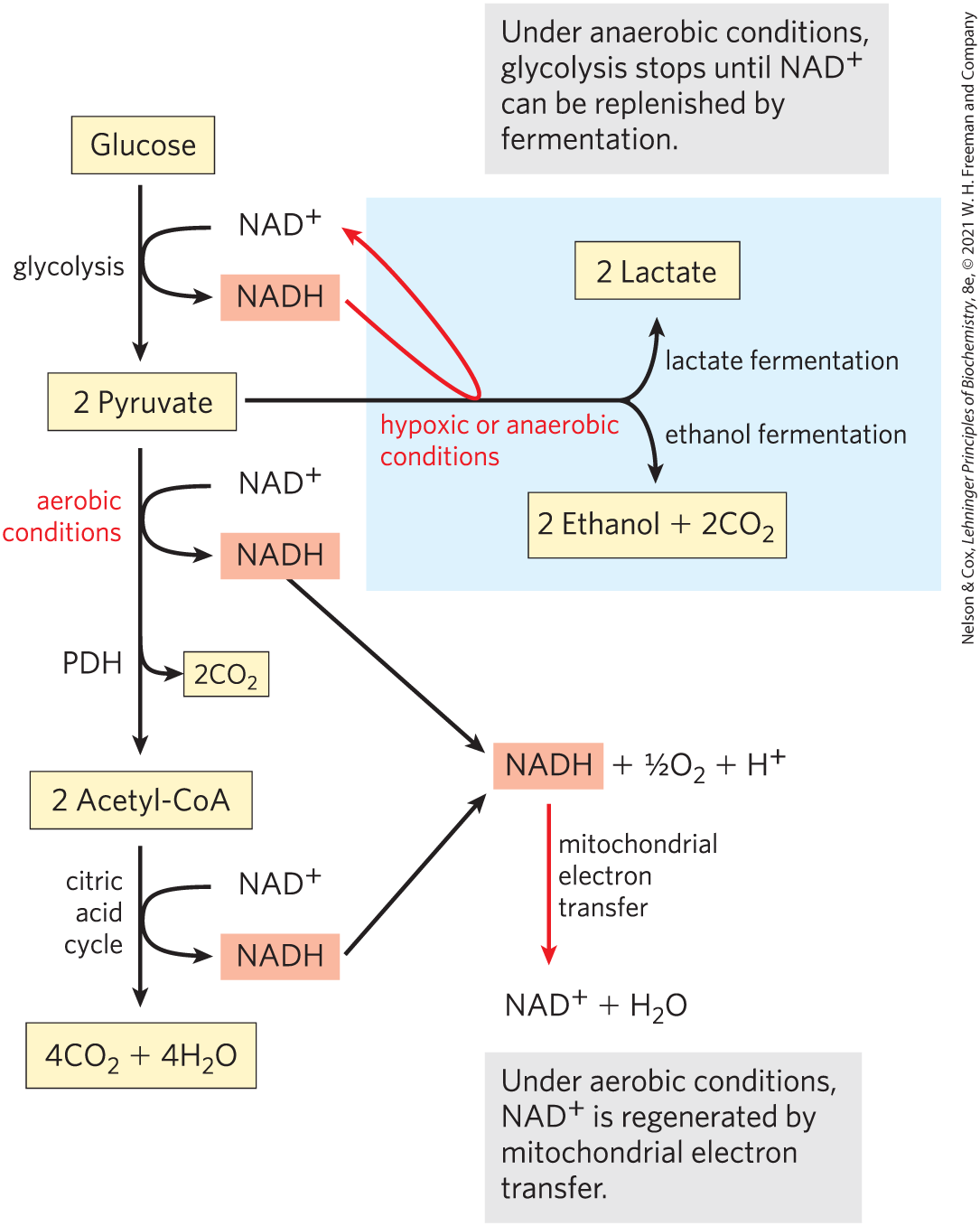
FIGURE 14-11 Three possible catabolic fates of the pyruvate formed in glycolysis and the recycling of NADH. Red arrows follow the regeneration of from NADH. Under aerobic conditions, pyruvate is activated to acetyl-CoA and is completely oxidized to and water through the citric acid cycle and mitochondrial oxidative phosphorylation. NADH produced in this pathway is oxidized to through mitochondrial electron transfer. Under anaerobic conditions, pyruvate reduction to lactate or to ethanol is required to produce the needed for glycolysis to continue. Pyruvate also serves as a precursor in many anabolic reactions, not shown here.
The earliest cells lived in an atmosphere almost devoid of oxygen and evolved deriving energy from fuel molecules under anaerobic conditions. Under anaerobic or low-oxygen conditions (hypoxia), NADH cannot be reoxidized to by passing its electrons to . But for glycolysis to continue, must be regenerated. Under these conditions, glucose is degraded by fermentation (defined below), which leads to one of two different fates for the pyruvate formed by glycolysis. In lactic acid fermentation, pyruvate accepts electrons from NADH and is reduced to lactate while regenerating the necessary for glycolysis. In ethanol (alcohol) fermentation, pyruvate is further catabolized to ethanol (Fig. 14-11).
The Pasteur and Warburg Effects Are Due to Dependence on Glycolysis Alone for ATP Production
During his studies on the fermentation of glucose by yeast, Louis Pasteur discovered that both the rate and the total amount of glucose consumption under anaerobic conditions were many times greater than under aerobic conditions. Later studies of muscle showed the same large difference in the rates of glycolysis under anaerobic and aerobic conditions. The biochemical basis of this “Pasteur effect” is now clear. The ATP yield from glycolysis alone (2 ATP per molecule of glucose) is much smaller than that from the complete oxidation of glucose to under aerobic conditions (30 or 32 ATP per glucose; see Table 19-5). About 15 times as much glucose must therefore be consumed anaerobically as aerobically to yield the same amount of ATP.
The flux of glucose through the glycolytic pathway is regulated to maintain nearly constant ATP levels (as well as adequate supplies of glycolytic intermediates that serve biosynthetic roles). The required adjustment in the rate of glycolysis is achieved by a complex interplay among ATP consumption, regeneration from NADH formed in glycolysis, and allosteric regulation of several glycolytic enzymes — including hexokinase, PFK-1, and pyruvate kinase — and by second-to-second fluctuations in the concentration of key metabolites that reflect the cellular balance between ATP production and consumption. On a slightly longer time scale, glycolysis is regulated by the hormones glucagon, epinephrine, and insulin, and by changes in the expression of the genes for several glycolytic enzymes. An especially interesting case is glycolysis in tumors. The German biochemist Otto Warburg first observed in 1928 that tumors of nearly all types carry out glycolysis at a much higher rate than normal tissue, even when oxygen is available. This “Warburg effect” is the basis for several methods of detecting and treating cancer (Box 14-1).

Otto Warburg, 1883–1970
Warburg is generally considered the preeminent biochemist of the first half of the twentieth century. He made seminal contributions to many other areas of biochemistry, including respiration, photosynthesis, and the enzymology of intermediary metabolism. Beginning in 1930, Warburg and his associates purified and crystallized seven of the enzymes of glycolysis. They developed an experimental tool that revolutionized biochemical studies of oxidative metabolism: the Warburg manometer, which directly measured the oxygen consumption of tissues by monitoring changes in gas volume, and thus allowed quantitative measurement of any enzyme with oxidase activity.
Pyruvate Is the Terminal Electron Acceptor in Lactic Acid Fermentation
When animal tissues cannot be supplied with sufficient oxygen to support aerobic oxidation of the pyruvate and NADH produced in glycolysis, is regenerated from NADH by the reduction of pyruvate to lactate. Some tissues and cell types (such as erythrocytes, which have no mitochondria and thus cannot oxidize pyruvate to ) produce lactate from glucose even under aerobic conditions. The reduction of pyruvate in this pathway is catalyzed by lactate dehydrogenase, which forms the l isomer of lactate at pH 7:

Pyruvate is a vertical three-carbon chain with C 1 double bonded to O to the upper left and bonded to O minus to the right, C 2 double bonded to O with both in a red box, and C 3 bonded to 3 H. A longer arrow points to the right, and a much shorter arrow points to the left above text reading lactate dehydrogenase. A curved arrow above the right-hand arrow shows that N A D H plus H plus is added and N A D plus is removed. This yields L-lactate, which has C 1 double bonded to O to the upper left and bonded to O minus to the upper right, C 2 bonded to O H on the left and to H on the right with all three in a light red box, and C 3 bonded to 3 H.
The overall equilibrium of the reaction strongly favors lactate formation, as shown by the large negative standard free-energy change.
In glycolysis, dehydrogenation of the two molecules of glyceraldehyde 3-phosphate derived from each molecule of glucose converts two molecules of to two of NADH. Because the reduction of two molecules of pyruvate to two of lactate regenerates two molecules of , there is no net change in or NADH:
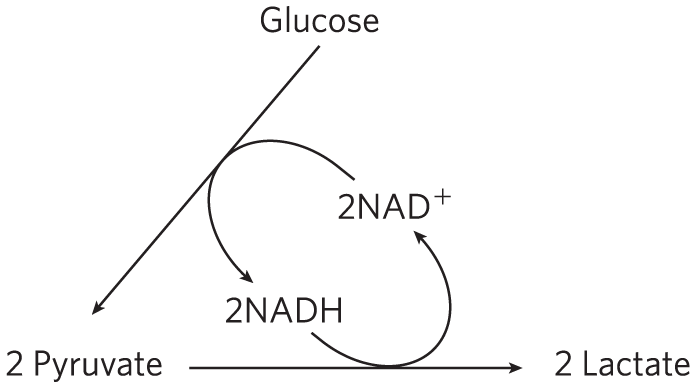
The lactate formed by active skeletal muscles (or by erythrocytes or retinal cells) can be recycled; it is carried in the blood to the liver, where it is converted to glucose during the recovery from strenuous muscular activity. When lactate is produced in large quantities during vigorous muscle contraction (during a sprint, for example), the acidification that results from ionization of lactic acid in muscle and blood limits the period of vigorous activity. The best-conditioned athletes can sprint at top speed for no more than a minute (Box 14-2).
Box 14-2
Glucose Catabolism at Limiting Concentrations of Oxygen
Most vertebrates are essentially aerobic organisms; they convert glucose to pyruvate by glycolysis, then use molecular oxygen to oxidize the pyruvate completely to and . Anaerobic catabolism of glucose to lactate occurs during short bursts of extreme muscular activity — for example, in a sprint — during which oxygen cannot be carried to the muscles fast enough to oxidize pyruvate. Instead, the muscles use their stored glucose (glycogen) as fuel to generate ATP by fermentation, with lactate as the end product. In a sprint, lactate in the blood builds up to high concentrations. It is slowly converted back to glucose by gluconeogenesis in the liver in the subsequent rest or recovery period, during which oxygen is consumed at a gradually diminishing rate until the breathing rate returns to normal. The excess oxygen consumed in the recovery period represents a repayment of the oxygen debt. This is the amount of oxygen required to supply ATP for gluconeogenesis during recovery respiration, in order to regenerate the glycogen “borrowed” from liver and muscle to carry out intense muscular activity in the sprint. The cycle of reactions that includes glucose conversion to lactate in muscle and lactate conversion to glucose in liver is called the Cori cycle, for Carl and Gerty Cori, whose studies in the 1930s and 1940s clarified the pathway and its role (see Box 15-1).
The circulatory systems of most small vertebrates can carry oxygen to their muscles fast enough to avoid having to use muscle glycogen anaerobically. For example, migrating birds often fly great distances at high speeds without rest and without incurring an oxygen debt. Many running animals of moderate size also maintain an essentially aerobic metabolism in their skeletal muscle. However, the circulatory systems of larger animals, including humans, cannot completely sustain aerobic metabolism in skeletal muscles over long periods of intense muscular activity. These animals generally are slow-moving under normal circumstances and engage in intense muscular activity only in the gravest emergencies, because such bursts of activity require long recovery periods to repay the oxygen debt.
Alligators and crocodiles, for example, are normally sluggish animals. Yet, when provoked, they are capable of lightning-fast charges and dangerous lashings of their powerful tails. Such intense bursts of activity are short and must be followed by long periods of recovery. The fast emergency movements require lactic acid fermentation to generate ATP in skeletal muscles. The stores of muscle glycogen are rapidly expended in intense muscular activity, and lactate reaches very high concentrations in myocytes and extracellular fluid. Whereas a trained athlete can recover from a 100 m sprint in 30 min or less, an alligator may require many hours of rest and extra oxygen consumption to clear the excess lactate from its blood and regenerate muscle glycogen after a burst of activity.
Other large animals, such as the elephant and the rhinoceros, have similar metabolic characteristics, as do diving mammals such as whales and seals. Dinosaurs and other huge, now-extinct animals probably had to depend on lactic acid fermentation to supply energy for muscular activity, followed by very long recovery periods during which they were vulnerable to attack by smaller predators that were better able to use oxygen and thus better adapted to continuous, sustained muscular activity.
Deep-sea explorations have revealed many species of marine life at great ocean depths, where the oxygen concentration is near zero. For example, the primitive coelacanth, a large fish recovered from depths of 4,000 m or more off the coast of South Africa, has an essentially anaerobic metabolism in virtually all its tissues. It converts carbohydrates to lactate and other products, most of which must be excreted. Some marine vertebrates ferment glucose to ethanol and in order to generate ATP.

Francena McCorory, Olympic sprinter
Although conversion of glucose to lactate includes two oxidation-reduction steps, there is no net change in the oxidation state of carbon; in glucose and lactic acid , the H:C ratio is the same. Nevertheless, some of the energy of the glucose molecule has been extracted by its conversion to lactate — enough to give a net yield of two molecules of ATP for every glucose molecule consumed. Fermentation is the general term for such processes, which extract energy (as ATP) but do not consume oxygen or change the concentrations of or NADH.
Ethanol Is the Reduced Product in Ethanol Fermentation
Yeast and other microorganisms ferment glucose to ethanol and , rather than to lactate. Glucose is metabolized to pyruvate by glycolysis, and the pyruvate is converted to ethanol and in a two-step process:
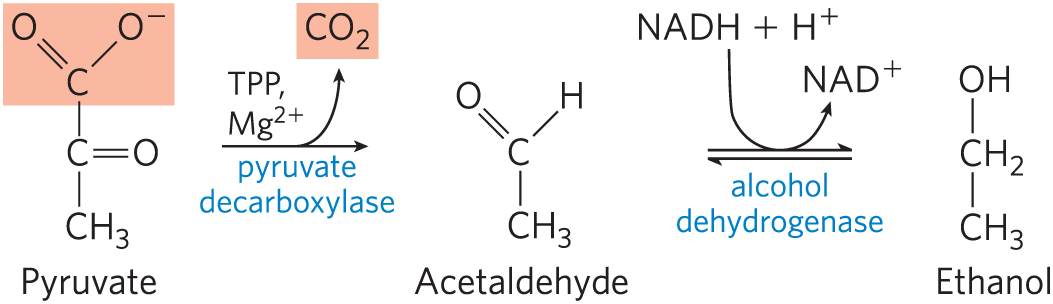
Pyruvate is a vertical three-carbon chain with C 1 double bonded to O to the upper left and bonded to O minus to the upper right and all three in a red box, C 2 double bonded to O to the right, and C 3 bonded to 3 H. An arrow points right above pyruvate decarboxylase and beneath T P P, M g 2 plus with an arrow curving up to show the loss of a light red square labeled C O 2. This yields acetaldehyde, which has C double bonded to O to the upper left, bonded to H to the upper right, and bonded to C H 3 below. Right- and left-pointing arrows above alcohol dehydrogenase show that a reversible reaction occurs. An accompanying curved arrow shows that N A D H plus H plus is added and N A D plus is lost. This yields ethanol, which has C H 2 bonded to O H above and to C H 3 below.
In the first step, pyruvate is decarboxylated to form acetaldehyde in an irreversible reaction catalyzed by pyruvate decarboxylase. This reaction is a simple decarboxylation and does not involve the net oxidation of pyruvate. Pyruvate decarboxylase requires and has a tightly bound coenzyme, thiamine pyrophosphate, which is discussed below. In the second step, acetaldehyde is reduced to ethanol through the action of alcohol dehydrogenase, with the reducing power furnished by NADH derived from the dehydrogenation of glyceraldehyde 3-phosphate. This reaction is a well-studied case of hydride transfer from NADH (Fig. 14-12). Ethanol and are thus the end products of ethanol fermentation, and the overall equation is
As in lactic acid fermentation, there is no net change in the ratio of hydrogen to carbon atoms when glucose is fermented to two ethanol and two (combined ). In all fermentations, the H:C ratio of the reactants and products remains the same.
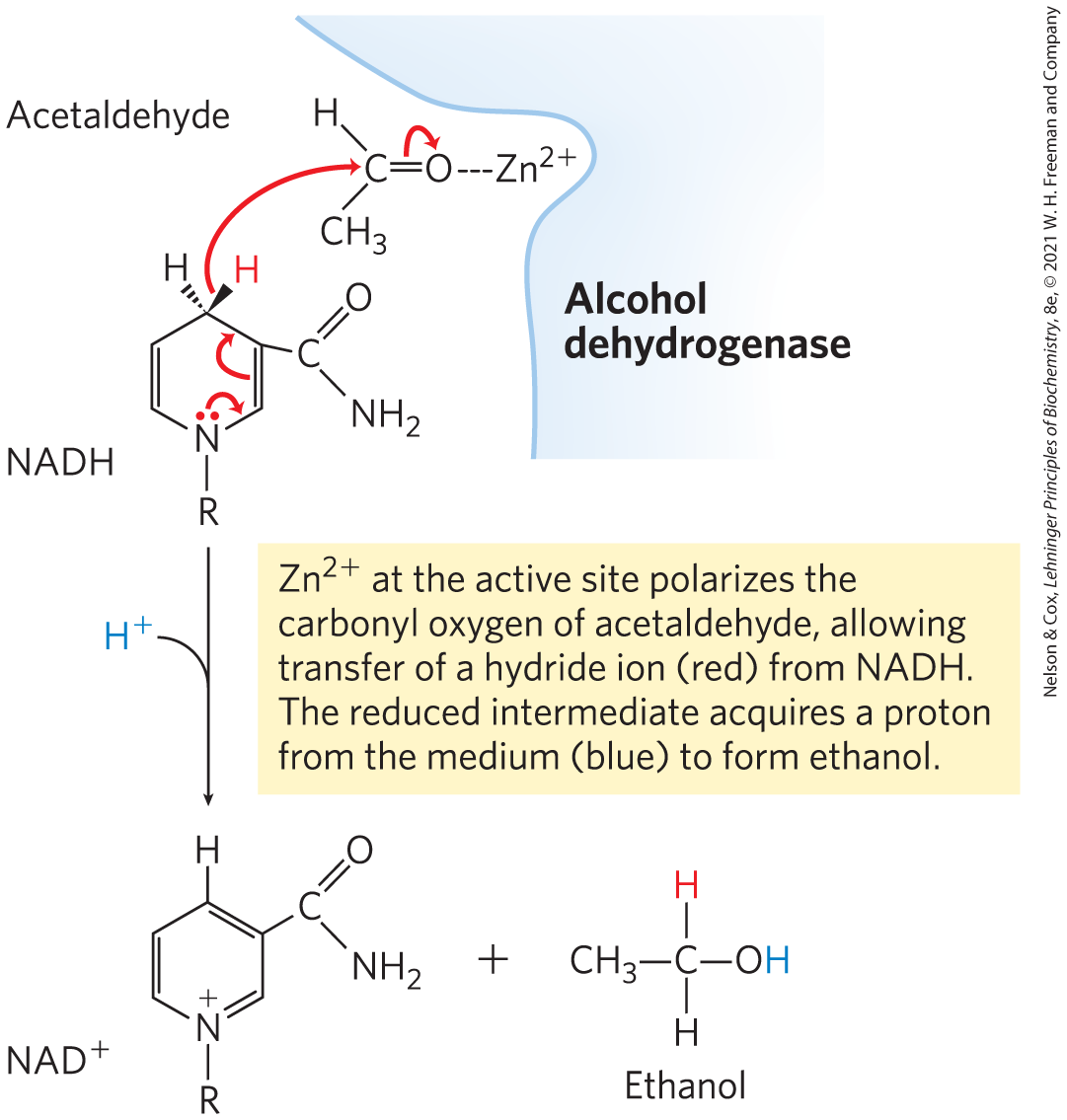
MECHANISM FIGURE 14-12 The alcohol dehydrogenase reaction.
An irregular line begins near the top center of the frame, has an invagination around Z n 2 plus, then curves back to end near the bottom center. This represents alcohol dehydrogenase. Acetaldehyde is shown as C bonded to H to the upper left, bonded to C H 3 to the lower left, and double bonded to O to the right with a dashed line to Z n 2 plus in the invagination in alcohol dehydrogenase. N A D H is shown as a ring with N substituted for C at the bottom vertex with a red pair of electrons and bonded to R below, double bonds at the left and right sides, the upper right vertex bonded to C double bonded to O to the upper right and bonded to N H 2 below, and the top vertex hashed wedge bonded to H to the upper left and solid wedge bonded to red highlighted H to the upper right. Curved red arrows point from the red pair of electrons on N to the adjacent lower right side bond, from the double bond on the right side to the upper right side bond, from the solid wedge bond to red highlighted H to the central C of acetaldehyde, and from the double bond between the same central C and O to the O. An arrow pointing down is joined by a line showing the addition of blue highlighted H plus. Accompanying text reads, Z n 2 plus at the active site polarizes the carbonyl oxygen of acetaldehyde, allowing transfer of a hydride ion (red) from N A D H. The reduced intermediate acquires a proton from the medium (blue) to form ethanol. This yields N A D plus and ethanol. N A D plus is similar to N A D H except that N at the bottom vertex of the ring has a positive charge, there are double bonds at the upper and lower right sides, and the top vertex is bonded to H. Ethanol has a central C bonded to C H 3 to the left, a red highlighted H above, O on the right further bonded to blue highlighted H, and H below.
Pyruvate decarboxylase is present in brewer’s and baker’s yeast (different strains of the species Saccharomyces cerevisiae) and in all other organisms that ferment glucose to ethanol, including some plants. The produced by pyruvate decarboxylation in brewer’s yeast is responsible for the characteristic carbonation of champagne. The ancient art of brewing beer involves several enzymatic processes in addition to the reactions of ethanol fermentation. In baking, released by pyruvate decarboxylase when yeast is mixed with a fermentable sugar causes dough to rise. The enzyme is absent in vertebrate tissues and in other organisms that carry out lactic acid fermentation.
Alcohol dehydrogenase is present in many organisms that metabolize ethanol, including humans. In the liver it catalyzes the oxidation of ethanol, either ingested or produced by intestinal microorganisms, with the concomitant reduction of to NADH. In this case, the reaction proceeds in the direction opposite to that involved in the production of ethanol by fermentation.
The pyruvate decarboxylase reaction provides our first encounter with thiamine pyrophosphate (TPP) (Fig. 14-13), a coenzyme derived from vitamin . TPP plays an important role in the cleavage of bonds adjacent to a carbonyl group, such as the decarboxylation of α-keto acids, and in chemical rearrangements in which an activated acetaldehyde group is transferred from one carbon atom to another (Table 14-1). The functional part of TPP, the thiazolium ring, has a relatively acidic proton at C-2. Loss of this proton produces a carbanion that is the active species in TPP-dependent reactions. The carbanion readily adds to carbonyl groups, and the thiazolium ring is thereby positioned to act as an “electron sink” that greatly facilitates reactions such as the decarboxylation catalyzed by pyruvate decarboxylase.
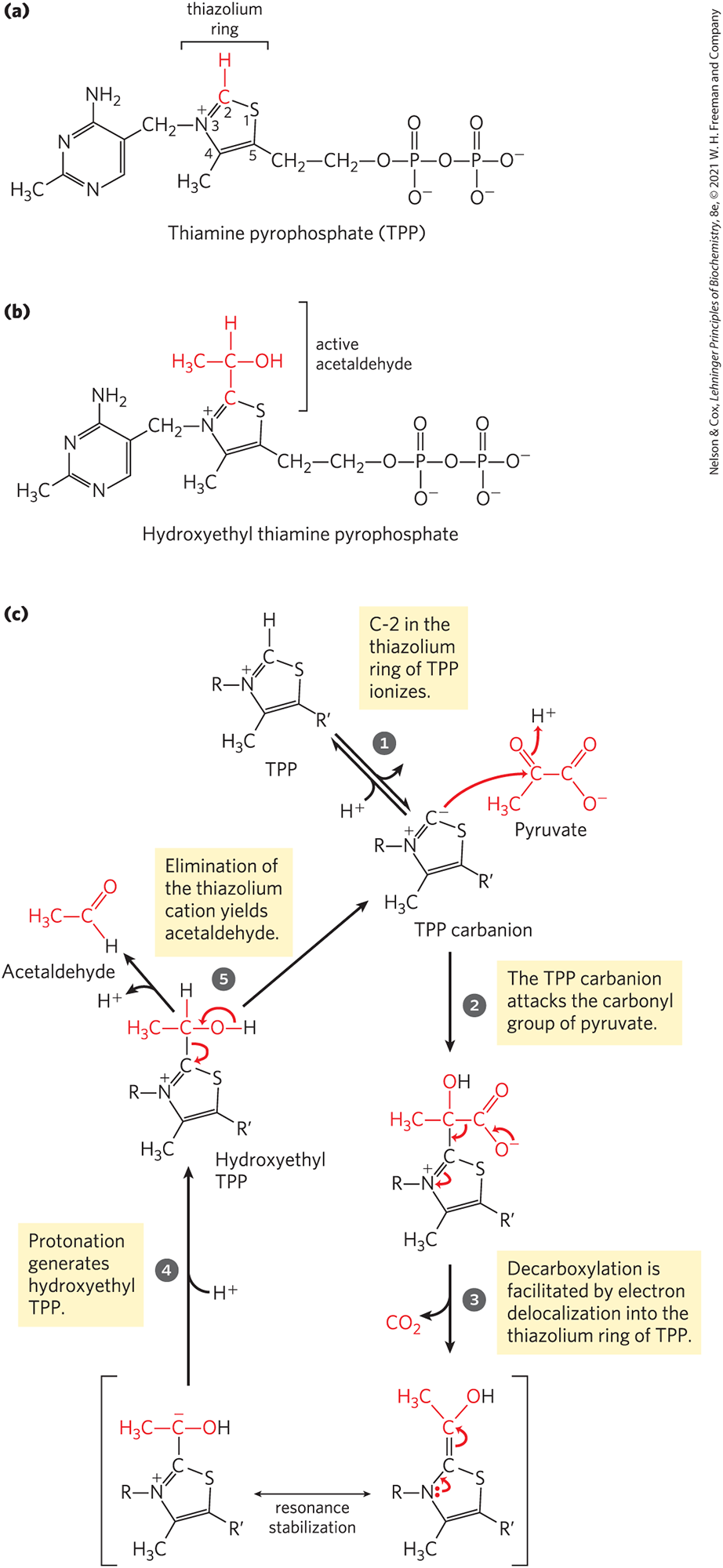
MECHANISM FIGURE 14-13 Thiamine pyrophosphate (TPP) and its role in pyruvate decarboxylation. (a) TPP is the coenzyme form of vitamin (thiamine). The reactive carbon atom in the thiazolium ring of TPP is shown in red. In the reaction catalyzed by pyruvate decarboxylase, two of the three carbons of pyruvate are carried transiently on TPP in the form of a hydroxyethyl, or “active acetaldehyde,” group (b), which is subsequently released as acetaldehyde. (c) The thiazolium ring of TPP stabilizes carbanion intermediates by providing an electrophilic (electron-deficient) structure into which the carbanion electrons can be delocalized by resonance. Structures with this property, often called “electron sinks,” play a role in many biochemical reactions — here, facilitating carbon–carbon bond cleavage. Dietary insufficiency of thiamine causes the serious disease beriberi and the Wernicke-Korsakoff syndrome.
Thiamine pyrophosphate (T P P) has a six-membered ring on the left with N substituted for C at the upper left and bottom vertices; double bonds at the upper left side, lower left side, and right side; the lower left vertex bonded to C H 3; the top vertex bonded to N H 2; and the upper right vertex bonded to C H 2 further bonded to N plus of the thiazolium ring. The thiazolium ring is a five-membered ring angled so that the top vertex is slightly to the left, and the positions in the ring are numbered, beginning with S at the upper right. The ring has S substituted for C at position 1 at the upper right, red highlighted C at position 2 connected by a red bond to red highlighted H above, a double bond between positions 2 and 3, N substituted for C at position 3 with a positive charge and a bond to C H 2 to the left already mentioned, C 4 bonded to C H 3, and C 5 bonded to C H 2 outside of the ring that is further bonded to C H 2 bonded to O bonded to P double bonded to O above, bonded to O minus below, and bonded to O to the right bonded to P double bonded to O above and bonded to O minus to the right and below. Part b shows hydroxyethyl thiamine pyrophosphate. This molecule has a similar structure to T P P except that the red highlighted C in position 2 of the ring is now bonded to C bonded to C H 3 to the left, H above, and O H to the right. These new atoms are highlighted in red and labeled active acetaldehyde. Part c shows the thiazolium ring of T P P with the chains bonded to positions 3 and 5 each shown as R. Diagonal arrows point down to the right and up to the left. The downward arrow has an arrow curving away from it and the upward arrow has a line showing the addition of H plus. Step 1: C-2 in the thiazolium ring of T P P ionizes. This yields a similar molecule labeled T P P carbanion in which the C at position 1 has a negative charge and is no longer bonded to H. Pyruvate is shown in red to its upper right as a left-hand C double bonded to O to its upper left, bonded to C H 3 to its lower right, and bonded to a right-hand C that is further double bonded to O to the upper right and bonded to O minus to the lower right. Curved red arrows point from the negative charge on C in position 1 of the T P P carbanion to the left-hand C of pyruvate and from the double-bond between the left-hand C of pyruvate and O and the same O. An arrow points down. Step 2: The T P P carbanion attacks the carbonyl group of pyruvate. This produces a similar molecule in which C at position 2 of the T P P carbanion is bonded to red highlighted central C bonded to red highlighted C H 3 to the left, bonded to red highlighted O above further bonded to nonhighlighted H, and bonded to red highlighted C to the right further double bonded to red highlighted O to the upper right and bonded to red highlighted O minus to the lower right. Curved red arrows point from the O minus to the right-hand C, from the bond between the right-hand C and the bond between the central C and C in position 2 of the ring, and from the double bond between positions 2 and 3 on the ring to the N plus in position 3 of the ring. An arrow points down and branches to show red highlighted C O 2 leaving. Step 3: Decarboxylation is facilitated by electron delocalization into the thiazolium ring of T P P. The arrow points to a similar molecule with a red pair of electrons on N at position 3 of the ring, no double bond between positions 2 and 3 of the ring, a double bond between C in position 2 of the ring and the red-highlighted C above it, and this red highlighted C further bonded to red highlighted C H 3 to the upper left and to red highlighted O to the upper right further bonded to nonhighlighted H. Curved red arrows point from the pair of electrons on N in position 3 to the bond between positions 2 and 3 on the ring and from the double bond between the C in position 2 and the red highlighted C above it to the same red highlighted C. This molecule has a double-headed arrow on its left indicating another molecule, and both are enclosed within brackets. The arrow is labeled resonance stabilization. The left-hand molecule is similar except that N at position 3 of the ring has a positive charge, there is a double bond between N in position 3 and C in position 2, C in position 2 is bonded to red highlighted C minus above that is bonded to red highlighted C H 3 to the left and to O on the right further bonded to nonhighlighted H. An arrow points upward from the left-hand molecule and is joined by a line showing the addition of H plus. Step 4: Protonation generates hydroxyethyl T P P. This yields a similar molecule in which C minus bonded to C in position 2 of the ring has lost its negative charge and is connected by a red bond to H above. Curved red arrows point from the red bond between H and O to the bond between the same O and the central C bonded to C in position 2 of the ring below and from the bond between the same central C and the C in position 2 of the ring below. Two arrows point away from this molecule. The left-hand molecule points to the upper left and branches to show the loss of H plus. It produces acetaldehyde, which is a mostly red highlighted molecule with a central C bonded to C H 3 to the left, double bonded to O to the upper right, and bonded to a nonhighlighted H to the lower right. The second arrow points up to the right to the T P P carbanion. Step 5: Elimination of the thiazolium cation yields acetaldehyde.
Enzyme |
Pathway(s) |
Bond cleaved |
Bond formed |
|---|---|---|---|
Pyruvate decarboxylase |
Ethanol fermentation |
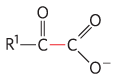
|
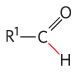
|
Pyruvate dehydrogenase α-Ketoglutarate dehydrogenase |
Synthesis of acetyl-CoA Citric acid cycle |
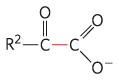
|
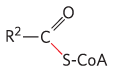
|
Transketolase |
Carbon-assimilation reactions Pentose phosphate pathway |
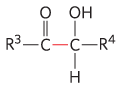
|
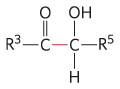
|
Fermentations Produce Some Common Foods and Industrial Chemicals
Our progenitors learned millennia ago to use fermentation in the production and preservation of foods and beverages. Certain microorganisms present in raw food products ferment the carbohydrates and yield metabolic products that give the foods their characteristic forms, textures, and tastes. In modern times, industrial fermentation produces organic chemicals and fuels.
Fermented Foods
Yogurt, already known in biblical times, is produced when the bacterium Lactobacillus bulgaricus ferments the carbohydrate in milk, producing lactic acid; the resulting drop in pH causes the milk proteins to precipitate, producing the thick texture and sour taste of unsweetened yogurt. Another bacterium, Propionibacterium freudenreichii, ferments milk to produce propionic acid and ; the propionic acid precipitates milk proteins, and bubbles of cause the holes characteristic of Swiss cheese. Many other food products are the result of fermentations: pickles, sauerkraut, sausage, soy sauce, kimchi, kefir, dahi, and kombucha. The drop in pH associated with fermentation also helps to preserve foods, because most of the microorganisms that cause food spoilage cannot grow at low pH. In agriculture, plant byproducts such as corn stalks are preserved for use as animal feed by packing them into a large container (a silo) with limited access to air; microbial fermentation produces acids that lower the pH. The silage that results from this fermentation process can be kept as animal feed for long periods without spoilage.
Fermented Beverages
Beer brewing was a science learned early in human history, and later refined for larger-scale production (Fig. 14-14). Brewers prepare beer by ethanol fermentation of the carbohydrates in cereal grains (seeds) such as barley, carried out by yeast glycolytic enzymes. The carbohydrates, largely polysaccharides, must first be degraded to disaccharides and monosaccharides. In the malting process, the barley seeds are allowed to germinate until they form the hydrolytic enzymes required to break down their polysaccharides, at which point germination is stopped by controlled heating. The product is malt, which contains enzymes that catalyze the hydrolysis of the β linkages of cellulose and other cell wall polysaccharides of the barley husks, and enzymes such as α-amylase and maltase. The malt is mixed with water, mashed, and boiled with hops to add flavor. Yeast cells added to this mixture grow and reproduce rapidly, using energy obtained from available sugars. No ethanol forms during this stage, because the yeast, amply supplied with oxygen, oxidizes the pyruvate formed by glycolysis to and via the citric acid cycle.

FIGURE 14-14 Beer brewing. Large breweries and microbreweries produce beers with a wide variety of flavors, the result of differences in materials and fermentation conditions.
When all the dissolved oxygen in the vat has been consumed, the yeast cells switch to anaerobic metabolism and ferment the sugars into ethanol and . The fermentation process is controlled in part by the concentration of the ethanol formed, by the pH, and by the amount of remaining sugar. After fermentation has been stopped, the cells are removed and the “raw” beer is ready for final processing.
Chemical Production by Fermentation
In 1910, Chaim Weizmann (later to become the first president of Israel) discovered that the bacterium Clostridium acetobutyricum ferments starch to butanol and acetone. This discovery opened the field of industrial fermentations, in which some readily available material rich in carbohydrate is supplied to a pure culture of a specific microorganism, which ferments it into a product of greater commercial value. Microbial fermentations produce formic, acetic, propionic, butyric, and succinic acids, and ethanol, glycerol, methanol, isopropanol, butanol, and butanediol. Industrial fermentations are also used to produce certain antibiotics, including penicillin, streptomycin, and chloramphenicol. These fermentations are generally carried out in huge closed vats in which temperature and access to air are controlled to favor the multiplication of the desired microorganism and to exclude contaminating organisms. The beauty of industrial fermentations is that complicated, multistep chemical transformations are carried out in high yields and with few side products by chemical factories that reproduce themselves — microbial cells.
Fuel Production by Fermentation
Much of the technology developed for large-scale production of alcoholic beverages can be applied to the production of ethanol as a renewable fuel. The principal advantage of ethanol as a fuel is that it can be produced from relatively inexpensive and renewable resources rich in sucrose, starch, or cellulose — starch from corn or wheat; sucrose from beets or cane; and cellulose from straw, forest industry waste, or municipal solid waste. Typically, the raw material (feedstock) is converted chemically to monosaccharides, then fed to a hardy strain of yeast in an industrial-scale fermenter. The fermentation can yield not only ethanol for fuel but also side products such as proteins that can be used as animal feed.
SUMMARY 14.3 Fates of Pyruvate
- The NADH formed in glycolysis must be recycled to regenerate , which is required as an electron acceptor in the first step of the payoff phase. Under aerobic conditions, electrons pass from NADH to in mitochondrial respiration. The Warburg effect is the observation that tumor cells have high rates of glycolysis, with fermentation of glucose to lactate, even in the presence of oxygen. It is the basis of PET scanning used to diagnose tumors.
- Under anaerobic or hypoxic conditions, many organisms regenerate by transferring electrons from NADH to pyruvate, forming lactate. Other organisms, such as yeast, regenerate by reducing pyruvate to ethanol and .
- A variety of microorganisms can ferment sugar in fresh foods, resulting in changes in pH, taste, and texture, and preserving food from spoilage. Fermentations are used in industry to produce many commercially valuable organic compounds from inexpensive starting materials.
 Under aerobic conditions, glycolysis is only the first stage in the complete degradation of glucose. The pyruvate formed in the final step of glycolysis is oxidized to acetate (acetyl-CoA), which enters the citric acid cycle and is oxidized completely to and (
Under aerobic conditions, glycolysis is only the first stage in the complete degradation of glucose. The pyruvate formed in the final step of glycolysis is oxidized to acetate (acetyl-CoA), which enters the citric acid cycle and is oxidized completely to and ( The NADH formed in glycolysis must be recycled to regenerate
The NADH formed in glycolysis must be recycled to regenerate