16.2 Reactions of the Citric Acid Cycle
We are now ready to trace the process by which acetyl-CoA undergoes oxidation. This chemical transformation is carried out by the citric acid cycle, the first cyclic pathway we have encountered (Fig. 16-7). To begin a turn of the cycle, acetyl-CoA donates its acetyl group to the four-carbon compound oxaloacetate to form the six-carbon citrate. Citrate is then transformed into isocitrate, also a six-carbon molecule, which is dehydrogenated with loss of to yield the five-carbon compound α-ketoglutarate (also called 2-oxoglutarate). α-Ketoglutarate undergoes loss of a second molecule of and ultimately yields the four-carbon compound succinate. Succinate is enzymatically converted in three steps into the four-carbon oxaloacetate — which is then ready to react with another molecule of acetyl-CoA. In each turn of the cycle, one acetyl group (two carbons) enters as acetyl-CoA and two molecules of leave; one molecule of oxaloacetate is used to form citrate and one molecule of oxaloacetate is regenerated. No net removal of oxaloacetate occurs; one molecule of oxaloacetate can theoretically bring about oxidation of an infinite number of acetyl groups, effectively acting catalytically; the steady-state concentration of oxaloacetate is very low (micromolar). Four of the eight steps in this process are oxidations, in which the energy of oxidation is very efficiently conserved in the form of the reduced coenzymes NADH and . These two carriers donate their electrons to the respiratory chain where electron flow drives ATP synthesis.
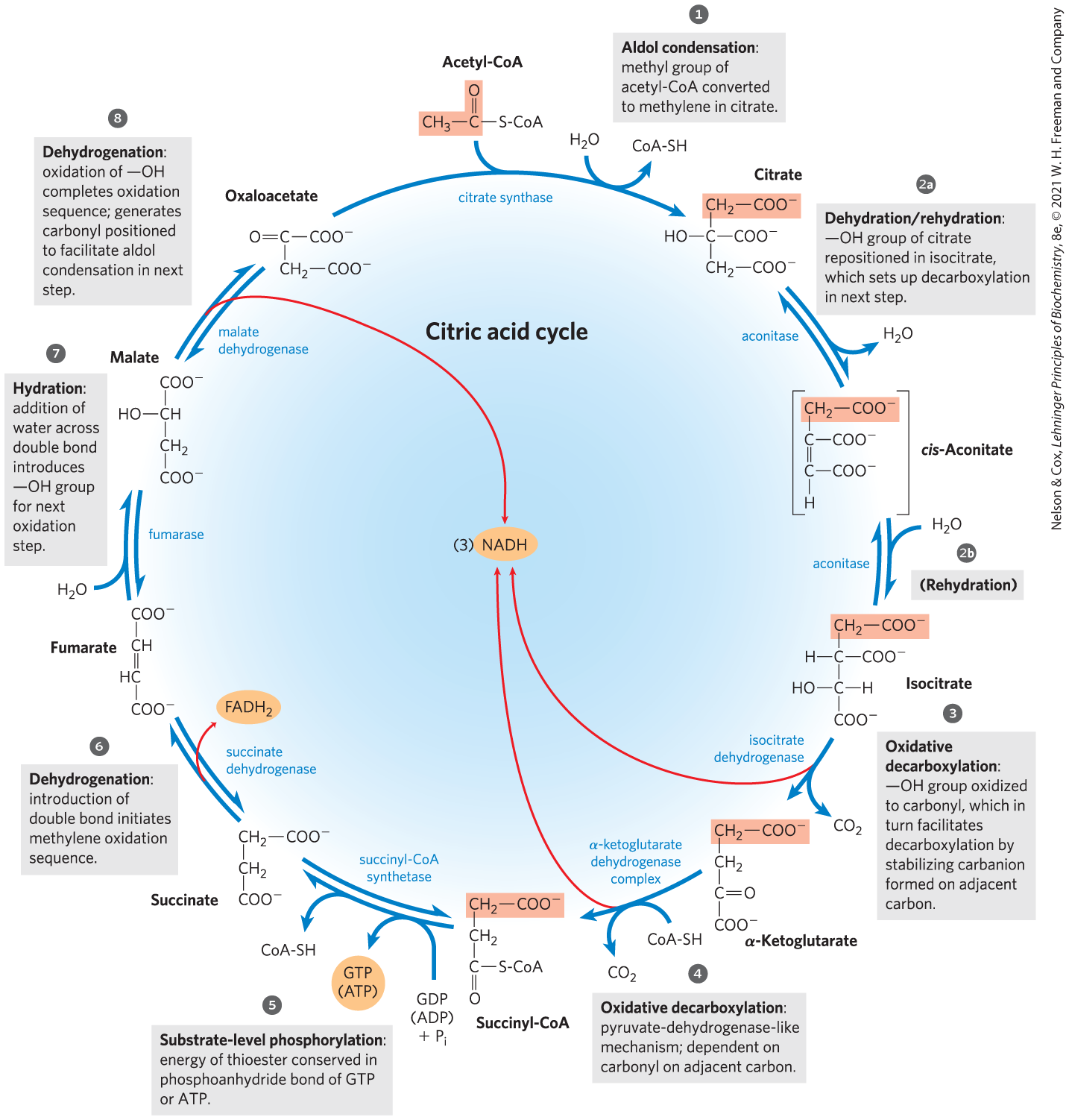
FIGURE 16-7 Reactions of the citric acid cycle. The carbon atoms shaded in pink are those derived from the acetate of acetyl-CoA in the first turn of the cycle; these are not the carbons released as in the first turn. Note that in succinate and fumarate, the two-carbon group derived from acetate can no longer be specifically denoted; because succinate and fumarate are symmetric molecules, C-1 and C-2 are indistinguishable from C-4 and C-3. The red arrows show where energy is conserved by electron transfer to FAD or , forming or . Steps , , and are essentially irreversible in the cell; all other steps are reversible. The nucleoside triphosphate product of step may be either ATP or GTP, depending on which succinyl-CoA synthetase isozyme is the catalyst.
Although the citric acid cycle is central to energy-yielding metabolism, its role is not limited to energy conservation. Four- and five-carbon intermediates of the cycle serve as precursors for a wide variety of products. To replace intermediates removed for this purpose, cells employ anaplerotic (replenishing) reactions, which are described below.
Eugene Kennedy and Albert Lehninger showed in 1948 that, in eukaryotes, the entire set of reactions of the citric acid cycle takes place in mitochondria. Isolated mitochondria were found to contain not only all the enzymes and coenzymes required for the citric acid cycle, but also all the enzymes and proteins necessary for the last stage of respiration — electron transfer and ATP synthesis by oxidative phosphorylation. As we shall see in later chapters, mitochondria also contain the enzymes for the oxidation of fatty acids and some amino acids to acetyl-CoA, and the oxidative degradation of other amino acids to α-ketoglutarate, succinyl-CoA, or oxaloacetate. Thus, in nonphotosynthetic eukaryotes, the mitochondrion is the site of most energy-yielding oxidative reactions and of the coupled synthesis of ATP. In photosynthetic eukaryotes, mitochondria are the major site of ATP production in the dark, but in daylight, chloroplasts produce most of the organism’s ATP. In most bacteria, the enzymes of the citric acid cycle are in the cytosol, and the plasma membrane plays a role analogous to that of the inner mitochondrial membrane in ATP synthesis (Chapter 19).
The Sequence of Reactions in the Citric Acid Cycle Makes Chemical Sense
Acetyl-CoA produced in the breakdown of carbohydrates, fats, and proteins must be completely oxidized to if the maximum potential energy is to be extracted from these fuels. However, it is not biochemically feasible to directly oxidize acetate (or acetyl-CoA) to . Decarboxylation of this two-carbon acid would yield and methane . Methane is chemically rather stable, and except for certain methanotrophic bacteria that grow in methane-rich niches, organisms do not have the cofactors and enzymes needed to oxidize methane. Methylene groups , however, are readily metabolized by enzyme systems present in most organisms. In typical oxidation sequences, two adjacent methylene groups are involved, at least one of which is adjacent to a carbonyl group. As we noted in Chapter 13 (p. 473), carbonyl groups are particularly important in the chemical transformations of metabolic pathways. The carbon of the carbonyl group has a partial positive charge due to the electron-withdrawing property of the carbonyl oxygen and is therefore an electrophilic center. A carbonyl group can facilitate the formation of a carbanion on an adjoining carbon by delocalizing the carbanion’s negative charge. We see an example of the oxidation of a methylene group in the citric acid cycle, as succinate is oxidized (steps to in Fig. 16-7) to form a carbonyl (in oxaloacetate) that is more chemically reactive than either a methylene group or methane.
In short, if acetyl-CoA is to be oxidized efficiently, the methyl group of the acetyl-CoA must be attached to something. The first step of the citric acid cycle — the condensation of acetyl-CoA with oxaloacetate — neatly solves the problem of the unreactive methyl group. The carbonyl of oxaloacetate acts as an electrophilic center, which is attacked by the methyl carbon of acetyl-CoA in an aldol condensation (p. 473) to form citrate (step in Fig. 16-7). The methyl group of acetate has been converted into a methylene in citric acid. This tricarboxylic acid then readily undergoes a series of oxidations that eliminate two carbons as . Note that all steps featuring the breakage or formation of carbon–carbon bonds (steps , , and ) rely on properly positioned carbonyl groups. As in all metabolic pathways, there is a chemical logic to the sequence of steps in the citric acid cycle: each step either involves an energy-conserving oxidation or is a necessary prelude to the oxidation, placing functional groups in position to facilitate oxidation or oxidative decarboxylation. As you learn the steps of the cycle, keep in mind the chemical rationale for each; it will make the process easier to understand and remember.
The Citric Acid Cycle Has Eight Steps
In examining the eight successive reaction steps of the citric acid cycle, we place special emphasis on the chemical transformations taking place as citrate formed from acetyl-CoA and oxaloacetate is oxidized to yield and the energy of this oxidation is conserved in the reduced coenzymes NADH and .
Formation of Citrate
The first reaction of the cycle is the condensation of acetyl-CoA with oxaloacetate to form citrate, catalyzed by citrate synthase.
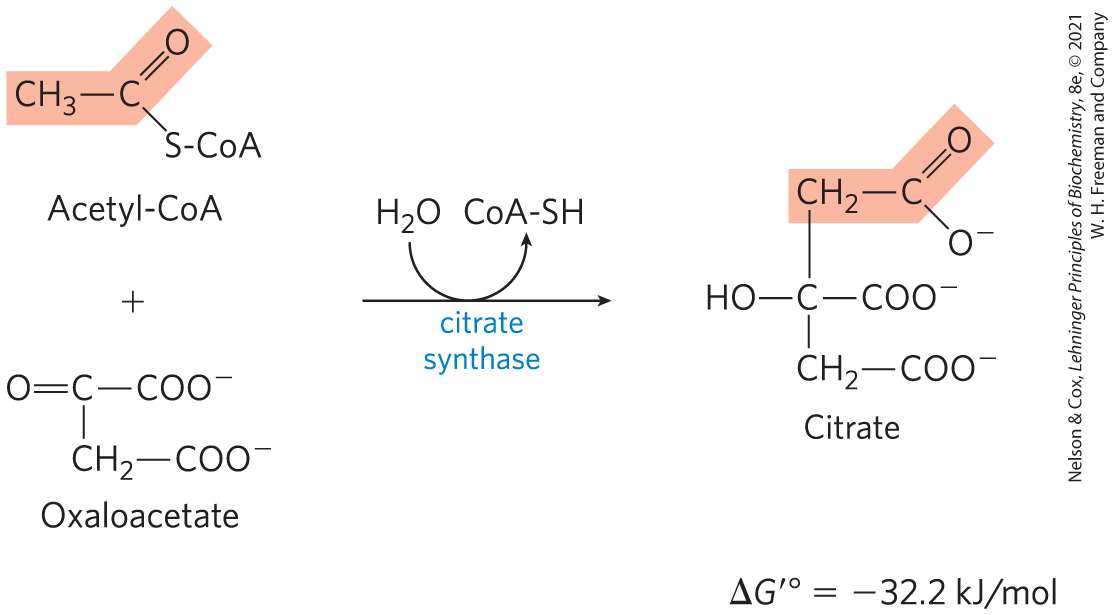
In this reaction, the methyl carbon of the acetyl group is joined to the carbonyl group (C-2) of oxaloacetate. Citroyl-CoA is a transient intermediate formed on the active site of the enzyme (see Fig. 16-9). It undergoes hydrolysis to free CoA and citrate, which are released from the active site. The hydrolysis of this high-energy thioester intermediate makes the forward reaction highly exergonic. The large, negative standard free-energy change of the forward citrate synthase reaction is essential to the operation of the cycle because the concentration of oxaloacetate is normally very low (micromolar). The CoA liberated in this reaction is recycled to participate in the oxidative decarboxylation of another molecule of pyruvate by the PDH complex.
Citrate synthase from mitochondria is a homodimer (Fig. 16-8). Each subunit is a single polypeptide with two domains, one large and rigid, the other smaller and more flexible, with the active site between them. Oxaloacetate, the first substrate to bind to the enzyme, induces a large conformational change in the flexible domain, creating a binding site for the second substrate, acetyl-CoA. When citroyl-CoA has formed in the enzyme active site, another conformational change brings about thioester hydrolysis, releasing CoA-SH. This induced fit of the enzyme first to its substrate and then to its reaction intermediate decreases the likelihood of premature and unproductive cleavage of the thioester bond of acetyl-CoA. Kinetic studies of the enzyme are consistent with this ordered bisubstrate mechanism (see Fig. 6-15). The reaction catalyzed by citrate synthase is essentially an aldol condensation (p. 473), involving a thioester (acetyl-CoA) and a ketone (oxaloacetate) (Fig. 16-9).
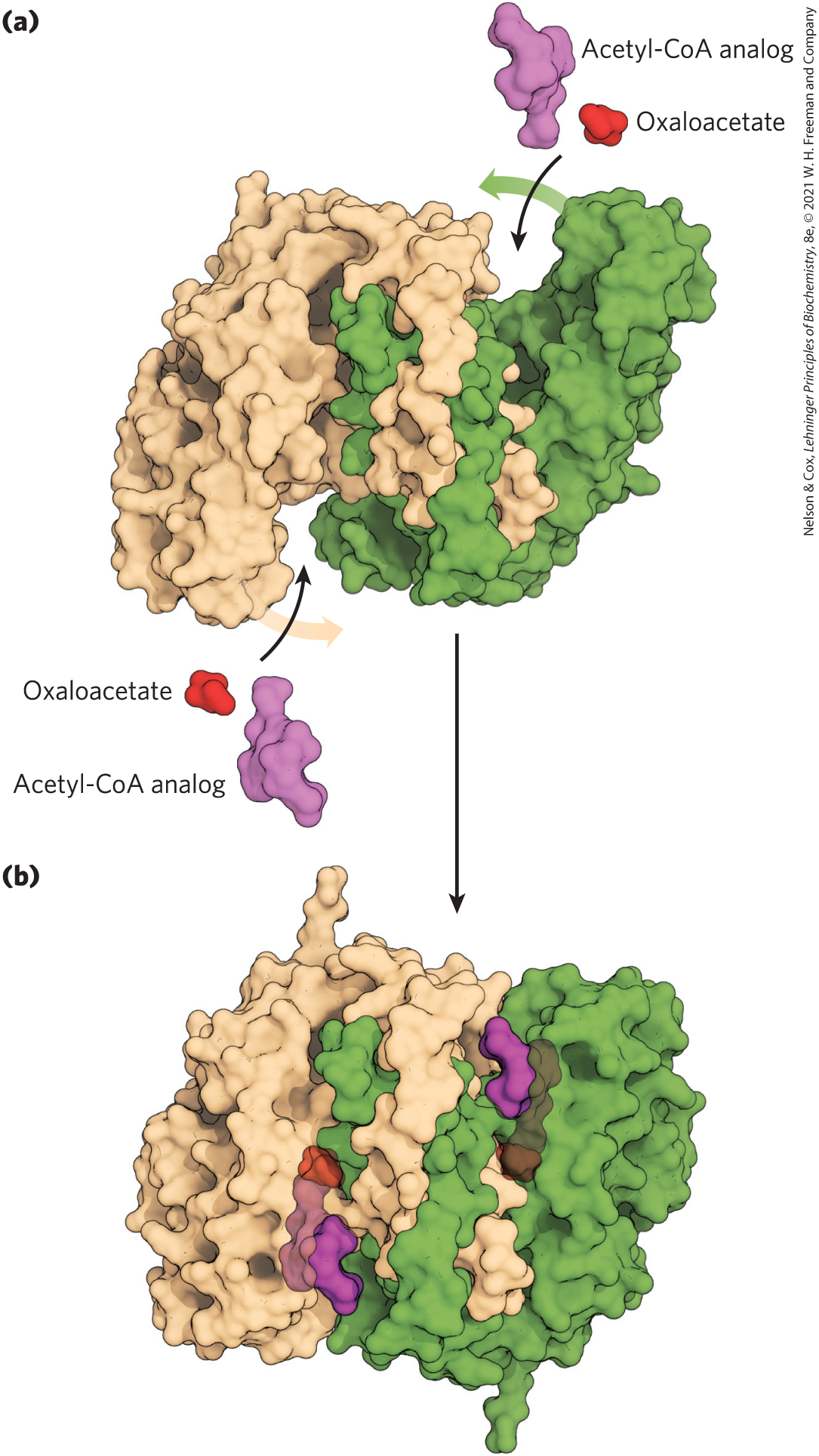
FIGURE 16-8 Structure of citrate synthase. The flexible domain of each subunit undergoes a large conformational change on binding oxaloacetate, creating a binding site for acetyl-CoA. (a) Open form of the enzyme alone; (b) closed form with bound oxaloacetate and a stable analog of acetyl-CoA (carboxymethyl-CoA). In these representations one subunit is colored tan and one green. [Data from (a) PDB ID 5CSC, D.-I. Liao et al., Biochemistry 30:6031, 1991;]
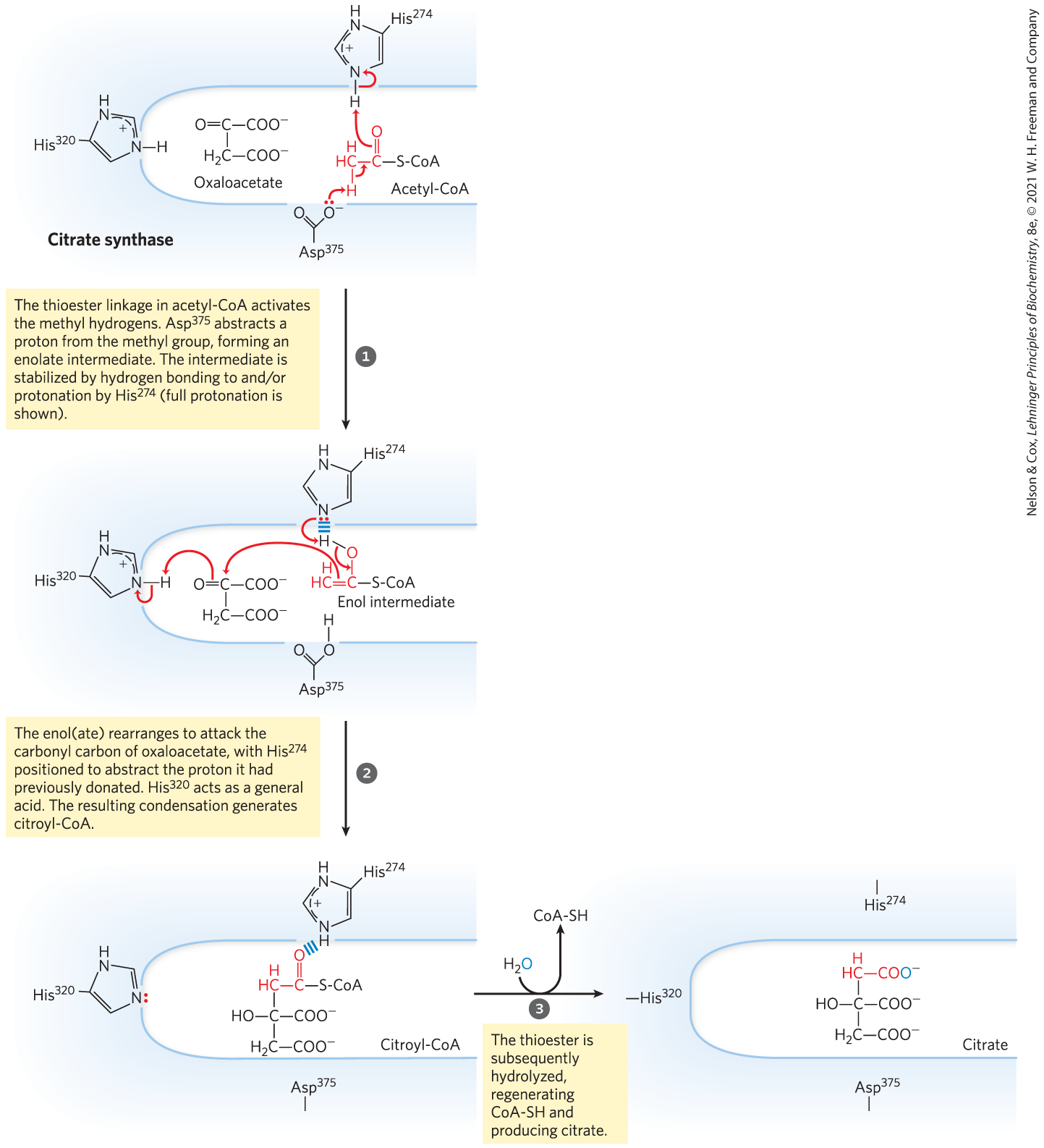
MECHANISM FIGURE 16-9 Citrate synthase. In the citrate synthase reaction in mammals, oxaloacetate binds first, in a strictly ordered reaction sequence. This binding triggers a conformation change that opens up the binding site for acetyl-CoA. Oxaloacetate is specifically oriented in the active site of citrate synthase by interaction of its two carboxylates with two positively charged Arg residues (not shown here). [Information from S. J. Remington, Curr. Opin. Struct. Biol. 2:730, 1992.]
Formation of Isocitrate via cis-Aconitate
The enzyme aconitase (more formally, aconitate hydratase) catalyzes the reversible transformation of citrate to isocitrate, through the intermediary formation of the tricarboxylic acid cis-aconitate, which normally does not dissociate from the active site. Aconitase can promote the reversible addition of to the double bond of enzyme-bound cis-aconitate in two different ways, one leading to citrate and the other to isocitrate:
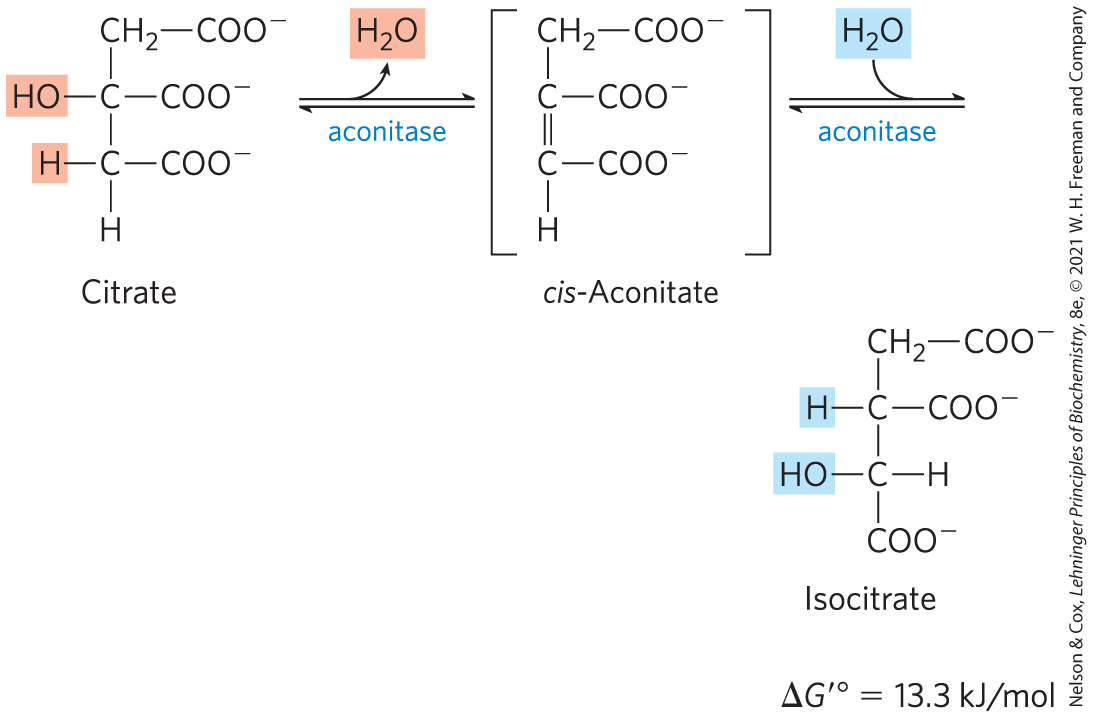
Although the equilibrium mixture at pH 7.4 and 25 °C contains less than 10% isocitrate, in the cell the reaction is pulled to the right because isocitrate is immediately consumed in the next step of the cycle, lowering its steady-state concentration. Aconitase contains an iron-sulfur center (Fig. 16-10), which acts both in the binding of the substrate at the active site and in the catalytic addition or removal of . In iron-depleted cells, acon-itase loses its iron-sulfur center and acquires a new role in the regulation of iron homeostasis. Aconitase is one of many enzymes known to “moonlight” in a second role (Box 16-1).
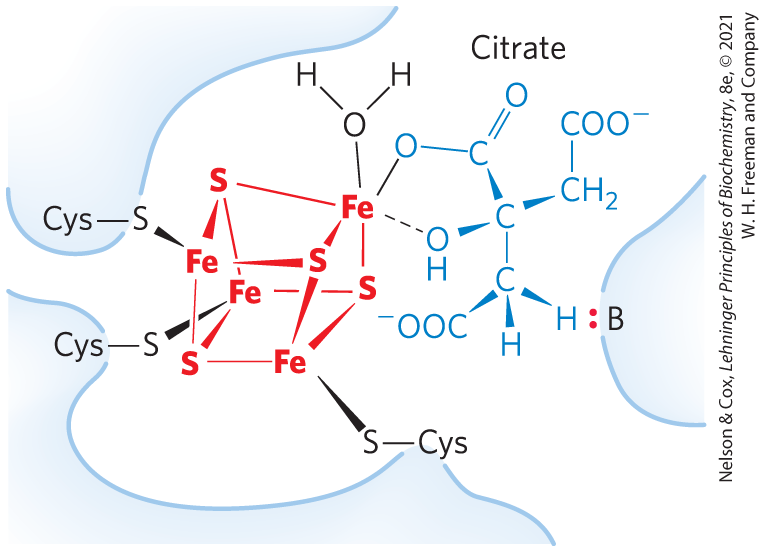
FIGURE 16-10 Iron-sulfur center in aconitase. The iron-sulfur center is in red, the citrate molecule in blue. Three Cys residues of the enzyme bind three iron atoms; the fourth iron is bound to one of the carboxyl groups of citrate and also interacts noncovalently with a hydroxyl group of citrate (dashed bond). A basic residue (:B) in the enzyme helps to position the citrate in the active site. The iron-sulfur center acts in both substrate binding and catalysis. The general properties of iron-sulfur proteins are discussed in Chapter 19.
Oxidation of Isocitrate to α-Ketoglutarate and
In the next step, isocitrate dehydrogenase catalyzes oxidative decarboxylation of isocitrate to form α-ketoglutarate (Fig. 16-11). in the active site interacts with the carbonyl group of the intermediate oxalosuccinate, which is formed transiently but does not leave the binding site until decarboxylation converts it to α-ketoglutarate. also stabilizes the enol formed transiently by decarboxylation.
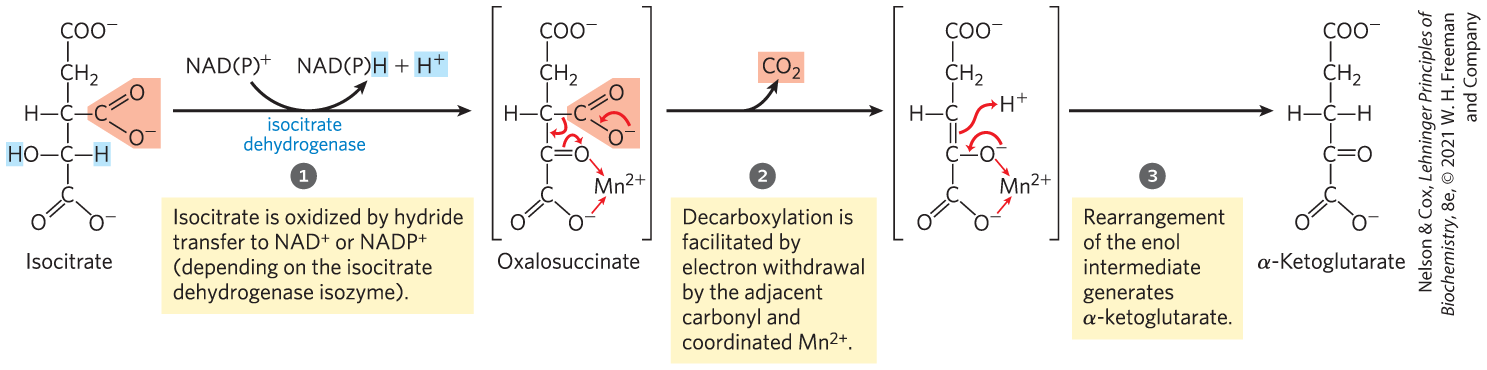
MECHANISM FIGURE 16-11 Isocitrate dehydrogenase. In this reaction, the substrate, isocitrate, loses one carbon by oxidative decarboxylation.
Isocitrate is shown as a five-carbon chain with the top C forming C O O minus; the second carbon bonded to 2 H; the third carbon bonded to H on the left and to C on the right in a light red box and further double bonded to O to the upper left and bonded to O minus to the lower right, all within a light red box; the further carbon bonded to O on the left further bonded to H in a light blue box and to H on the right in a light blue box; and the bottom carbon double bonded to O to the lower left and bonded to O minus to the lower right. Step 1: Isocitrate is oxidized by hydride transfer to N A D plus or N A D P plus (depending on the isocitrate dehydrogenase isozyme). An arrow points right above isocitrate dehydrogenase accompanied by a curved arrow showing the addition of N A D (P) plus and the loss of N A D (P) H, with H in a light blue box, plus H plus in a blue box. This yields oxalosuccinate, shown within brackets as a similar molecule in which the fourth carbon is double bonded to O on the right. Additionally, there are curved red arrows from O minus to the bond between it and C within the red box bonded to the third carbon, between C in the red box bonded to the third carbon and the bond between the third and fourth carbons in the vertical chain, and between the double bond between the fourth carbon in the vertical chain and O and the same O. There are also red arrows from this double bonded O and from the O minus on the bottom carbon to M n 2 plus between them. Step 2: Decarboxylation is facilitated by electron withdrawal by the adjacent carbonyl and coordinated M n 2 plus. An arrow points right accompanied by a branched arrow showing the loss of C O 2 in a red box. This yields an intermediate shown in brackets. It is similar except that the third carbon in the vertical chain is bonded to H on the left and double bonded to the fourth carbon below and the further carbon has a single bond to O minus to its right. Curved red arrows point from the double bond between the third and fourth carbons in the vertical chain to H plus to the right and from the negative charge on O bonded to the further carbon in the chain to the bond between this O and carbon. Step 3: Rearrangement of the enol intermediate generates Greek letter alpha-ketoglutarate. An arrow points right. This yields Greek letter alpha-ketoglutarate, shown as a vertical five-carbon chain with the top carbon forming C O O minus, the second carbon bonded to 2 H, the third carbon bonded to H to the left and H to the right, the fourth carbon double bonded to O, and the fifth carbon double bonded to O to the left and bonded to O minus to the right.
There are two different forms of isocitrate dehydrogenase in all cells, one requiring as electron acceptor and the other requiring . The overall reactions are otherwise identical. In eukaryotic cells, the NAD-dependent enzyme occurs in the mitochondrial matrix and serves in the citric acid cycle. The main function of the NADP-dependent enzyme, found in both the mitochondrial matrix and the cytosol, is the generation of NADPH, which is essential for reductive anabolic pathways such as fatty acid and sterol synthesis.
Oxidation of α-Ketoglutarate to Succinyl-CoA and
The next step is another oxidative decarboxylation, in which α-ketoglutarate is converted to succinyl-CoA and by the action of the α-ketoglutarate dehydrogenase complex; serves as electron acceptor and CoA as the carrier of the succinyl group. The energy of oxidation of α-ketoglutarate is conserved in the formation of the thioester bond of succinyl-CoA:
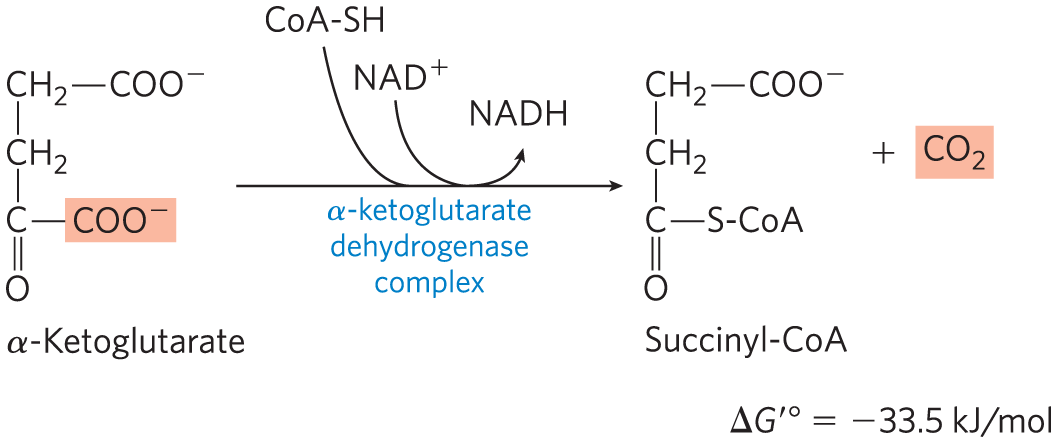
Greek letter alpha-ketoglutarate is shown as a vertical three-carbon chain with the top carbon bonded to 2 H and to C O O minus, the middle carbon bonded to 2 H, and the third carbon bonded to C O O minus in a light red box and double bonded to O. An arrow points right above Greek letter alpha-ketoglutarate dehydrogenase complex accompanied by an line showing the addition of Co A – S H and a curved arrow showing the addition of N A D plus and loss of N A D H. This yields succinyl Co A, which is similar except that the third carbon is bonded to S on the right further bonded to C o A and double bonded to O below. A red box labeled C O 2 is also present.
This reaction is virtually identical to the pyruvate dehydrogenase reaction discussed above and to the reaction sequence responsible for the breakdown of branched-chain amino acids (Fig. 16-12). The α-ketoglutarate dehydrogenase complex closely resembles the PDH complex and the complex that degrades branched-chain α-keto acids in both structure and function. All three have homologous and components, identical components, enzyme-bound TPP and lipoate, coenzyme A, FAD, and NAD. These related enzymes can employ the same subunit because the substrate for — a reduced lipoate — is the same for both complexes. They are certainly derived from a common evolutionary ancestor by gene duplication and subsequent divergent evolution, as described in Figure 1-32.

FIGURE 16-12 A conserved mechanism for oxidative decarboxylation. The pathways shown employ the same five cofactors (thiamine pyrophosphate, coenzyme A, lipoate, FAD, and ), closely similar multienzyme complexes, and the same enzymatic mechanism to carry out oxidative decarboxylations of pyruvate (by the pyruvate dehydrogenase complex), α-ketoglutarate (in the citric acid cycle), and the carbon skeletons of the three branched-chain amino acids, isoleucine (shown here), leucine, and valine.
Three examples are shown. Pyruvate dehydrogenase complex: Pyruvate is converted to acetyl-Co A. Pyruvate is shown in a red box as C H 3 bonded to C double bonded to O above and bonded to C O O minus to the right. An arrow points down accompanied by a branched arrow showing the loss of C O 2 next to a curved line showing the addition of H S – Co A and a curved arrow showing the addition of N A D plus and loss of an orange oval labeled N A D H. This yields acetyl Co A, shown as C H 3 bonded to C double bonded to O to the upper right and bonded to S to the lower right further bonded to Co A. Citric acid cycle: Greek letter alpha-ketoglutarate is converted to succinyl Co A. Greek letter alpha-ketoglutarate is shown as C O O minus bonded to C H 2 bonded to C H 2 in a red box that is bonded to C double bonded to O above and to C O O minus to the right within the red box. An arrow points down accompanied by a branched arrow showing the loss of C O 2 next to a curved line showing the addition of H S – Co A and a curved arrow showing the addition of N A D plus and loss of an orange oval labeled N A D H. This yields succinyl Co A, shown as C O O minus bonded to C H 2 bonded to C H 2 bonded to C double bonded to O to the upper right and bonded to S to the lower right further bonded to C o A. Oxidation of isoleucine (leucine, valine): A Greek letter alpha-keto acid from isoleucine is converted to Greek letter alpha-methylbutyryl-Co A. Greek letter alpha-keto acid from isoleucine is shown as C H 3 bonded to C H 2 bonded to C H 2 bonded to C H in a red box bonded to C H 3 above outside of the box and to C double bonded to O above and to C O O minus to the right within the light red box. An arrow points down accompanied by a branched arrow showing the loss of C O 2 next to a curved line showing the addition of H S – Co A and a curved arrow showing the addition of N A D plus and loss of an orange oval labeled N A D H. This yields Greek letter alpha-methylbutyryl-Co A, shown as C H 3 C H 2 C H bonded to C H 3 above and bonded to C on the right further double bonded to O to the upper right and to S to the lower right further bonded to Co A.
Conversion of Succinyl-CoA to Succinate
Succinyl-CoA, like acetyl-CoA, has a thioester bond with a strongly negative standard free energy of hydrolysis . In the next step of the citric acid cycle, energy released in the breakage of this bond is used to drive the synthesis of a phosphoanhydride bond in either GTP or ATP, with a net of only –2.9 kJ/mol. Succinate is formed in the process:
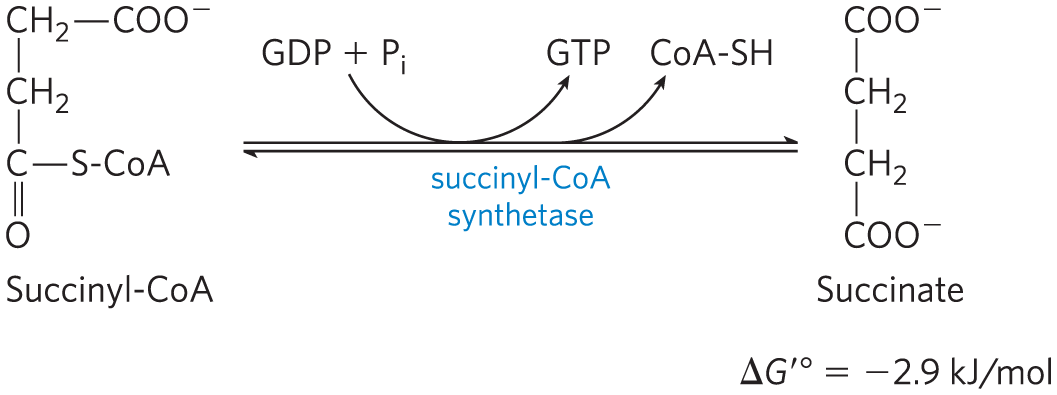
Succinyl-Co A is shown as a vertical three-carbon chain with the top C bonded to 2 H and to C O O minus to the right, the middle C bonded to 2 H, and the bottom C double bonded to O below and bonded to S to the right further bonded to Co A. Right- and left-pointing arrows are shown above succinyl-Co A synthetase. The forward arrow is accompanied by a curved arrow above that shows the addition of G T P plus P subscript i and loss of G T P. An addition arrow branches away to show the loss of Co A – S H. This yields succinate, which is shown as a four-carbon vertical chain with the top carbon forming C O O minus, the second carbon bonded to 2 H, the third carbon bonded to 2 H, and the fourth carbon bonded to C O O minus.
The enzyme that catalyzes this reversible reaction is called succinyl-CoA synthetase or succinic thiokinase; both names indicate the participation of a nucleoside triphosphate in the reaction.
This energy-conserving reaction involves an intermediate step in which the enzyme molecule itself becomes phosphorylated at a His residue in the active site (Fig. 16-13a). This phosphoryl group, which has a high group transfer potential, is transferred to ADP (or GDP) to form ATP (or GTP). Animal cells have two isozymes of succinyl-CoA synthetase, one specific for ADP and the other for GDP. The enzyme has two subunits, , which has the –His residue and the binding site for CoA, and , which confers specificity for either ADP or GDP. The active site is at the interface between subunits. The crystal structure of succinyl-CoA synthetase reveals two “power helices” (one from each subunit), oriented so that their electric dipoles situate partial positive charges close to the negatively charged –His (Fig. 16-13b), stabilizing the phosphoenzyme intermediate. (Recall the similar role of helix dipoles in stabilizing ions in the channel; see Fig. 11-45.)

FIGURE 16-13 The succinyl-CoA synthetase reaction. (a) In step a phosphoryl group replaces the CoA of succinyl-CoA bound to the enzyme, forming a high-energy acyl phosphate. In step the succinyl phosphate donates its phosphoryl group to a His residue of the enzyme, forming a high-energy phosphohistidyl enzyme. In step the phosphoryl group is transferred from the His residue to the terminal phosphate of GDP (or ADP), forming GTP (or ATP). (b) Active site of succinyl-CoA synthetase of Escherichia coli. The active site includes part of both the α (blue) and the β (brown) subunits. The power helices (blue, brown) place the partial positive charges of the helix dipole near the phosphate group of in the α chain, stabilizing the phosphohistidyl enzyme. The bacterial and mammalian enzymes have similar amino acid sequences and three-dimensional structures. [Data from PDB ID 1SCU, W. T. Wolodko et al., J. Biol. Chem. 269:10,883, 1994.]
Part a shows a clockwise cycle of reactions. Step 1 is shown at the three o’clock position. Succinyl Co A is shown as a four-carbon chain with the top carbon double bonded to O to the upper left and bonded to O minus to the upper right, the second and third carbons bonded to 2 H, and the bottom carbon double bonded to O to the lower left and bonded to S to the lower left further bonded to S to the lower right further bonded to C o A. Succinyl Co A is added at the two o’clock position, then a new arrow starts from the three o’clock to five o’clock positions accompanied by a curved arrow labeled 1 showing the addition of red highlighted P subscript I and the loss of Co A – S H. This yields enzyme-bound succinyl phosphate, which is similar to succinyl Co A except that the third carbon is enclosed in a blue oval with H i s at the far right with a bond to the right, and the bottom carbon has a bond to O at the lower right that is further bonded to a red circle labeled P. An arrow points from enzyme-bound succinyl phosphate to phosphohistidyl enzyme at the ten o’clock position. At the seven o’clock position, an arrow labeled 2 curves away and points to succinate, shown as C double bonded to O to the upper left, bonded to O minus to the lower left, and bonded to C H 2 to the right further bonded to C H 2 bonded to C double bonded to O to the upper right and bonded to O minus to the lower right. All data are approximate. The arrow within the circle continues up to a structure labeled phosphohidtidyl enzyme at the ten o’clock position. This is a blue oval with H i s at the right side with a bond to the right and a bond to the lower right to a red circle labeled P outside of the blue oval. An arrow points from this point to a similar blue oval labeled succinyl-Co A synthetase at the twelve o’clock position with H i s at the right side with a bond to the right but with no bond to a red circle labeled P. A curved arrow labeled 3 at the eleven o’clock position shows the addition of G D P and loss of G T P with P highlighted in red. An arrow points from succinyl-Co A synthetase to the three o’clock position to continue the cycle. Part b shows a brown enzyme labeled Greek letter beta subunit in the background with helices and strands. It has a vertical purple piece labeled coenzyme A containing a ball-and-stick model that bends toward the left near its top. At the bottom of coenzyme A, several structures come together and the brown Greek letter beta subunit above meets the blue Greek letter alpha subunit below. A ball-and-stick model of phosphate is visible between the structures with a red structure behind. There are circles containing minus signs next to the two lower O atoms of the phosphate. To the left, a yellow structure contains a ball-and-stick model and is labeled H i s superscript 246 bonded to a red circle labeled P. A purple helix labeled Greek letter alpha subunit power helix has a circle with a minus symbol near its bottom and extends up from just to the right of the center bottom to the phosphate group. It has an arrow running through its center that ends with a blue point containing a circle containing a plus sign near one of the negatively charged O atoms. A brown helix labeled Greek letter beta subunit power helix extends from the upper center right to the phosphate and has a circle with a minus symbol near its left end. It also contains an arrow with a blue point containing a circle with a plus sign next to a negatively charged O atom.
The formation of ATP (or GTP) at the expense of the energy released by the oxidative decarboxylation of α-ketoglutarate is a substrate-level phosphorylation, like the synthesis of ATP in the glycolytic reactions catalyzed by phosphoglycerate kinase and pyruvate kinase (see Fig. 14-2). The GTP formed by succinyl-CoA synthetase can donate its terminal phosphoryl group to ADP to form ATP, in a reversible reaction catalyzed by nucleoside diphosphate kinase (p. 487):
Thus the net result of the activity of either isozyme of succinyl-CoA synthetase is the conservation of energy as ATP. There is no change in free energy for the nucleoside diphosphate kinase reaction; ATP and GTP are energetically equivalent.
Oxidation of Succinate to Fumarate
The succinate formed from succinyl-CoA is oxidized to fumarate by the flavoprotein succinate dehydrogenase:
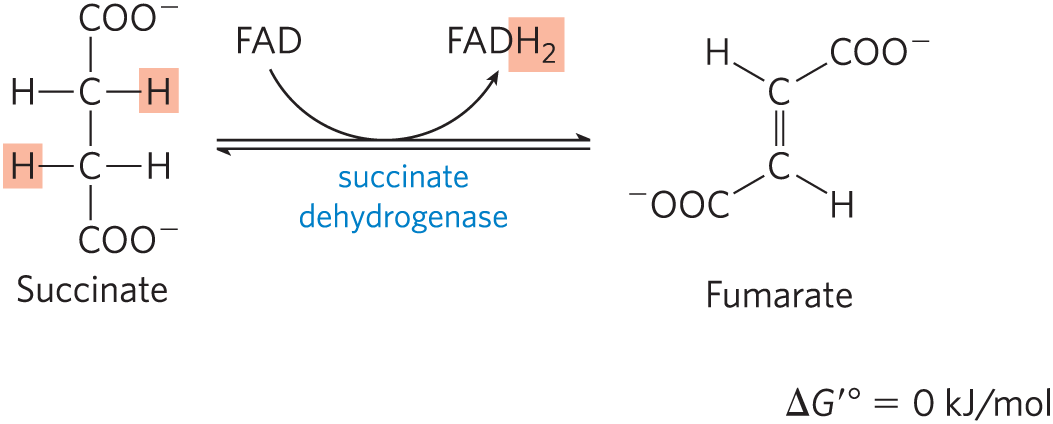
Succinate is shown as a four-carbon chain with the top carbon forming C O O minus, the second carbon bonded to H on each side with the right-hand H in a red box, the third carbon bonded to H on each side with the left-hand H in a red box, and the fourth carbon forming C O O minus. Right- and left-pointing arrows are shown above succinate dehydrogenase accompanied by a curved arrow above the right-pointing arrow that shows the addition of F A D and loss of F A D H 2 with a red box around H 2. This yields fumarate, which is shown as C bonded to H to the upper left, bonded to C O O minus to the upper right, and double bonded to C below further bonded to C O O minus to the lower left and bonded to H to the lower right.
In eukaryotes, succinate dehydrogenase is an integral protein of the mitochondrial inner membrane; in bacteria, of the plasma membrane. The enzyme contains three different iron-sulfur clusters and one molecule of covalently bound FAD (see Fig. 19-9). Electrons pass from succinate through the FAD and iron-sulfur centers before entering the chain of electron carriers in the mitochondrial inner membrane (the plasma membrane in bacteria). Electron flow from succinate through these carriers to the final electron acceptor, , is coupled to the synthesis of about 1.5 ATP molecules per pair of electrons (respiration-linked phosphorylation). Malonate, an analog of succinate not normally present in cells, is a strong competitive inhibitor of succinate dehydrogenase, and its addition to mitochondria in the laboratory blocks the activity of the citric acid cycle.
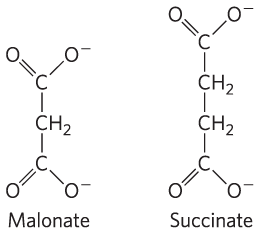
Malonate has C double bonded to O to the upper left bonded to O to the upper right, and bonded to C H 2 below further bonded to O double bonded to O to the lower left and bonded to O to the lower right. Succinate has C double bonded to O to the upper left bonded to O to the upper right, and bonded to C H 2 below bonded to C H 2 below further bonded to O double bonded to O to the lower left and bonded to O to the lower right.
Hydration of Fumarate to Malate
The reversible hydration of fumarate to l-malate is catalyzed by fumarase (formally, fumarate hydratase). The transition state in this reaction is a carbanion:
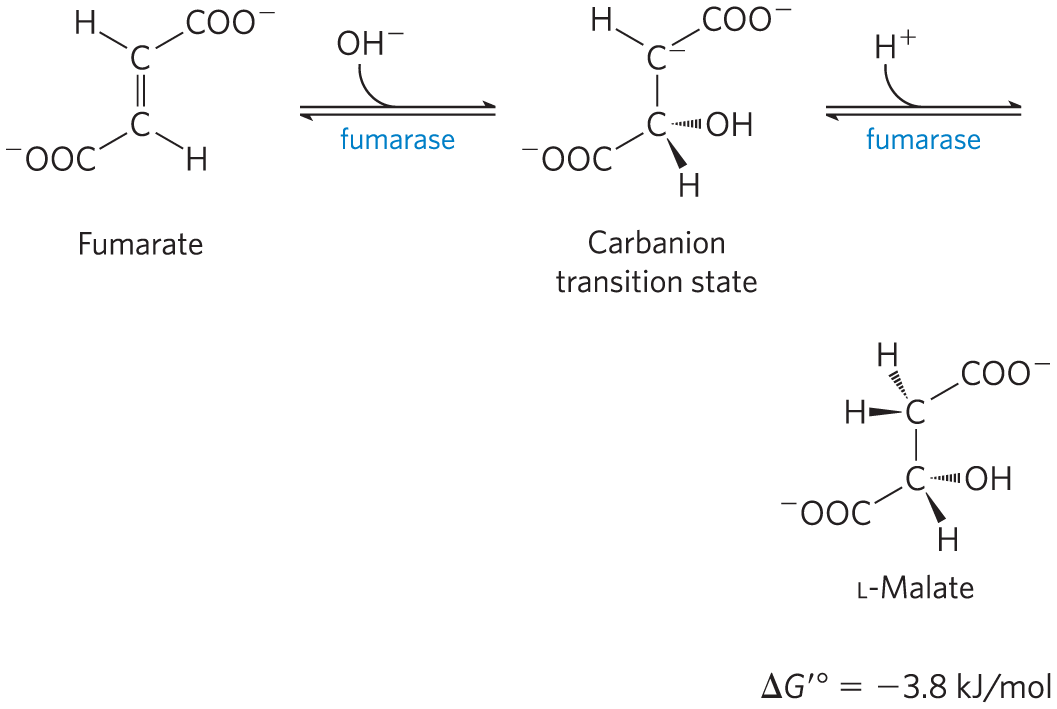
Fumarate is shown as C bonded to H to the upper left, bonded to C O O minus to the upper right, and double bonded to C below bonded to C O O minus to the lower left and to H to the lower right. Right- and left-pointing arrows are shown above the word fumarase. A line shows the addition of O H minus to the right-pointing arrow. This yields a carbanion transition state, shown as C with a negative charge bonded to H to the upper left, bonded to C O O minus to the upper right, and bonded to C below that is bonded to C O O minus to the lower left, solid wedge bonded to the lower right, and hashed wedge bonded to O H and slightly down. Upward- and downward- pointing arrows are shown below accompanied by the word fumarase. A curved line shows the addition of H plus to the downward arrow. This yields L-malate, shown as C with a solid wedge bond to H to the left, a hashed wedge bond to H to the upper left, a bond to C O O minus to the upper right, and a bond to C below bonded to C O O minus to the lower left, solid wedge bonded to H to the lower right, and hashed bonded to O H to the right and slightly down.
This enzyme is highly stereospecific; it catalyzes hydration of the trans double bond of fumarate but not the cis double bond of maleate. In the reverse direction (from l-malate to fumarate), fumarase is equally stereospecific: d-malate is not a substrate.

Fumarate has C bonded to H to the upper left, bonded to C O O minus to the upper right, and double bonded to C below bonded to C O O minus to the lower left and to H to the lower right. Maleate has C bonded to H to the upper left, bonded to C O O minus to the upper right, and double bonded to C below bonded to H to the lower left and to C O O minus to the lower right. L-malate has a vertical four-carbon chain with the top carbon forming C O O minus, the second carbon bonded to O H to the left and H to the right, the third carbon bonded to 2 H, and the fourth carbon forming C O O minus. D-malate is similar to L-malate, except that the second carbon is bonded to H on the left and O H on the right.
Oxidation of Malate to Oxaloacetate
In the last reaction of the citric acid cycle, l-malate dehydrogenase catalyzes the oxidation of l-malate to oxaloacetate, coupled to the reduction of to NADH:
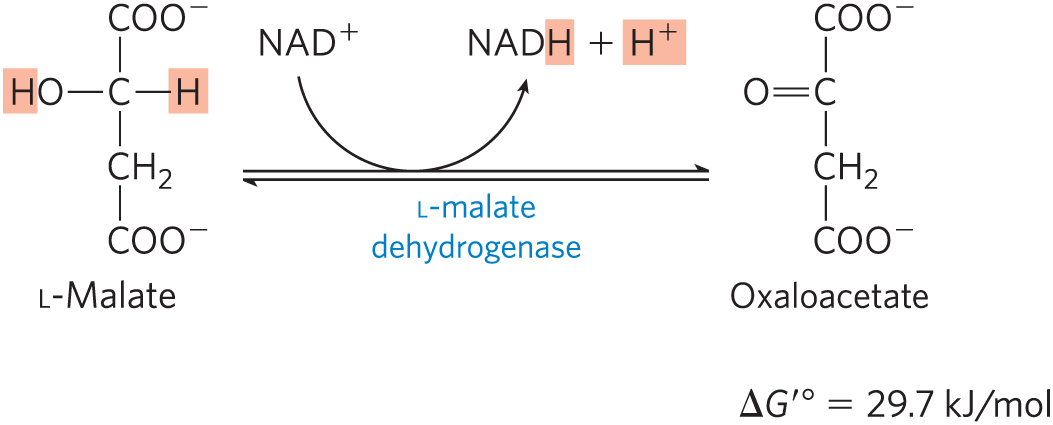
L-malate is shown as a vertical four-carbon chain with the top carbon forming C O O minus, the second carbon bonded to O H with H in a red box on the left and to H in a red box on the right, the third carbon bonded to 2 H, and the fourth carbon forming C O O minus. Right- and left-pointing arrows are shown above L-malate dehydrogenase accompanied by a curved arrow above the right-pointing arrow that shows the addition of N A D plus and loss of N A D H with H in a red box plus H plus in a red box. This yields oxaloacetate, which has a similar structure except that the second carbon is double bonded to O to the left.
The equilibrium of this reaction lies far to the left under standard thermodynamic conditions, but in intact cells oxaloacetate is continually removed by the highly exergonic citrate synthase reaction (step of Fig. 16-7). This keeps the concentration of oxaloacetate in the cell extremely low , pulling the malate dehydrogenase reaction toward the formation of oxaloacetate.
Although the individual reactions of the citric acid cycle were initially worked out in vitro, using minced muscle tissue, the pathway and its regulation have also been studied extensively in vivo. By using precursors isotopically labeled with , researchers have traced the fate of individual carbon atoms through the citric acid cycle. Some of the earliest experiments with produced an unexpected result, however, which aroused considerable controversy about the pathway and mechanism of the citric acid cycle. In fact, these experiments at first seemed to show that citrate was not the first tricarboxylic acid to be formed. Box 16-2 gives some details of this episode in the history of citric acid cycle research. Today biochemists use -labeled precursors and whole-tissue NMR spectroscopy to monitor metabolic flux through the cycle in living tissue. Because the NMR signal is unique to the compound containing the , precursor carbons can be traced into each cycle intermediate and into compounds derived from the intermediates. This technique makes it possible to study regulation of the citric acid cycle and its interconnections with other metabolic pathways.
Box 16-2
Citrate: A Symmetric Molecule That Reacts Asymmetrically
When compounds enriched in the heavy-carbon isotope and the radioactive carbon isotopes and became available in the mid-1940s, they were soon put to use in tracing the pathway of carbon atoms through the citric acid cycle. One such experiment initiated the controversy over the role of citrate. Acetate labeled in the carboxyl group (designated acetate) was incubated aerobically with an animal tissue preparation. Acetate is enzymatically converted to acetyl-CoA in animal tissues, and the pathway of the labeled carboxyl carbon of the acetyl group in the cycle reactions could thus be traced. α-Ketoglutarate was isolated from the tissue after incubation, then degraded by known chemical reactions to establish the position(s) of the isotopic carbon.
Condensation of unlabeled oxaloacetate with carboxyl-labeled acetate would be expected to produce citrate labeled in one of the two primary carboxyl groups. Citrate is a symmetric molecule, its two terminal carboxyl groups being chemically indistinguishable. Therefore, half the labeled citrate molecules were expected to yield α-ketoglutarate labeled in the α-carboxyl group and the other half to yield α-ketoglutarate labeled in the γ-carboxyl group; that is, the α-ketoglutarate isolated was expected to be a mixture of the two types of labeled molecules (Fig. 1, pathways and ). Contrary to this expectation, the labeled α-ketoglutarate isolated from the tissue suspension contained only in the γ-carboxyl group (Fig. 1, pathway ). The investigators concluded that citrate (or any other symmetric molecule) could not be an intermediate in the pathway from acetate to α-ketoglutarate. Rather, an asymmetric tricarboxylic acid, presumably cis-aconitate or isocitrate, must be the first product formed from condensation of acetate and oxaloacetate.
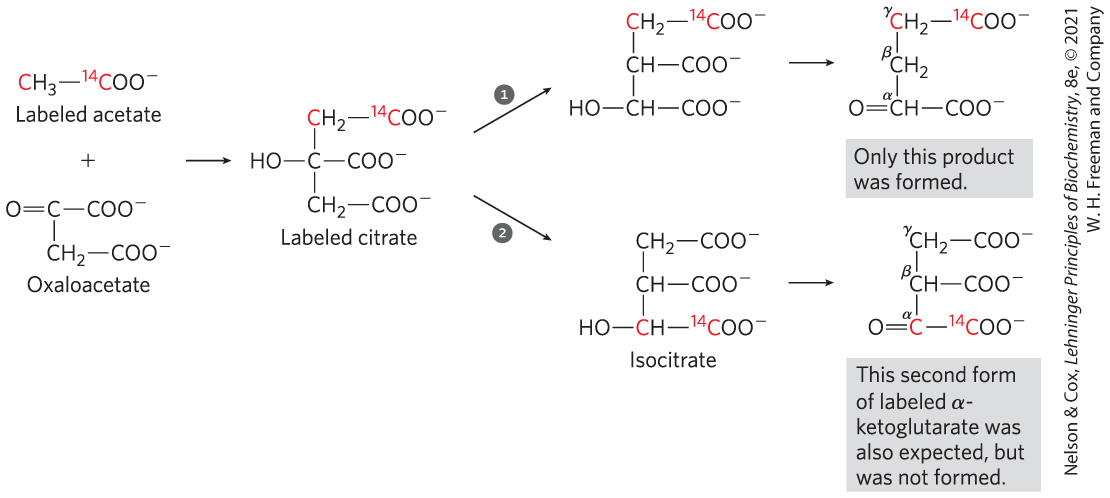
FIGURE 1 Incorporation of the isotopic carbon of the labeled acetyl group into α-ketoglutarate by the citric acid cycle. The carbon atoms of the entering acetyl group are shown in red.
Labeled acetate is shown as red highlighted C bonded to nonhighlighted 3 H bonded to red highlighted superscript 14 end superscript C nonhighlighted O O minus. Oxaloacetate is shown as C double bonded to O to the left, bonded to C O O minus to the right, and bonded to C H 2 below further bonded to C O O minus. Labeled acetate and oxaloacetate are added and a right-pointing arrow shows that this yields labeled citrate, shown as a vertical three-carbon chain with the top carbon highlighted in red, bonded to 2 H, and further bonded to red highlighted superscript 14 end superscript C nonhighlighted O O minus, the middle carbon bonded to O H to the left and C O O minus to the right, and the bottom carbon bonded to 2 H and to C O O minus. An arrow pointing up and to the right is numbered 1, and an arrow pointing down and to the right is numbered 2. Both reactions yield isocitrate, but the labeled carbon atoms are in different positions. Arrow 1 points to a product shown as a vertical three-carbon chain with the top carbon highlighted in red, bonded to 2 H, and further bonded to red highlighted superscript 14 end superscript C nonhighlighted O O minus, the middle carbon bonded to H and to C O O minus to the right, and the bottom carbon bonded to O H to the left and to C O O minus to the right. A right-pointing arrow shows that this yields a vertical three-carbon chain shown with the top superscript Greek letter gamma end superscript red highlighted carbon bonded to 2 H bonded to red highlighted superscript 14 end superscript C nonhighlighted O O minus, the middle superscript Greek letter beta end superscript carbon bonded to 2 H, and the bottom superscript Greek letter alpha end superscript C bonded to H, double bonded to O to the left, and bonded to C O O minus to the right. Accompanying text reads, Only this product was formed. Arrow 2 points to a product shown as a vertical three-carbon chain with the top carbon bonded to 2 H and to C O O minus, the middle carbon bonded to H and to C O O minus to the right, and the bottom carbon highlighted in red, bonded to O H to the left, and bonded to red highlighted superscript 14 end superscript C nonhighlighted O O minus on the right. A right-pointing arrow shows that this yields a vertical three-carbon chain shown with the top superscript Greek letter gamma end superscript carbon bonded to 2 H bonded to C O O minus to the right, the middle superscript Greek letter beta end superscript carbon bonded to H and to C O O minus to the right, and the bottom superscript Greek letter alpha end superscript red highlighted C double bonded to O to the left and bonded to red highlighted superscript 14 end superscript C nonhighlighted O O minus to the right. Accompanying text reads, This second form of labeled Greek letter alpha-ketoglutarate was also expected, but was not formed.
In 1948, however, Alexander Ogston pointed out that although citrate has no chiral center (see Fig. 1-19), it has the potential to react asymmetrically if an enzyme with which it interacts has an active site that is asymmetric. He suggested that the active site of aconitase may have three points to which the citrate must be bound and that the citrate must undergo a specific three-point attachment to these binding points. As seen in Figure 2, the binding of citrate to three such points could happen in only one way, and this would account for the formation of only one type of labeled α-ketoglutarate. Organic molecules such as citrate that have no chiral center but are potentially capable of reacting asymmetrically with an asymmetric active site are now called prochiral molecules.
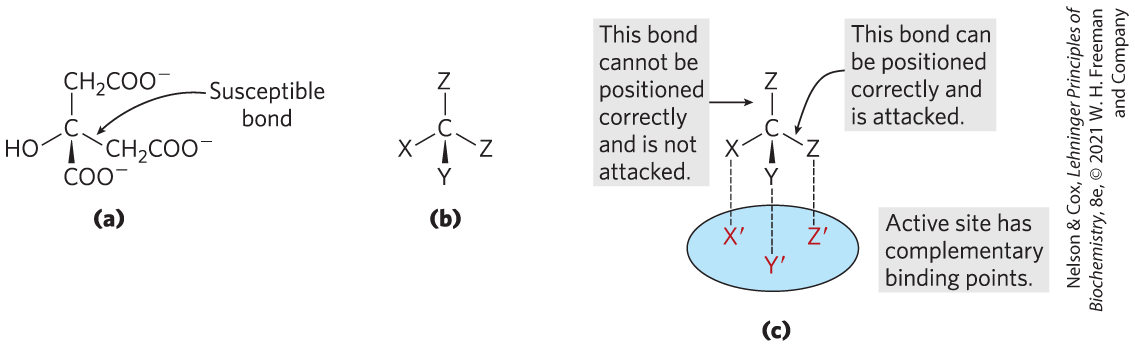
FIGURE 2 The prochiral nature of citrate. (a) Structure of citrate; (b) schematic representation of citrate: ; ; . (c) Correct complementary fit of citrate to the binding site of aconitase. There is only one way in which the three specified groups of citrate can fit on the three points of the binding site. Thus only one of the two groups is bound by aconitase.
Part a shows citrate with a central C bonded to C H 2 C O O minus above, bonded to C H 2 C O O minus to the lower right, solid wedge bonded to C O O minus below to the front, and bonded to O H to the lower left. The bond between C and C H 2 C O O minus to the lower right is labeled susceptible bond. Part b shows C bonded to Z above, bonded to Z to the lower right, solid wedge bonded to Y below to the front, and bonded to X to the lower left. Part c shows the schematic illustration in part b above a blue oval with three labeled positioned. The bond between C and Z to the lower right is accompanied by text reading, This bond can be positioned correctly and attached. A dashed vertical line extends down from this Z to red highlighted Z prime at the rear right of the blue oval. The solid wedge bond between C and Y below to the front has a dashed vertical line extending down to red highlighted Y prime at the lower front of the blue oval. The bond between C and X to the lower left has a dashed vertical line extending down to red highlighted X prime at the rear left of the blue oval. The bond between C and Z above is accompanied by text reading, This bond cannot be positioned correctly and is not attached.
The Energy of Oxidations in the Cycle Is Efficiently Conserved
We have now covered one complete turn of the citric acid cycle (Fig. 16-14). A two-carbon acetyl group entered the cycle by combining with oxaloacetate. Two carbon atoms emerged from the cycle as from the oxidation of isocitrate and α-ketoglutarate. The energy released by these oxidations was conserved in the reduction of three and one FAD and the production of one ATP or GTP. At the end of the cycle a molecule of oxaloacetate was regenerated. Note that the two carbon atoms appearing as are not the same two carbons that entered in the form of the acetyl group; additional turns around the cycle are required to release these carbons as (Fig. 16-7).
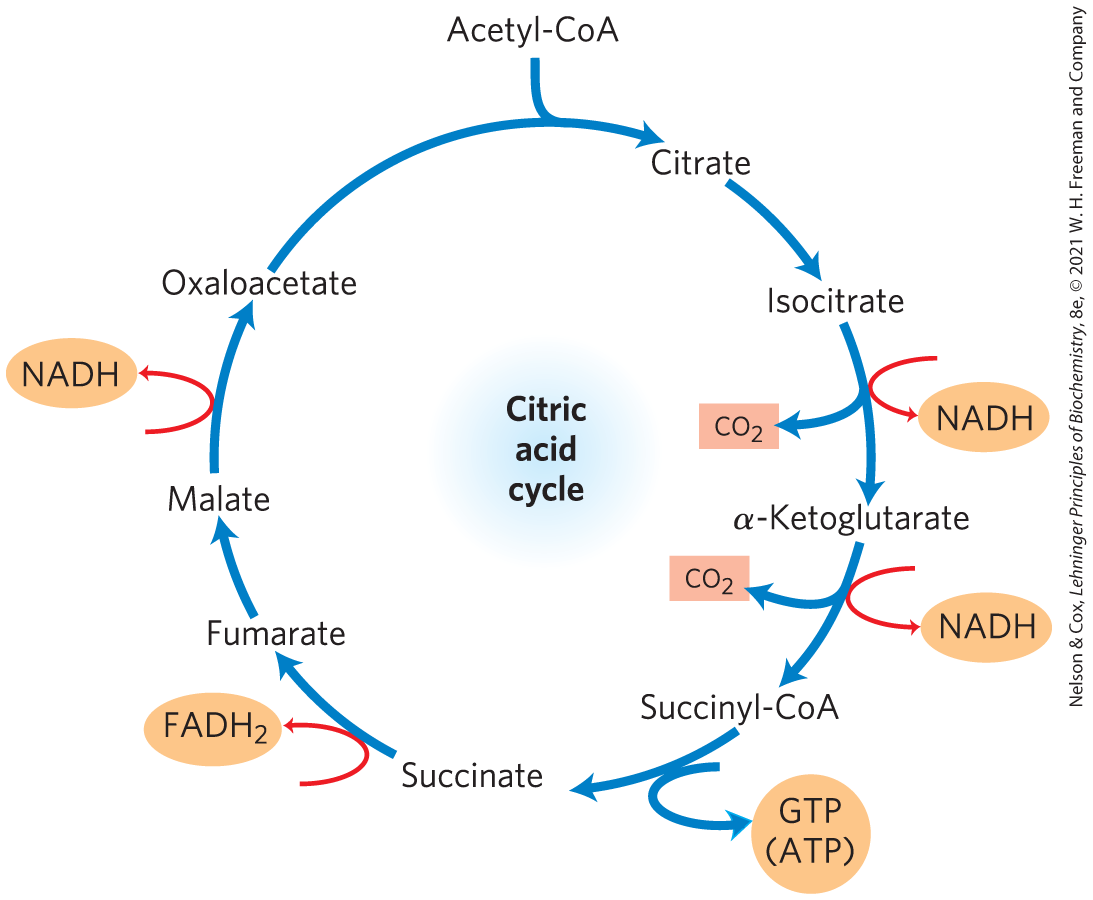
FIGURE 16-14 Products of one turn of the citric acid cycle. At each turn of the cycle, three NADH, one , one GTP (or ATP), and two are released in oxidative decarboxylation reactions. Here and in several of the following figures, all cycle reactions are shown as proceeding in one direction only, but keep in mind that most of the reactions are reversible.
The cycle consists of clockwise blue arrows. The first blue arrow begins at oxaloacetate at the eleven o’clock position and points to citrate at the one o’clock position. A curved blue line shows the addition of acetyl Co A to this line at the twelve o’clock position. A short blue arrow points from citrate to isocitrate at the two o’clock position. A blue arrow points from isocitrate to Greek letter alpha-ketoglutarate at the three o’clock position. This arrow has a blue arrow that branches off to show the loss of a red box labeled C O 2 and is accompanied by a curved red arrow showing that something is added and an orange oval of N A D H is produced. A blue arrow points from Greek letter alpha-ketoglutarate to succinyl-Co A at the five o’clock position. This arrow has a blue arrow that branches off to show the loss of a red box labeled C O 2 and is accompanied by a curved red arrow showing that something is added and an orange oval of N A D H is produced. An arrow points from succinyl-Co A to succinate at just past the six o’clock position. This arrow is accompanied by a curved blue arrow showing the production of an orange oval labeled G T P (A T P). A blue arrow points from succinate to fumarate at the seven o’clock position and is accompanied by a red arrow showing that something is added and that an orange oval labeled F A D H 2 is produced. A short blue arrow points from fumarate to malate at the nine o’clock position. An arrow points from malae to oxaloacetate at the eleven o’clock position and is accompanied by a red arrow showing that something is added and that an orange oval labeled F A D H 2 is produced. All data are approximate.
Although the citric acid cycle directly generates only one ATP per turn (in the conversion of succinyl-CoA to succinate), the four oxidation steps in the cycle provide a large flow of electrons into the respiratory chain via NADH and and thus lead to formation of almost 10 times more ATP during oxidative phosphorylation.
We saw in Chapter 14 that the production of two molecules of pyruvate from one molecule of glucose in glycolysis yields 2 ATP and 2 NADH. In oxidative phosphorylation (Chapter 19), passage of two electrons from NADH to drives the formation of about 2.5 ATP, and passage of two electrons from to yields about 1.5 ATP. This stoichiometry allows us to calculate the overall yield of ATP from the complete oxidation of glucose. When both pyruvate molecules are oxidized to 6 via the pyruvate dehydrogenase complex and the citric acid cycle, and the electrons are transferred to via oxidative phosphorylation, as many as 32 ATP are obtained per glucose (Table 16-1). In round numbers, this represents the conservation of , or 34% of the theoretical maximum of about 2,840 kJ/mol available from the complete oxidation of glucose. These calculations employ the standard free-energy changes; when corrected for the actual free energy required to form ATP within cells (see Worked Example 13-2, p. 480), the calculated efficiency of the process is closer to 65%. When cells under anaerobic conditions depend on glycolysis for ATP, one glucose yields just 2 ATP. Aerobic metabolism is far more effective in capturing the energy in glucose as a fuel.
| Reaction | Number of ATP or reduced coenzyme directly formed | Number of ATP ultimately formeda |
|---|---|---|
2 NADH |
3 or 5b |
|
2 ATP |
2 |
|
2 ATP |
2 |
|
2 NADH |
5 |
|
2 NADH |
5 |
|
2 NADH |
5 |
|
2 ATP (or 2 GTP) |
2 |
|
3 |
||
2 NADH |
5 |
|
Total |
30–32 |
|
|
aThis is calculated as 2.5 ATP per NADH and 1.5 ATP per . A negative value indicates consumption. bThis number is either 3 or 5, depending on the mechanism used to shuttle NADH equivalents from the cytosol to the mitochondrial matrix; see Figures 19-31 and 19-32. |
||
SUMMARY 16.2 Reactions of the Citric Acid Cycle
- The citric acid cycle (Krebs cycle, TCA cycle) is a nearly universal central catabolic pathway in which compounds derived from the breakdown of carbohydrates, fats, and proteins are oxidized to , with most of the energy of oxidation temporarily held in the electron carriers and NADH. During aerobic metabolism, these electrons are transferred to and the energy of electron flow is conserved as ATP.
- Acetyl-CoA enters the citric acid cycle as citrate synthase catalyzes its condensation with oxaloacetate to form citrate. In seven sequential reactions, including two decarboxylations, the citric acid cycle converts citrate to oxaloacetate and releases two . The pathway is cyclic in that the intermediates of the cycle are not used up; for each oxaloacetate consumed in the path, one is produced.
- For each acetyl-CoA oxidized by the citric acid cycle, the energy gain consists of three molecules of NADH, one , and one nucleoside triphosphate (either ATP or GTP).
 one molecule of oxaloacetate can theoretically bring about oxidation of an infinite number of acetyl groups, effectively acting catalytically; the steady-state concentration of oxaloacetate is very low (micromolar). Four of the eight steps in this process are oxidations, in which the energy of oxidation is very efficiently conserved in the form of the reduced coenzymes NADH and . These two carriers donate their electrons to the respiratory chain where electron flow drives ATP synthesis.
one molecule of oxaloacetate can theoretically bring about oxidation of an infinite number of acetyl groups, effectively acting catalytically; the steady-state concentration of oxaloacetate is very low (micromolar). Four of the eight steps in this process are oxidations, in which the energy of oxidation is very efficiently conserved in the form of the reduced coenzymes NADH and . These two carriers donate their electrons to the respiratory chain where electron flow drives ATP synthesis. ,
,  , and
, and  are essentially irreversible in the cell; all other steps are reversible. The nucleoside triphosphate product of step
are essentially irreversible in the cell; all other steps are reversible. The nucleoside triphosphate product of step 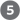 may be either ATP or GTP, depending on which succinyl-CoA synthetase isozyme is the catalyst.
may be either ATP or GTP, depending on which succinyl-CoA synthetase isozyme is the catalyst. to
to  in
in  Formation of Isocitrate via cis-Aconitate
Formation of Isocitrate via cis-Aconitate –His residue and the binding site for CoA, and , which confers specificity for either ADP or GDP. The active site is at the interface between subunits. The crystal structure of succinyl-CoA synthetase reveals two “power helices” (one from each subunit), oriented so that their electric dipoles situate partial positive charges close to the negatively charged
–His residue and the binding site for CoA, and , which confers specificity for either ADP or GDP. The active site is at the interface between subunits. The crystal structure of succinyl-CoA synthetase reveals two “power helices” (one from each subunit), oriented so that their electric dipoles situate partial positive charges close to the negatively charged  in the α chain, stabilizing the phosphohistidyl enzyme. The bacterial and mammalian enzymes have similar amino acid sequences and three-dimensional structures. [Data from PDB ID 1SCU, W. T. Wolodko et al., J. Biol. Chem. 269:10,883, 1994.]
in the α chain, stabilizing the phosphohistidyl enzyme. The bacterial and mammalian enzymes have similar amino acid sequences and three-dimensional structures. [Data from PDB ID 1SCU, W. T. Wolodko et al., J. Biol. Chem. 269:10,883, 1994.] Hydration of Fumarate to Malate
Hydration of Fumarate to Malate The citric acid cycle (Krebs cycle, TCA cycle) is a nearly universal central catabolic pathway in which compounds derived from the breakdown of carbohydrates, fats, and proteins are oxidized to , with most of the energy of oxidation temporarily held in the electron carriers and NADH. During aerobic metabolism, these electrons are transferred to and the energy of electron flow is conserved as ATP.
The citric acid cycle (Krebs cycle, TCA cycle) is a nearly universal central catabolic pathway in which compounds derived from the breakdown of carbohydrates, fats, and proteins are oxidized to , with most of the energy of oxidation temporarily held in the electron carriers and NADH. During aerobic metabolism, these electrons are transferred to and the energy of electron flow is conserved as ATP.