13.3 Phosphoryl Group Transfers and ATP
Having developed some fundamental principles of energy changes in chemical systems and reviewed the common classes of reactions, we can now examine the energy cycle in cells and the special role of ATP as the energy currency that links catabolism and anabolism (see Fig. 1-28). Heterotrophic cells obtain free energy in a chemical form by the catabolism of nutrient molecules, and they use that energy to make ATP from ADP and . ATP then donates some of its chemical energy to endergonic processes such as the synthesis of metabolic intermediates and macromolecules from smaller precursors, the transport of substances across membranes against concentration gradients, and mechanical motion. This donation of energy from ATP generally involves the covalent participation of ATP in the reaction that is to be driven, with the eventual result that ATP is converted to ADP and or, in some reactions, to AMP and 2 . We discuss here the chemical basis for the large free-energy changes that accompany hydrolysis of ATP and other high-energy phosphate compounds, and we show that most cases of energy donation by ATP involve group transfer, not simple hydrolysis of ATP. To illustrate the range of energy transductions in which ATP provides the energy, we consider the synthesis of information-rich macromolecules, the transport of solutes across membranes, and motion produced by muscle contraction.
The Free-Energy Change for ATP Hydrolysis Is Large and Negative
Figure 13-11 summarizes the chemical basis for the relatively large, negative, standard free energy of hydrolysis of ATP. The hydrolytic cleavage of the terminal phosphoric acid anhydride (phosphoanhydride) bond in ATP separates one of the three negatively charged phosphates and thus relieves some of the internal electrostatic repulsion in ATP; the released is stabilized by the formation of several resonance forms not possible in ATP.
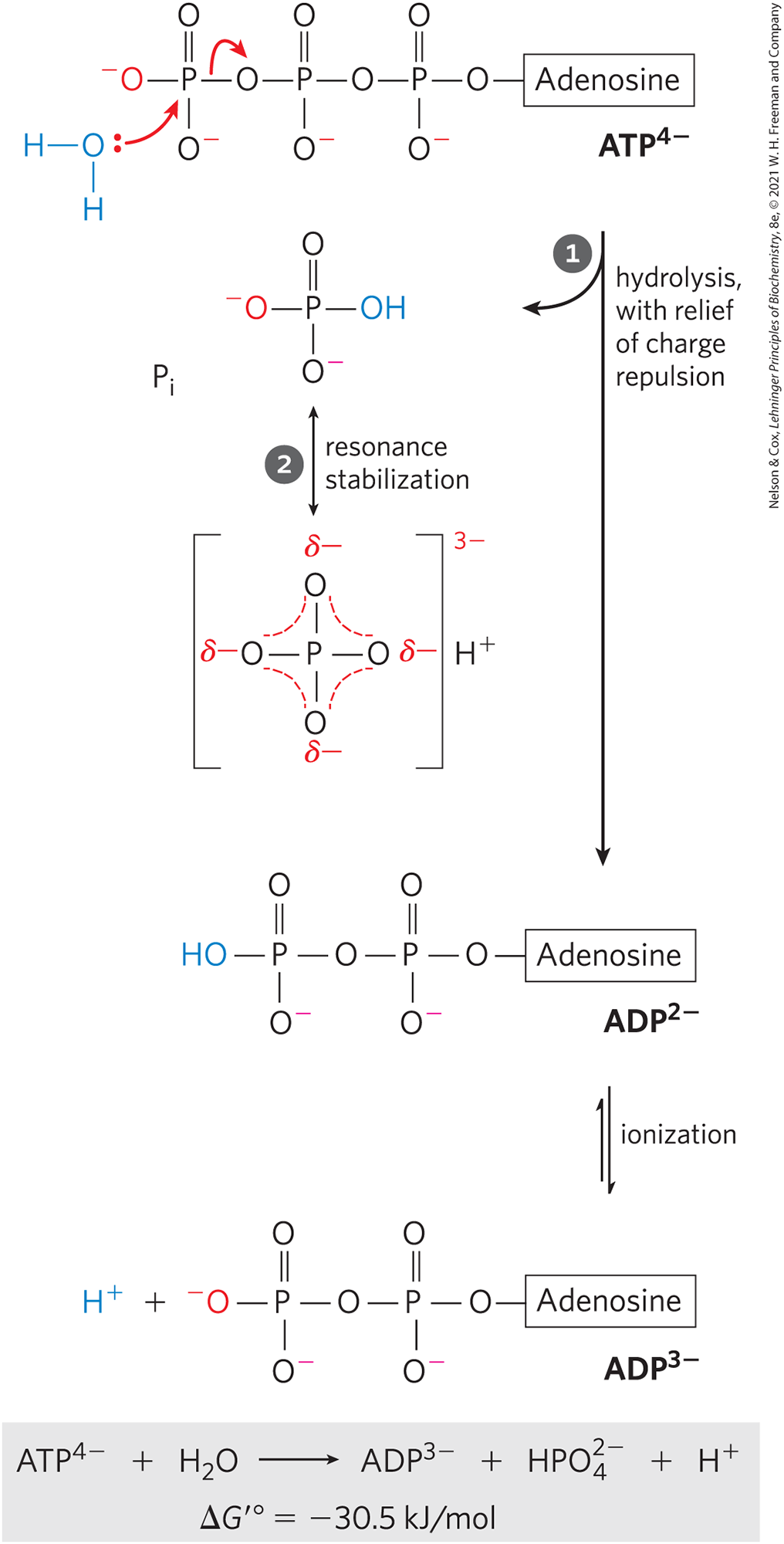
FIGURE 13-11 Chemical basis for the large free-energy change associated with ATP hydrolysis. The charge separation that results from hydrolysis relieves electrostatic repulsion among the four negative charges on ATP. The product inorganic phosphate is stabilized by formation of a resonance hybrid, in which each of the four phosphorus–oxygen bonds has the same degree of double-bond character and the hydrogen ion is not permanently associated with any one of the oxygens. (Some degree of resonance stabilization also occurs in phosphates involved in ester or anhydride linkages, but fewer resonance forms are possible than for .) A third factor (not shown) that favors ATP hydrolysis is the greater degree of solvation (hydration) of the products and ADP relative to ATP, which further stabilizes the products relative to the reactants.
The free-energy change for ATP hydrolysis is under standard conditions, but the actual free energy of hydrolysis (ΔG) of ATP in living cells is very different: the cellular concentrations of ATP, ADP, and are not identical and are much lower than the 1.0 m of standard conditions (Table 13-5). Furthermore, in the cytosol binds to ATP and ADP (Fig. 13-12), and for most enzymatic reactions that involve ATP as phosphoryl group donor, the true substrate is . The relevant is therefore that for hydrolysis. We can calculate ΔG for ATP hydrolysis using data such as those in Table 13-5. The actual free energy of hydrolysis of ATP under intracellular conditions is often called its phosphorylation potential, , for reasons we will explain.
| Concentration (mm)a | |||||
|---|---|---|---|---|---|
| Cell type | ATP | ADPb | AMP | PCr | |
Rat hepatocyte |
3.38 |
1.32 |
0.29 |
4.8 |
0 |
Rat myocyte |
8.05 |
0.93 |
0.04 |
8.05 |
27 |
Rat neuron |
2.59 |
0.73 |
0.06 |
2.72 |
4.7 |
Human erythrocyte |
2.25 |
0.25 |
0.02 |
1.65 |
0 |
E. coli cell |
9.6 |
0.56 |
0.28 |
— |
— |
|
a For erythrocytes, the concentrations are those of the cytosol (human erythrocytes lack a nucleus and mitochondria). In the other types of cells, the data are for the entire cell contents, although the cytosol and the mitochondria have very different concentrations of ADP. PCr is phosphocreatine, discussed on p. 487. b This value reflects total concentration; the true value for free ADP may be much lower (Worked Example 13-2). Mammalian data from R. L. Veech et al., J. Biol. Chem. 254:6538, 1979. E. coli data from B. D. Bennett et al., Nat. Chem. Biol. 5:593, 2009. |
|||||
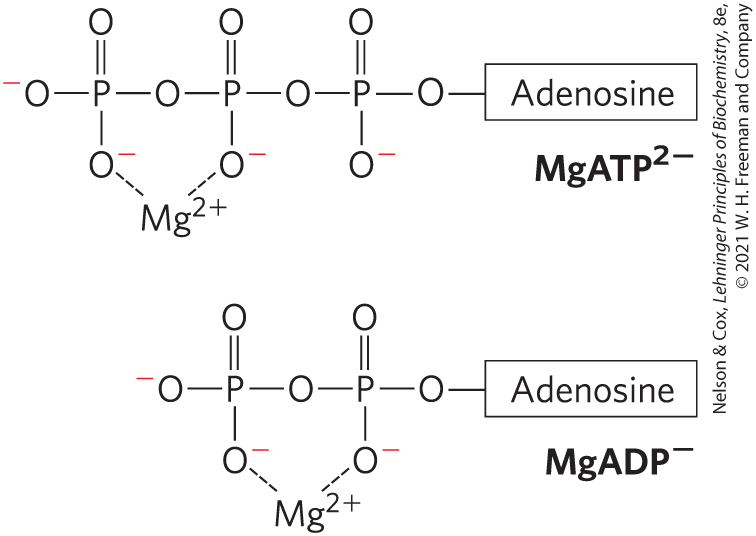
FIGURE 13-12 and ATP. Formation of complexes partially shields the negative charges and influences the conformation of the phosphate groups in nucleotides such as ATP and ADP.
Because the concentrations of ATP, ADP, and differ from one cell type to another, for ATP likewise differs among cells. Moreover, in any given cell, can vary from time to time, depending on the metabolic conditions and how they influence the concentrations of ATP, ADP, , and (pH). We can calculate the actual free-energy change for any given metabolic reaction as it occurs in a cell, provided we know the concentrations of all the reactants and products and other factors (such as pH, temperature, and ) that may affect the actual free-energy change.
WORKED EXAMPLE 13-2 Calculation of
Calculate the actual free energy of hydrolysis of ATP, , in human erythrocytes. The standard free energy of hydrolysis of ATP is , and the concentrations of ATP, ADP, and in erythrocytes are as shown in Table 13-5. Assume that the pH is 7.0 and the temperature is (human body temperature). What does this reveal about the amount of energy required to synthesize ATP under the same cellular conditions?
SOLUTION:
The concentrations of ATP, ADP, and in human erythrocytes are 2.25, 0.25, and 1.65 mm, respectively. The actual free energy of hydrolysis of ATP under these conditions is given by the relationship (see Eqn 13-4)
Substituting the appropriate values, we get
Thus , the actual free-energy change for ATP hydrolysis in the intact erythrocyte , is much larger than the standard free-energy change . Similarly, the free energy required to synthesize ATP from ADP and under the conditions prevailing in the erythrocyte would be 52 kJ/mol.
To further complicate the issue, the total concentrations of ATP, ADP, and (and ) in a cell — such as the values given in Table 13-5 — may be substantially higher than the free concentrations, which are the thermodynamically relevant values. The difference is due to tight binding of ATP, ADP, and to cellular proteins. For example, the free [ADP] in resting muscle has been variously estimated at between 1 and 37 µm. Using the value 25 µm in Worked Example 13-2, we would get a of . Calculation of the exact value of , however, is perhaps less instructive than the generalization we can make about actual free-energy changes: in vivo, the energy released by ATP hydrolysis is greater than the standard free-energy change, .
In the following discussions we use the value for ATP hydrolysis because this allows comparisons, on the same basis, with the energetics of other cellular reactions. Always keep in mind, however, that in living cells ΔG is the relevant quantity — for ATP hydrolysis and all other reactions — and may be quite different from .
Here we must make an important point about cellular ATP levels. We have shown (and will discuss further) how the chemical properties of ATP make it a suitable form of energy currency in cells. But it is not merely the molecule’s intrinsic chemical properties that give it this ability to drive metabolic reactions and other energy-requiring processes. Even more important is that, in the course of evolution, there has been a very strong selective pressure for regulatory mechanisms that hold cellular ATP concentrations far above the equilibrium concentrations for the hydrolysis reaction. When the ATP level drops, not only does the amount of fuel decrease, but the fuel itself loses its potency: ΔG for its hydrolysis (that is, its phosphorylation potential, ) is diminished. As our discussions of the metabolic pathways that produce and consume ATP will show, living cells have developed elaborate mechanisms — often at what might seem to us the expense of efficiency — to maintain high concentrations of ATP.
Other Phosphorylated Compounds and Thioesters Also Have Large, Negative Free Energies of Hydrolysis
ATP is not the only biological compound with a large, negative free energy of hydrolysis. Table 13-6 lists the standard free energies of hydrolysis for some biologically important phosphorylated compounds. In all of these phosphate-releasing reactions, a few of which we describe below, the several resonance forms available to (Fig. 13-11) stabilize this product relative to the reactant, contributing to an already negative free-energy change.
| Compounds and Acetyl-CoA | (kJ/mol) | (kcal/mol) |
|---|---|---|
Phosphoenolpyruvate |
||
1,3-Bisphosphoglycerate |
||
Phosphocreatine |
||
ADP |
||
ATP |
||
ATP |
||
AMP |
||
Glucose 3-phosphate |
||
Fructose 6-phosphate |
||
Glucose 6-phosphate |
||
Glycerol 3-phosphate |
||
Acetyl-CoA |
||
Data mostly from W. P. Jencks, in Handbook of Biochemistry and Molecular Biology, 3rd edn (G. D. Fasman, ed.), Physical and Chemical Data, Vol. 1, p. 296, CRC Press, 1976. Value for the free energy of hydrolysis of from P. A. Frey and A. Arabshahi, Biochemistry 34:11,307, 1995. |
||
Phosphoenolpyruvate (PEP; Fig. 13-13), a central intermediate in the energy-conserving process of glycolysis (Chapter 14), contains a phosphate ester bond that undergoes hydrolysis to yield the enol form of pyruvate, and this direct product can tautomerize to the more stable keto form. Because the reactant (PEP) has only one form (enol) and the product (pyruvate) has two possible forms, the reaction occurs with a gain in entropy, and the product is therefore stabilized relative to the reactant. This is a major contributor to the high standard free energy of hydrolysis of phosphoenolpyruvate: . A second contributor is the greater resonance stabilization in the released by cleavage of PEP.
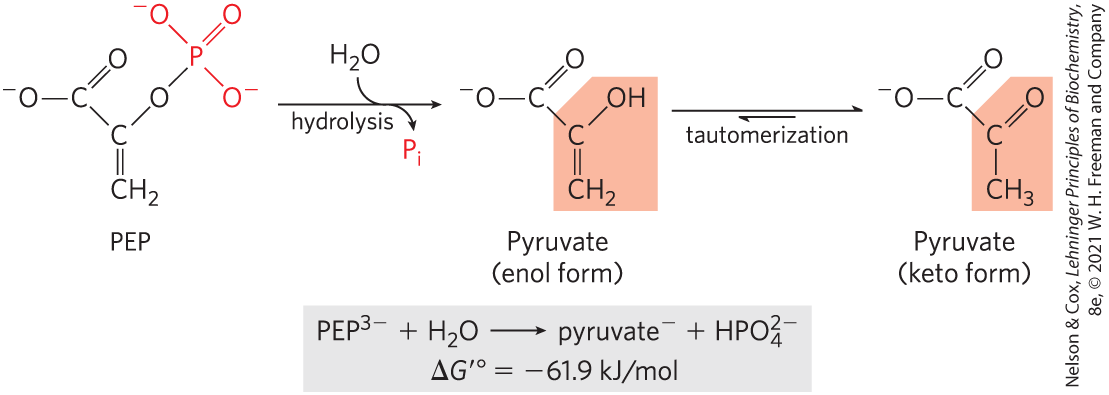
FIGURE 13-13 Hydrolysis of phosphoenolpyruvate (PEP). Catalyzed by pyruvate kinase, this reaction is followed by spontaneous tautomerization of the product, pyruvate. Tautomerization is not possible in PEP, and thus the products of hydrolysis are stabilized relative to the reactants. Resonance stabilization of also occurs, as shown in Figure 13-11.
Another intermediate in glycolysis, the three-carbon compound 1,3-bisphosphoglycerate (Fig. 13-14), contains an anhydride bond between the C-1 carboxyl group and phosphoric acid. Hydrolysis of this acyl phosphate is accompanied by a large, negative, standard free-energy change , which can, again, be explained in terms of the structure of reactant and products. When is added across the anhydride bond of 1,3-bisphosphoglycerate, one of the direct products, 3-phosphoglyceric acid, can lose a proton to give the carboxylate ion, 3-phosphoglycerate, which has two equally probable resonance forms. Removal of the direct product (3-phosphoglyceric acid) by its further metabolism and formation of the resonance-stabilized ion both favor the forward reaction.
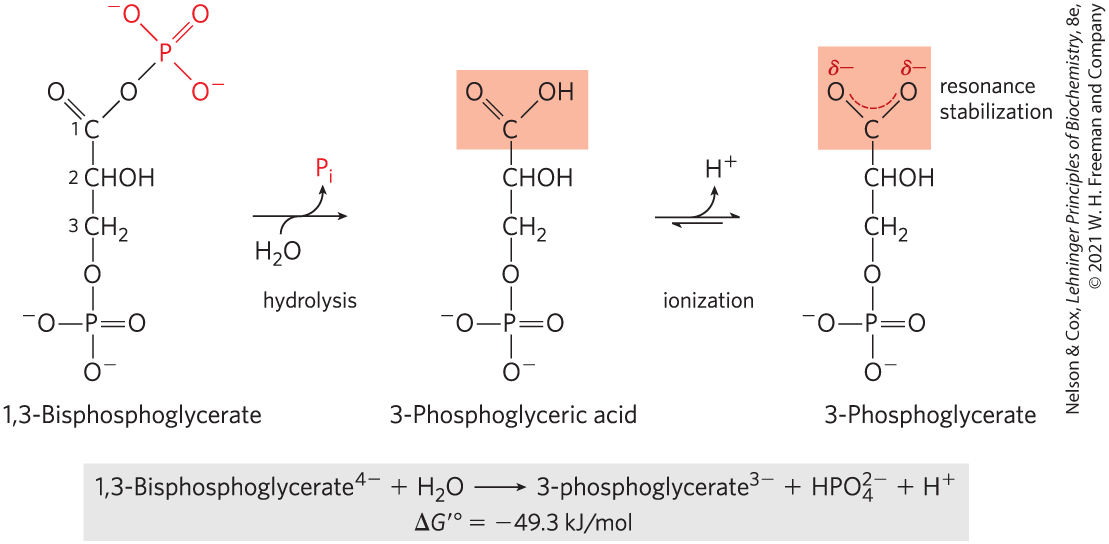
FIGURE 13-14 Hydrolysis of 1,3-bisphosphoglycerate. The direct product of hydrolysis is 3-phosphoglyceric acid, with an undissociated carboxylic acid. Its dissociation allows resonance structures that stabilize the product relative to the reactants. Resonance stabilization of further contributes to the negative free-energy change.
In phosphocreatine (Fig. 13-15), which is used in muscle tissue to replenish ATP after its use in contraction, the bond can be hydrolyzed to generate free creatine and . The release of and the resonance stabilization of creatine favor the forward reaction. The standard free-energy change of phosphocreatine hydrolysis is therefore large: .
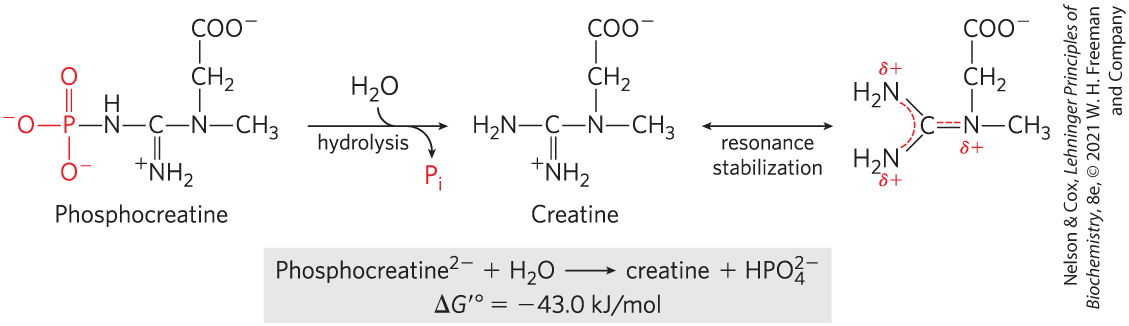
FIGURE 13-15 Hydrolysis of phosphocreatine. Breakage of the bond in phosphocreatine produces creatine, which is stabilized by formation of a resonance hybrid. The other product, , is also resonance stabilized.
Thioesters, in which a sulfur atom replaces the usual oxygen in the ester bond, also have large, negative, standard free energies of hydrolysis. Acetyl-coenzyme A, or acetyl-CoA (Fig. 13-16), is one of many thioesters important in metabolism. The acyl group in these compounds is activated for transacylation and condensation. Thioesters undergo much less resonance stabilization than do oxygen esters; consequently, the difference in free energy between the reactant and its hydrolysis products, which are resonance-stabilized, is greater for thioesters than for comparable oxygen esters (Fig. 13-17). In both cases, hydrolysis of the ester generates a carboxylic acid, which can ionize and assume several resonance forms. Together, these factors result in the large, negative for acetyl-CoA hydrolysis.
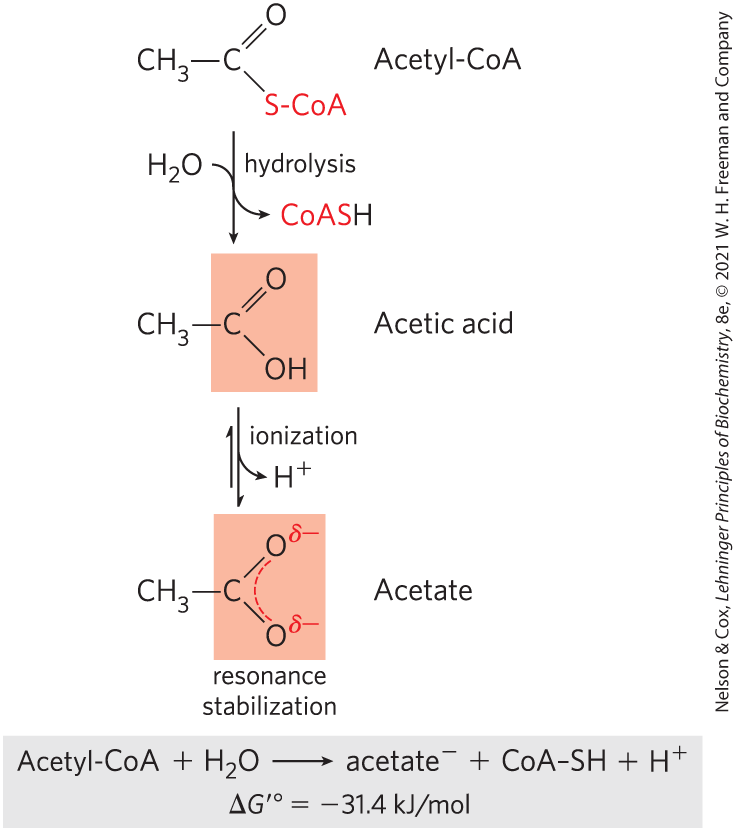
FIGURE 13-16 Hydrolysis of acetyl-coenzyme A. Acetyl-CoA is a thioester with a large, negative, standard free energy of hydrolysis. Thioesters contain a sulfur atom in the position occupied by an oxygen atom in oxygen esters. The complete structure of coenzyme A (CoA, or CoASH) is shown in Figure 8-41.
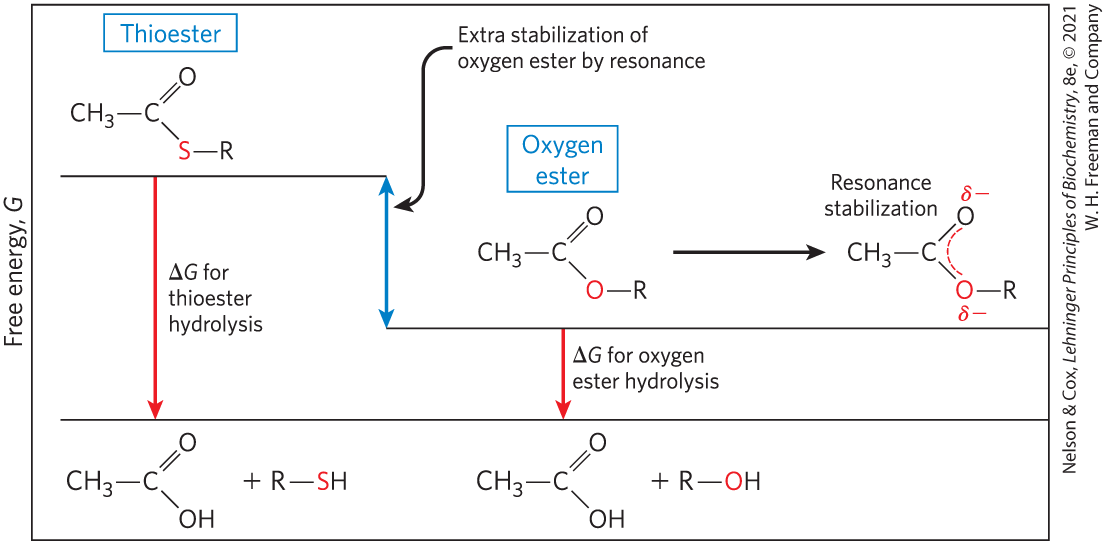
FIGURE 13-17 Free energy of hydrolysis for thioesters and oxygen esters. The products of both types of hydrolysis reaction have about the same free-energy content (G), but the thioester has a higher free-energy content than the oxygen ester. Orbital overlap between the O and C atoms allows resonance stabilization in oxygen esters; orbital overlap between S and C atoms is poorer and provides little resonance stabilization.
To summarize, for hydrolysis reactions with large, negative, standard free-energy changes, the products are more stable than the reactants for one or more of the following reasons: (1) the bond strain in reactants due to electrostatic repulsion is relieved by charge separation, as for ATP; (2) the products are stabilized by ionization, as for ATP, acyl phosphates, and thioesters; (3) the products are stabilized by isomerization (tautomerization), as for PEP; and/or (4) the products are stabilized by resonance, as for creatine released from phosphocreatine, carboxylate ion released from acyl phosphates and thioesters, and phosphate released from anhydride or ester linkages.
ATP Provides Energy by Group Transfers, Not by Simple Hydrolysis
Throughout this book you will encounter reactions or processes for which ATP supplies energy, and the contribution of ATP to these reactions is commonly indicated as in Figure 13-18a, with a single arrow showing the conversion of ATP to ADP and (or, in some cases, of ATP to AMP and pyrophosphate, ). When written this way, these reactions of ATP seem to be simple hydrolysis reactions in which water displaces (or ), and one is tempted to say that an ATP-dependent reaction is “driven by the hydrolysis of ATP.” This is not the case. ATP hydrolysis per se usually accomplishes nothing but the liberation of heat, which cannot drive a chemical process in an isothermal system. A single reaction arrow such as that in Figure 13-18a almost invariably represents a two-step process (Fig. 13-18b) in which part of the ATP molecule, a phosphoryl or pyrophosphoryl group or the adenylate moiety (AMP), is first transferred to a substrate molecule or to an amino acid residue in an enzyme, becoming covalently attached to the substrate or the enzyme and raising its free-energy content (activating it). Then, in a second step, the phosphate-containing moiety transferred in the first step is displaced, generating , , or AMP as the leaving group. Thus, ATP participates covalently in the enzyme-catalyzed reaction to which it contributes free energy.
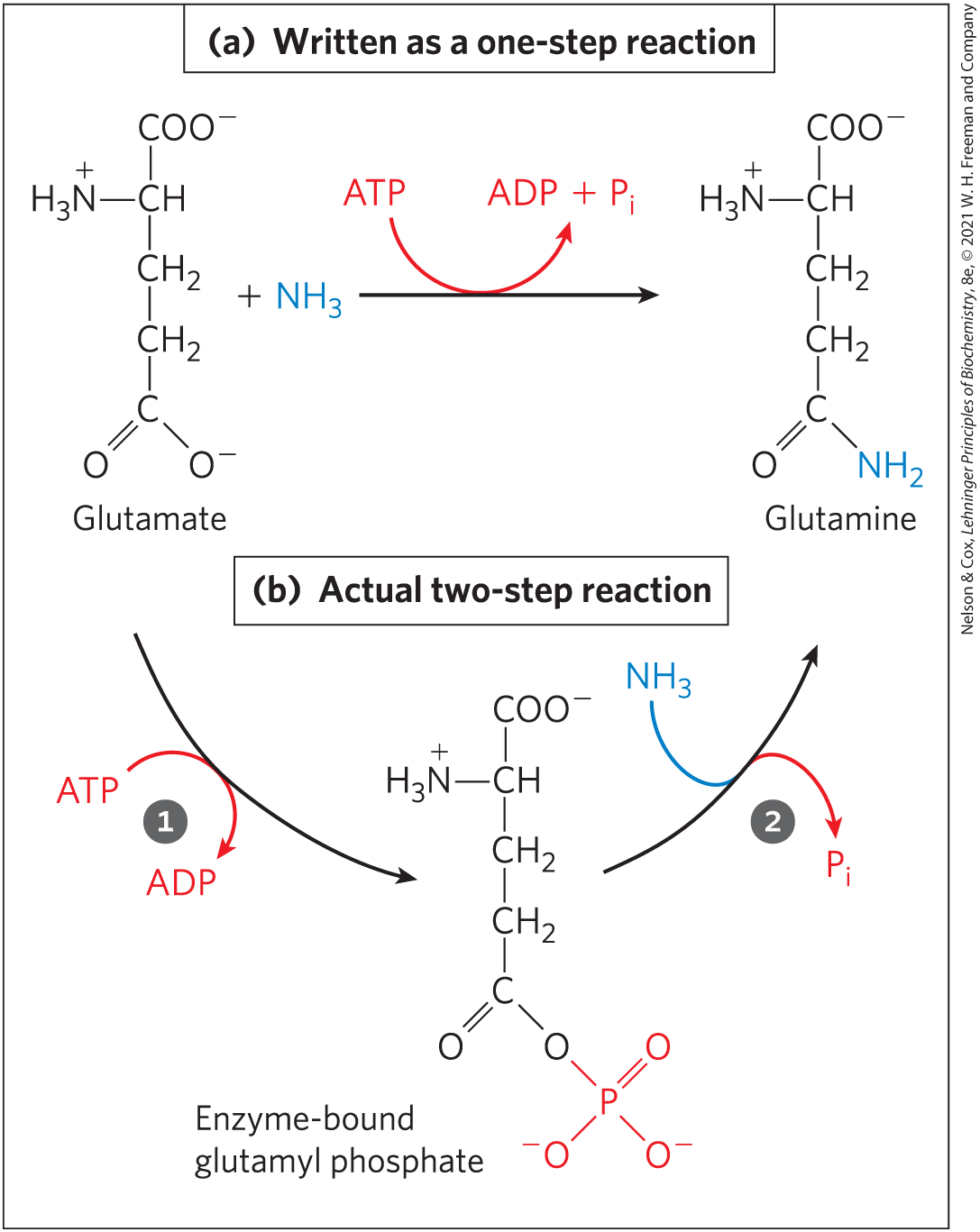
FIGURE 13-18 ATP hydrolysis in two steps. (a) The contribution of ATP to a reaction is often shown as a single step but is almost always a two-step process. (b) Shown here is the reaction catalyzed by ATP-dependent glutamine synthetase. A phosphoryl group is transferred from ATP to glutamate, then the phosphoryl group is displaced by and released as .
Some processes do involve direct hydrolysis of ATP (or the related GTP, guanosine triphosphate), however. For example, noncovalent binding of ATP (or GTP), followed by its hydrolysis to ADP (or GDP, guanosine diphosphate) and , can provide the energy to cycle some proteins between two conformations, producing mechanical motion. This occurs in muscle contraction (see Fig. 5-29) and in the movement of enzymes along DNA (see Fig. 25-30) or of ribosomes along messenger RNA (see Fig. 27-30). The energy-dependent reactions catalyzed by helicases, RecA protein, and some topoisomerases (Chapter 25) also involve direct hydrolysis of phosphoanhydride bonds. The ATPases involved in DNA replication and other processes described in Chapter 25 use ATP hydrolysis to cycle associated proteins between active and inactive forms. GTP-binding proteins that act in signaling pathways directly hydrolyze GTP to drive conformational changes that terminate signals triggered by hormones or by other extracellular factors (Chapter 12).
The phosphate compounds found in living organisms can be divided, somewhat arbitrarily, into two groups, based on their standard free energies of hydrolysis (Fig. 13-19). “High-energy” compounds have a of hydrolysis more negative than ; “low-energy” compounds have a less negative . Based on this criterion, ATP, with a of hydrolysis of , is a high-energy compound; glucose 6-phosphate, with a of hydrolysis of , is a low-energy compound.
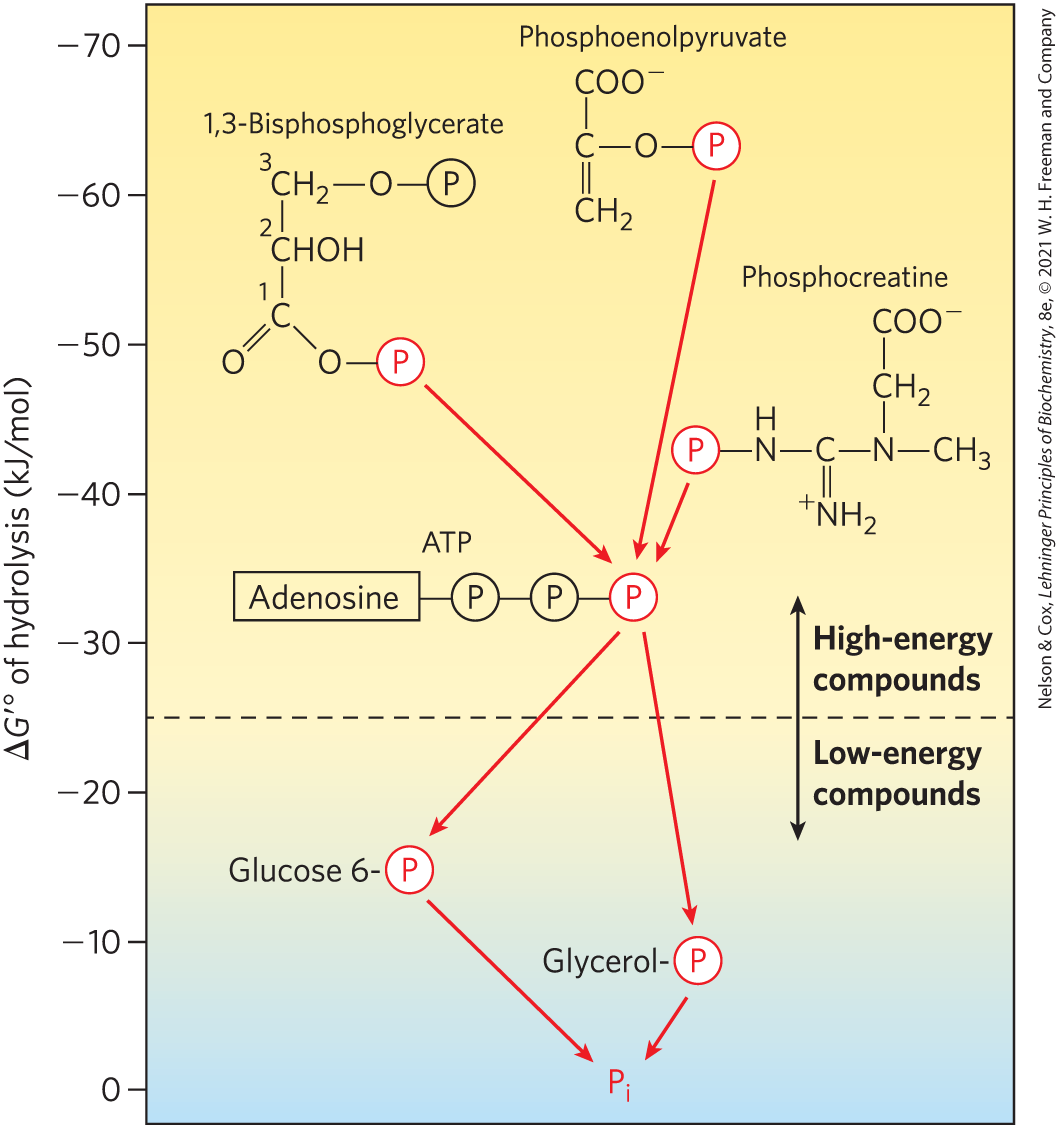
FIGURE 13-19 Ranking of biological phosphate compounds by standard free energies of hydrolysis. Phosphoryl groups, represented by , flow from high-energy phosphoryl group donors via ATP to acceptor molecules (such as glucose and glycerol) to form their low-energy phosphate derivatives. (The location of each compound’s donor phosphoryl group along the scale is an approximate indication of the compound’s of hydrolysis.) This flow of phosphoryl groups, catalyzed by kinases, proceeds with an overall loss of free energy under intracellular conditions. Hydrolysis of low-energy phosphate compounds releases , which has an even lower phosphoryl group transfer potential.
The term “high-energy phosphate bond,” long used by biochemists to describe the bond broken in hydrolysis reactions, is incorrect and misleading, as it wrongly suggests that the bond itself contains the energy. In fact, the breaking of all chemical bonds requires an input of energy. The free energy released by hydrolysis of phosphate compounds does not come from the specific bond that is broken; it results from the products of the reaction having a lower free-energy content than the reactants. For simplicity, we sometimes use the term “high-energy phosphate compound” when referring to ATP or other phosphate compounds with a large, negative, standard free energy of hydrolysis.
As is evident from the additivity of free-energy changes of sequential reactions (see Section 13.1), any phosphorylated compound can be synthesized by coupling the synthesis to the breakdown of another phosphorylated compound with a more negative free energy of hydrolysis. For example, because cleavage of from phosphoenolpyruvate releases more energy than is needed to drive the condensation of with ADP, the direct donation of a phosphoryl group from PEP to ADP is thermodynamically feasible:
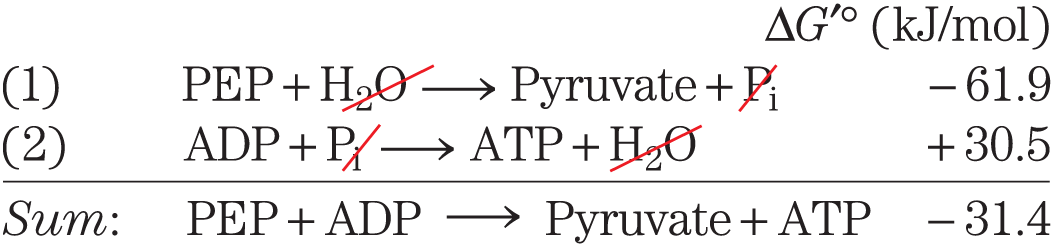
Notice that although the actual reaction is represented as the algebraic sum of the first two reactions, the actual reaction is a third, distinct reaction that does not involve ; PEP donates a phosphoryl group directly to ADP. We can describe phosphorylated compounds as having a high or low phosphoryl group transfer potential, on the basis of their standard free energies of hydrolysis (as listed in Table 13-6). The phosphoryl group transfer potential of PEP is very high, that of ATP is high, and that of glucose 6-phosphate is low (Fig. 13-19).
Much of catabolism is directed toward the synthesis of high-energy phosphate compounds, but their formation is not an end in itself; they are the means of activating a wide variety of compounds for further chemical transformation. The transfer of a phosphoryl group to a compound effectively puts free energy into that compound, so that it has more free energy to give up during subsequent metabolic transformations. We described above how the synthesis of glucose 6-phosphate is accomplished by phosphoryl group transfer from ATP. In the next chapter we see how this phosphorylation of glucose activates, or “primes,” the glucose for catabolic reactions that occur in nearly every living cell. Because of its intermediate position on the scale of group transfer potential, ATP can carry energy from high-energy phosphate compounds produced by catabolism (phosphoenolpyruvate, for example) to compounds such as glucose, converting them into more reactive species with better leaving groups. ATP thus serves as the universal energy currency in all living cells.
One more chemical feature of ATP is crucial to its role in metabolism: although, in aqueous solution, ATP is thermodynamically unstable and is therefore a good phosphoryl group donor, it is kinetically stable. Because of the huge activation energies (200 to 400 kJ/mol) required for uncatalyzed cleavage of its phosphoanhydride bonds, ATP does not spontaneously donate phosphoryl groups to water or to the hundreds of other potential acceptors in the cell. Only when specific enzymes are present to lower the energy of activation does phosphoryl group transfer from ATP proceed. The cell is therefore able to regulate the disposition of the energy carried by ATP by regulating the various enzymes that act on it.
ATP Donates Phosphoryl, Pyrophosphoryl, and Adenylyl Groups
The reactions of ATP are generally nucleophilic displacements (see Section 13.2) in which the nucleophile may be, for example, the oxygen of an alcohol or carboxylate, or a nitrogen of creatine or of the side chain of arginine or histidine. Each of the three phosphates of ATP is susceptible to nucleophilic attack (Fig. 13-20), and each position of attack yields a different type of product.
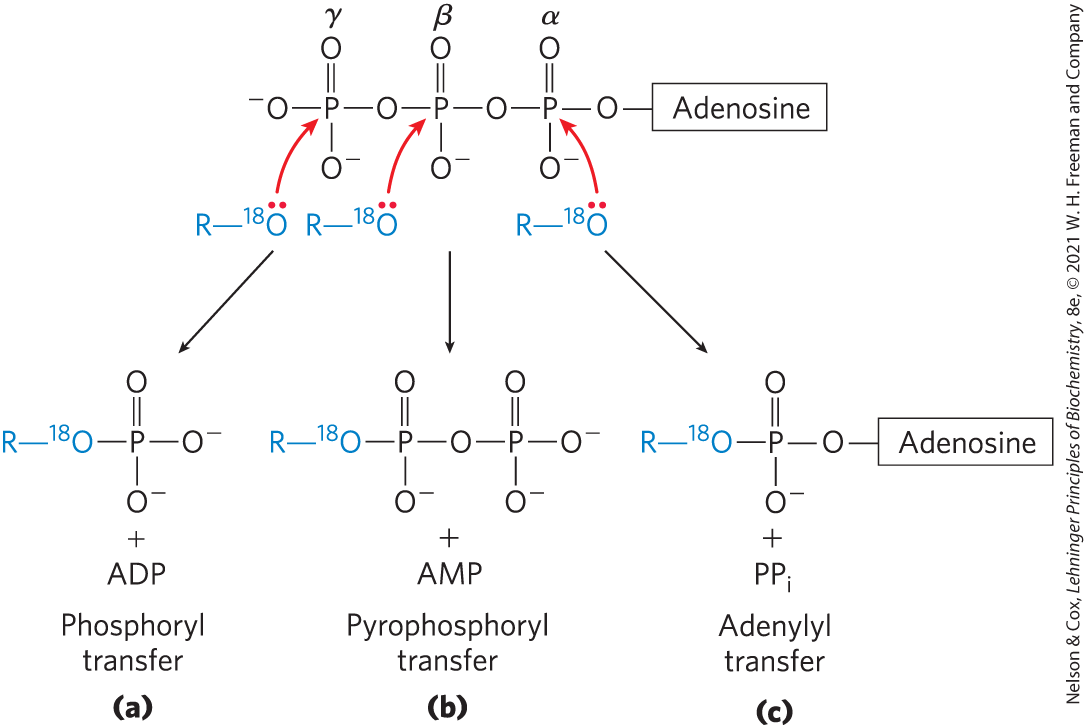
FIGURE 13-20 Three positions on ATP for attack by the nucleophile Any of the three P atoms (α, β, or γ) may serve as the electrophilic target for nucleophilic attack, in this case by the labeled nucleophile . The nucleophile may be an alcohol (ROH), a carboxyl group , or a phosphoanhydride (a nucleoside mono- or diphosphate, for example). (a) When the oxygen of the nucleophile attacks the γ position, the bridge oxygen of the product is labeled, indicating that the group transferred from ATP is a phosphoryl , not a phosphate . (b) Attack on the β position displaces AMP and leads to the transfer of a pyrophosphoryl (not pyrophosphate) group to the nucleophile. (c) Attack on the α position displaces and transfers the adenylyl group to the nucleophile.
Nucleophilic attack by an alcohol on the γ phosphate (Fig. 13-20a) displaces ADP and produces a new phosphate ester. Studies with reactants have shown that the bridge oxygen in the new compound is derived from the alcohol, not from ATP; the group transferred from ATP is therefore a phosphoryl , not a phosphate . Phosphoryl group transfer from ATP to glutamate (Fig. 13-18) or to glucose (p. 209) involves attack at the γ position of the ATP molecule.
Attack at the β phosphate of ATP displaces AMP and transfers a pyrophosphoryl (not pyrophosphate) group to the attacking nucleophile (Fig. 13-20b). For example, the formation of 5-phosphoribosyl-1-pyrophosphate (Chapter 22), a key intermediate in nucleotide synthesis, results from attack of an of the ribose on the β phosphate.
Nucleophilic attack at the α position of ATP displaces and transfers adenylate (-AMP) as an adenylyl group (Fig. 13-20c); the reaction is an adenylylation (, one of the most ungainly words in the biochemical language). Notice that hydrolysis of the α–β phosphoanhydride bond releases considerably more energy (~46 kJ/mol) than hydrolysis of the β–γ bond (~31 kJ/mol) (Table 13-6). Furthermore, the formed as a byproduct of the adenylylation is hydrolyzed to two by the ubiquitous enzyme inorganic pyrophosphatase, releasing 19 kJ/mol and thereby providing a further energy “push” for the adenylylation reaction. In effect, both phosphoanhydride bonds of ATP are split in the overall reaction. Adenylylation reactions are therefore thermodynamically very favorable. When the energy of ATP is used to drive a particularly unfavorable metabolic reaction, adenylylation is often the mechanism of energy coupling. Fatty acid activation is a good example of this energy-coupling strategy.
The first step in the activation of a fatty acid — either for energy-yielding oxidation or for use in the synthesis of more complex lipids — is the formation of its thiol ester (see Fig. 17-5). The direct condensation of a fatty acid with coenzyme A is endergonic, but the formation of a fatty acyl–CoA is made exergonic by stepwise removal of two phosphoryl groups from ATP. First, adenylate (AMP) is transferred from ATP to the carboxyl group of the fatty acid, forming a mixed anhydride (fatty acyl adenylate) and liberating . The thiol group of coenzyme A then displaces the adenylyl group and forms a thioester with the fatty acid. The sum of these two reactions is energetically equivalent to the exergonic hydrolysis of ATP to AMP and and the endergonic formation of fatty acyl–CoA. The formation of fatty acyl–CoA is made energetically favorable by hydrolysis of the by inorganic pyrophosphatase. Thus, in the activation of a fatty acid, both phosphoanhydride bonds of ATP are broken. The resulting is the sum of the values for the breakage of these bonds, or :
The activation of amino acids before their polymerization into proteins (see Fig. 27-19) is accomplished by an analogous set of reactions in which a transfer RNA molecule takes the place of coenzyme A. An interesting use of the cleavage of ATP to AMP and occurs in the firefly, which uses ATP as an energy source to produce flashes of light (Box 13-2).
Assembly of Informational Macromolecules Requires Energy
When simple precursors are assembled into high molecular weight polymers with defined sequences (DNA, RNA, proteins), as described in detail in Part III, energy is required both for the condensation of monomeric units and for the creation of ordered sequences. The precursors for DNA and RNA synthesis are nucleoside triphosphates, and polymerization is accompanied by cleavage of the phosphoanhydride linkage between the α and β phosphates, with the release of (Fig. 13-20). The moieties transferred to the growing polymer in these reactions are adenylate (AMP), guanylate (GMP), cytidylate (CMP), or uridylate (UMP) for RNA synthesis, and their deoxy analogs (with TMP in place of UMP) for DNA synthesis. As noted above, the activation of amino acids for protein synthesis involves the donation of adenylyl groups from ATP, and we shall see in Chapter 27 that several steps of protein synthesis on the ribosome are also accompanied by GTP hydrolysis. In all these cases, the exergonic breakdown of a nucleoside triphosphate is coupled to the endergonic process of synthesizing a polymer of a specific sequence.
ATP can supply the energy for transporting an ion or a molecule across a membrane into another aqueous compartment where its concentration is higher (see Fig. 11-39). Transport processes are major consumers of energy; in human kidney and brain, for example, as much as two-thirds of the energy consumed at rest is used to pump and across plasma membranes via the ATPase. The transport of and is driven by cyclic phosphorylation and dephosphorylation of the transporter protein, with ATP as the phosphoryl group donor. -dependent phosphorylation of the ATPase forces a change in the protein’s conformation, and -dependent dephosphorylation favors return to the original conformation. Each cycle in the transport process results in the conversion of ATP to ADP and , and it is the free-energy change of ATP hydrolysis that drives the cyclic changes in protein conformation that result in the electrogenic pumping of and . Note that in this case, ATP interacts covalently by phosphoryl group transfer to the enzyme, not to the substrate.
In the contractile system of skeletal muscle cells, myosin and actin are specialized to transduce the chemical energy of ATP into motion (see Fig. 5-29). ATP binds tightly but noncovalently to one conformation of myosin, holding the protein in that conformation. When myosin catalyzes the hydrolysis of its bound ATP, the ADP and dissociate from the protein, allowing it to relax into a second conformation until another molecule of ATP binds. The binding and subsequent hydrolysis of ATP (by myosin ATPase) provide the energy that forces cyclic changes in the conformation of the myosin head. The change in conformation of many individual myosin molecules results in the sliding of myosin fibrils along actin filaments (see Fig. 5-28), which translates into macroscopic contraction of the muscle fiber. As we noted earlier, this production of mechanical motion at the expense of ATP is one of the few cases in which ATP hydrolysis per se, rather than group transfer from ATP, is the source of the chemical energy in a coupled process.
Transphosphorylations between Nucleotides Occur in All Cell Types
Although we have focused on ATP as the cell’s energy currency and donor of phosphoryl groups, all other nucleoside triphosphates (GTP, UTP, and CTP) and all deoxynucleoside triphosphates (dATP, dGTP, dTTP, and dCTP) are energetically equivalent to ATP. The standard free-energy changes associated with hydrolysis of their phosphoanhydride linkages are very nearly identical with those shown in Table 13-6 for ATP. In preparation for their various biological roles, these other nucleotides are generated and maintained as the nucleoside triphosphate (NTP) forms by phosphoryl group transfer to the corresponding nucleoside diphosphates (NDPs) and monophosphates (NMPs).
ATP is the primary high-energy phosphate compound produced by catabolism, in the processes of glycolysis, oxidative phosphorylation, and, in photosynthetic cells, photophosphorylation. Several enzymes then carry phosphoryl groups from ATP to the other nucleotides. Nucleoside diphosphate kinase, found in all cells, catalyzes the reaction
Although this reaction is fully reversible, the relatively high [ATP]/[ADP] ratio in cells normally drives the reaction to the right, with the net formation of NTPs and dNTPs. The enzyme actually catalyzes a two-step phosphoryl group transfer, which is a classic case of a double-displacement (Ping-Pong) mechanism (Fig. 13-21; see also Fig. 6-15b). First, phosphoryl group transfer from ATP to an active-site His residue produces a phosphoenzyme intermediate; then the phosphoryl group is transferred from the –His residue to an NDP acceptor. Because the enzyme is nonspecific for the base in the NDP and works equally well on dNDPs and NDPs, it can synthesize all NTPs and dNTPs, given the corresponding NDPs and a supply of ATP.
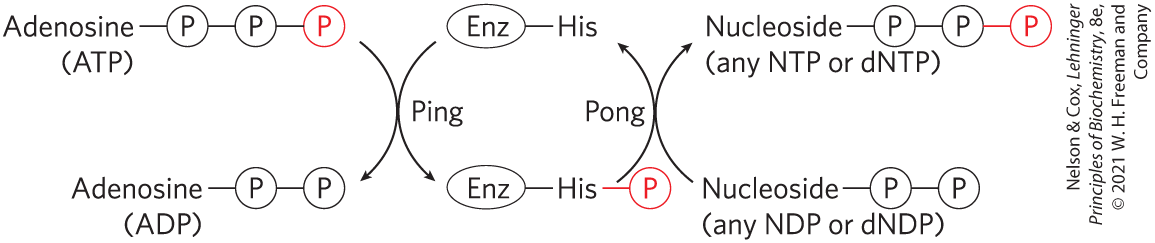
FIGURE 13-21 Ping-Pong mechanism of nucleoside diphosphate kinase. The enzyme binds its first substrate (ATP in our example), and a phosphoryl group is transferred to the side chain of a His residue. ADP departs, another nucleoside (or deoxynucleoside) diphosphate replaces it, and this is converted to the corresponding triphosphate by transfer of the phosphoryl group from the phosphohistidine residue.
All phosphates are shown as a circle labeled P. Adenosine is bonded to three phosphates, with the terminal phosphate highlighted in red. Text below reads, (A T P). A curved downward arrow meets a curved downward arrow to its right and the point where they meet is labeled ping. The left-hand arrow ends at adenosine bonded to two phosphates to the lower left above text reading, (A D P). The right-hand arrow ends at an oval labeled E n z bonded to H i s connected by a red bond to a red highlighted phosphate. A curved upward arrow meets a curved upward arrow to the right and the point where they meet is labeled pong. The right-hand arrow begins at nucleoside bonded to two phosphate with text below reading, (any N D P or d N D P) and ends at nucleoside bonded to three phosphates with the terminal phosphate highlighted in red above text reading, (any N T P or d N T P). The arrow immediately to its left, which it met at the point labeled pong, ends at an oval labeled E n z bonded to H i s. A curved downward arrow from this molecule meets the curved arrow to its left at the point labeled ping before reaching the oval labeled E n z bonded to His connected by a red highlighted bond to red highlighted P below.
Phosphoryl group transfers from ATP result in an accumulation of ADP; for example, when muscle is contracting vigorously, ADP accumulates and interferes with ATP-dependent contraction. During periods of intense demand for ATP, the cell lowers the ADP concentration, and at the same time replenishes ATP, by the action of adenylate kinase:
This reaction is fully reversible, so, after the intense demand for ATP ends, the enzyme can recycle AMP by converting it to ADP, which can then be phosphorylated to ATP in mitochondria. A similar enzyme, guanylate kinase, converts GMP to GDP at the expense of ATP. By pathways such as these, energy conserved in the catabolic production of ATP is used to supply the cell with all required NTPs and dNTPs.
Phosphocreatine (PCr; Fig. 13-15), also called creatine phosphate, serves as a ready source of phosphoryl groups for the quick synthesis of ATP from ADP. The PCr concentration in skeletal muscle is approximately 30 mm, nearly 10 times the concentration of ATP, and in other tissues such as smooth muscle, brain, and kidney, [PCr] is 5 to 10 mm. The enzyme creatine kinase catalyzes the reversible reaction
When a sudden demand for energy depletes ATP, the PCr reservoir is used to replenish ATP at a rate considerably faster than ATP can be synthesized by catabolic pathways. When the demand for energy slackens, ATP produced by catabolism is used to replenish the PCr reservoir by reversal of the creatine kinase reaction (see Box 23-1). Organisms in the lower phyla employ other PCr-like molecules (collectively called phosphagens) as phosphoryl reservoirs.
SUMMARY 13.3 Phosphoryl Group Transfers and ATP
- ATP is the chemical link between catabolism and anabolism. It is the energy currency of the living cell. The exergonic conversion of ATP to ADP and , or to AMP and , is coupled to many endergonic reactions and processes.
- The free-energy change for ATP hydrolysis under cellular conditions is its phosphorylation potential, .
- Direct hydrolysis of ATP is the source of energy in some processes driven by conformational changes. In general, however, it is not ATP hydrolysis but the transfer of a phosphoryl group from ATP to a substrate or an enzyme that couples the energy of ATP breakdown to endergonic transformations of substrates.
- Phosphate compounds with high free energies of hydrolysis can donate their phosphoryl group to form another phosphate compound with a smaller free energy of hydrolysis.
- ATP can also donate a pyrophosphoryl or adenylyl (AMP) group to a variety of metabolic intermediates, activating them for nucleophilic displacement reactions.
- Through these group transfer reactions, ATP provides the energy for a large number of anabolic reactions, including the synthesis of informational macromolecules, and for the transport of molecules and ions across membranes against concentration gradients and electrical potential gradients. Muscle contraction is also powered by ATP.
- To maintain its high group transfer potential, ATP concentration must be held far above the equilibrium concentration by energy-yielding reactions of catabolism.
- ATP can donate a phosphoryl group to nucleoside diphosphates by transphosphorylation to keep the levels of GTP, UTP, CTP, and the deoxynucleotides far above their equilibrium concentrations.
 The charge separation that results from hydrolysis relieves electrostatic repulsion among the four negative charges on ATP.
The charge separation that results from hydrolysis relieves electrostatic repulsion among the four negative charges on ATP.  The product inorganic phosphate is stabilized by formation of a resonance hybrid, in which each of the four phosphorus–oxygen bonds has the same degree of double-bond character and the hydrogen ion is not permanently associated with any one of the oxygens. (Some degree of resonance stabilization also occurs in phosphates involved in ester or anhydride linkages, but fewer resonance forms are possible than for .) A third factor (not shown) that favors ATP hydrolysis is the greater degree of solvation (hydration) of the products and ADP relative to ATP, which further stabilizes the products relative to the reactants.
The product inorganic phosphate is stabilized by formation of a resonance hybrid, in which each of the four phosphorus–oxygen bonds has the same degree of double-bond character and the hydrogen ion is not permanently associated with any one of the oxygens. (Some degree of resonance stabilization also occurs in phosphates involved in ester or anhydride linkages, but fewer resonance forms are possible than for .) A third factor (not shown) that favors ATP hydrolysis is the greater degree of solvation (hydration) of the products and ADP relative to ATP, which further stabilizes the products relative to the reactants. in the course of evolution, there has been a very strong selective pressure for regulatory mechanisms that hold cellular ATP concentrations far above the equilibrium concentrations for the hydrolysis reaction. When the ATP level drops, not only does the amount of fuel decrease, but the fuel itself loses its potency: ΔG for its hydrolysis (that is, its phosphorylation potential, ) is diminished. As our discussions of the metabolic pathways that produce and consume ATP will show, living cells have developed elaborate mechanisms — often at what might seem to us the expense of efficiency — to maintain high concentrations of ATP.
in the course of evolution, there has been a very strong selective pressure for regulatory mechanisms that hold cellular ATP concentrations far above the equilibrium concentrations for the hydrolysis reaction. When the ATP level drops, not only does the amount of fuel decrease, but the fuel itself loses its potency: ΔG for its hydrolysis (that is, its phosphorylation potential, ) is diminished. As our discussions of the metabolic pathways that produce and consume ATP will show, living cells have developed elaborate mechanisms — often at what might seem to us the expense of efficiency — to maintain high concentrations of ATP. , flow from high-energy phosphoryl group donors via ATP to acceptor molecules (such as glucose and glycerol) to form their low-energy phosphate derivatives. (The location of each compound’s donor phosphoryl group along the scale is an approximate indication of the compound’s of hydrolysis.) This flow of phosphoryl groups, catalyzed by kinases, proceeds with an overall loss of free energy under intracellular conditions. Hydrolysis of low-energy phosphate compounds releases , which has an even lower phosphoryl group transfer potential.
, flow from high-energy phosphoryl group donors via ATP to acceptor molecules (such as glucose and glycerol) to form their low-energy phosphate derivatives. (The location of each compound’s donor phosphoryl group along the scale is an approximate indication of the compound’s of hydrolysis.) This flow of phosphoryl groups, catalyzed by kinases, proceeds with an overall loss of free energy under intracellular conditions. Hydrolysis of low-energy phosphate compounds releases , which has an even lower phosphoryl group transfer potential. As is evident from the additivity of free-energy changes of sequential reactions (see
As is evident from the additivity of free-energy changes of sequential reactions (see  ATP is the chemical link between catabolism and anabolism. It is the energy currency of the living cell. The exergonic conversion of ATP to ADP and , or to AMP and , is coupled to many endergonic reactions and processes.
ATP is the chemical link between catabolism and anabolism. It is the energy currency of the living cell. The exergonic conversion of ATP to ADP and , or to AMP and , is coupled to many endergonic reactions and processes.