11.3 Solute Transport across Membranes
Every living cell must acquire from its surroundings the raw materials for biosynthesis and for energy production, and must release the byproducts of metabolism to its environment; both processes require that small compounds or inorganic ions cross the plasma membrane. Within the eukaryotic cell, different compartments have different concentrations of ions and of metabolic intermediates and products, and these, too, must move across intracellular membranes in tightly regulated processes. A few nonpolar compounds can dissolve in the lipid bilayer and cross a membrane unassisted, but for any polar compound or ion, a specific membrane protein carrier is essential. Approximately 2,000 genes in the human genome encode proteins that function in transporting solutes across membranes. In some cases, a membrane protein simply facilitates the diffusion of a solute down its concentration gradient; but transport can also occur against a gradient of concentration, electrical potential, or both, and in these cases, as we shall see, the transport process requires energy. Ions may also diffuse across membranes via ion channels formed by proteins, or they may be carried across by ionophores, small molecules that mask the charge of ions and allow them to diffuse through the lipid bilayer. Figure 11-27 summarizes the various types of transport mechanisms discussed in this section.
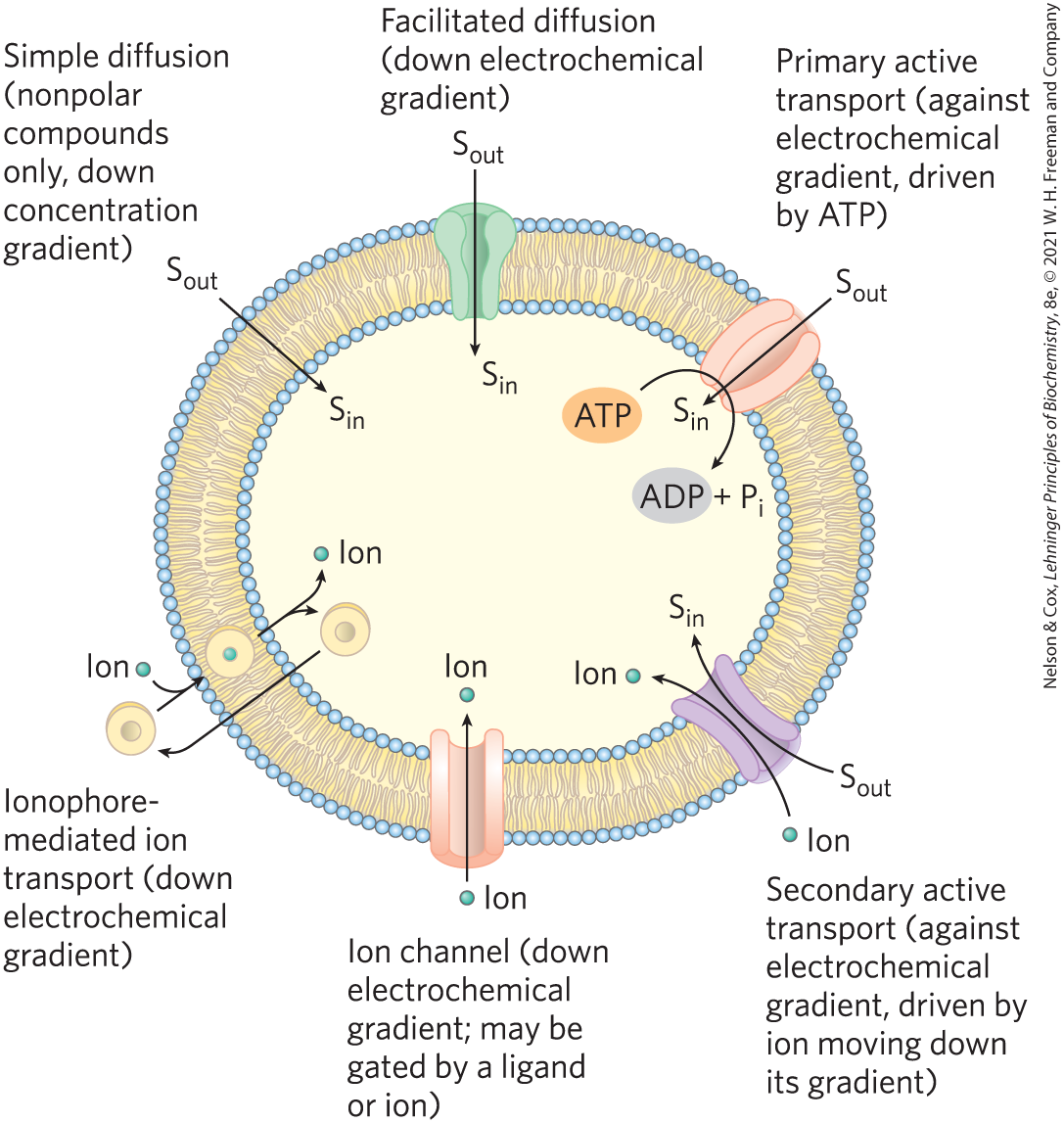
FIGURE 11-27 Summary of transporter types. Some types (ionophores, ion channels, and passive transporters) simply speed transmembrane movement of solutes (S) down their electrochemical gradients, whereas others (active transporters) can pump solutes against a gradient, using ATP or a gradient of a second solute to provide the energy.
Transport May Be Passive or Active
When two aqueous compartments containing unequal concentrations of a soluble compound or ion are separated by a permeable divider (membrane), the solute moves by simple diffusion from the region of higher concentration, through the membrane, to the region of lower concentration, until the two compartments have equal solute concentrations (Fig. 11-28a). When ions of opposite charge are separated by a permeable membrane, there is a transmembrane electrical gradient, a membrane potential, (expressed in millivolts). This membrane potential produces a force opposing ion movements that increase and driving ion movements that reduce (Fig. 11-28b). Thus, the direction in which a charged solute tends to move spontaneously across a membrane depends on both the chemical gradient (the difference in solute concentration) and the electrical gradient across the membrane. Together these two factors are referred to as the electrochemical gradient or electrochemical potential. This behavior of solutes is in accord with the second law of thermodynamics: molecules tend to spontaneously assume the distribution of greatest randomness and lowest energy.
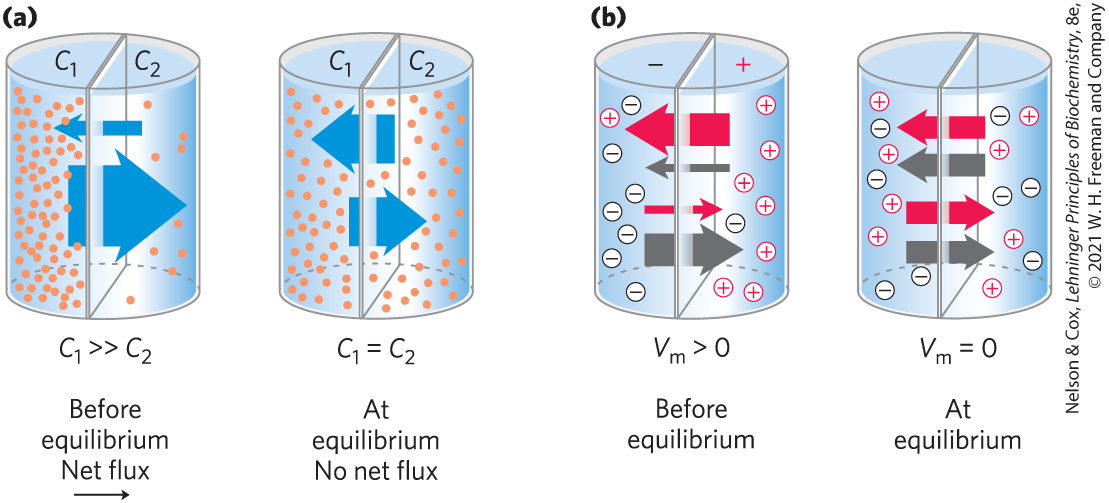
FIGURE 11-28 Movement of solutes across a permeable membrane. (a) Net movement of an electrically neutral solute is toward the side of lower solute concentration until equilibrium is achieved. The solute concentrations on the left and right sides of the membrane, as shown here, are designated and . The rate of transmembrane solute movement (indicated by the arrows) is proportional to the concentration ratio. (b) Net movement of an electrically charged solute is dictated by a combination of the electrical potential and the ratio of chemical concentrations across the membrane; net ion movement continues until this electrochemical potential reaches zero.
Membrane proteins that act by increasing the rate of solute movement across membranes are called transporters or carriers. Transporters are of two general types: passive and active. Passive transporters simply facilitate movement down a concentration gradient, increasing the transport rate. This process is called passive transport or facilitated diffusion. Active transporters (sometimes called pumps) can move substrates across a membrane against a concentration gradient or an electrical potential, a process called active transport. Primary active transporters use energy provided directly by a chemical reaction; secondary active transporters couple uphill transport of one substrate with downhill transport of another.
Transporters and Ion Channels Share Some Structural Properties but Have Different Mechanisms
To pass through a lipid bilayer, a polar or charged solute must first give up its interactions with the water molecules in its hydration shell, then diffuse about 3 nm (30 Å) through a substance (lipid) in which it is poorly soluble (Fig. 11-29a). The energy used to strip away the hydration shell and to move the polar compound from water into lipid, then through the lipid bilayer, is regained as the compound leaves the membrane on the other side and is rehydrated. However, the intermediate stage of transmembrane passage is a high-energy state comparable to the transition state in an enzyme-catalyzed chemical reaction. In both cases, an activation barrier must be overcome to reach the intermediate stage (Fig. 11-29; compare with Fig. 6-3). The energy of activation for translocation of a polar solute across the bilayer is so large that pure lipid bilayers are virtually impermeable to polar and charged species on time scales relevant to cell growth and division.
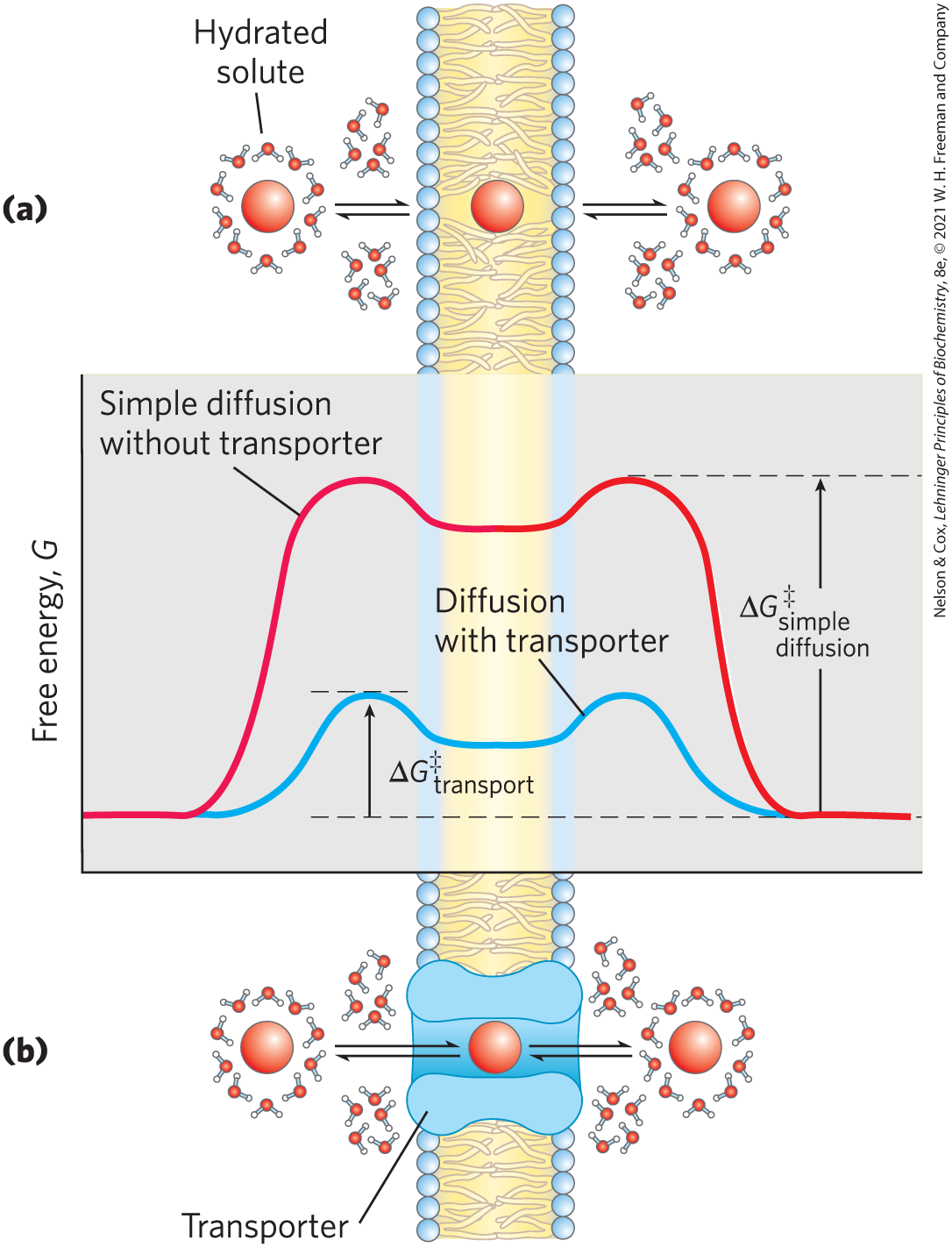
FIGURE 11-29 Energy changes accompanying passage of a hydrophilic solute through the lipid bilayer of a biological membrane. (a) In simple diffusion, removal of the hydration shell is highly endergonic, and the energy of activation for diffusion through the bilayer is very high. (b) A transporter protein reduces the for transmembrane diffusion of the solute. It does this by forming noncovalent interactions with the dehydrated solute to replace the hydrogen bonding with water and by providing a hydrophilic transmembrane pathway.
Membrane proteins lower the activation energy for transport of polar compounds and ions by providing an alternative path across the membrane for specific solutes. Lowering the activation energy greatly increases the rate of transmembrane movement (recall p. 182). Transporters are not enzymes in the usual sense; their “substrates” are moved from one compartment to another but are not chemically altered. Like enzymes, however, transporters bind their substrates with stereochemical specificity through multiple weak, noncovalent interactions. The negative free-energy change associated with these weak interactions, , counterbalances the positive free-energy change that accompanies loss of the water of hydration from the substrate, , thereby lowering for transmembrane passage (Fig. 11-29b). Transporter proteins span the lipid bilayer several times, forming a transmembrane pathway lined with hydrophilic amino acid side chains. The pathway provides an alternative route for a specific substrate to move across the lipid bilayer without its having to dissolve in the bilayer, further lowering for transmembrane diffusion. The result is an orders-of-magnitude increase in the substrate’s rate of passage across the membrane.
Ion channels use a different mechanism than transporters to move inorganic ions across membranes. Ion channels speed the passage of ions across membranes by providing an aqueous path across the membrane through which inorganic ions can diffuse at very high rates. Most ion channels have a “gate” (Fig. 11-30a) regulated by a biological signal. When the gate is open, ions move across the membrane, through the channel, in the direction dictated by the ion’s charge and the electrochemical gradient. Movement occurs at rates approaching the limit of unhindered diffusion (tens of millions of ions per second per channel — much higher than typical transporter rates). Ion channels typically show some specificity for an ion, but they are not saturable with their ion substrate. Flow through a channel stops either when the gating mechanism is closed (again, by a biological signal) or when there is no longer an electrochemical gradient providing the driving force for the movement. In contrast, transporters, which bind their “substrates” with high stereospecificity, catalyze transport at rates well below the limits of free diffusion, and they are saturable in the same sense as are enzymes: there is some substrate concentration above which further increases will not produce a greater rate of transport. Transporters have a gate on either side of the membrane, and the two gates are never open at the same time (Fig. 11-30b).
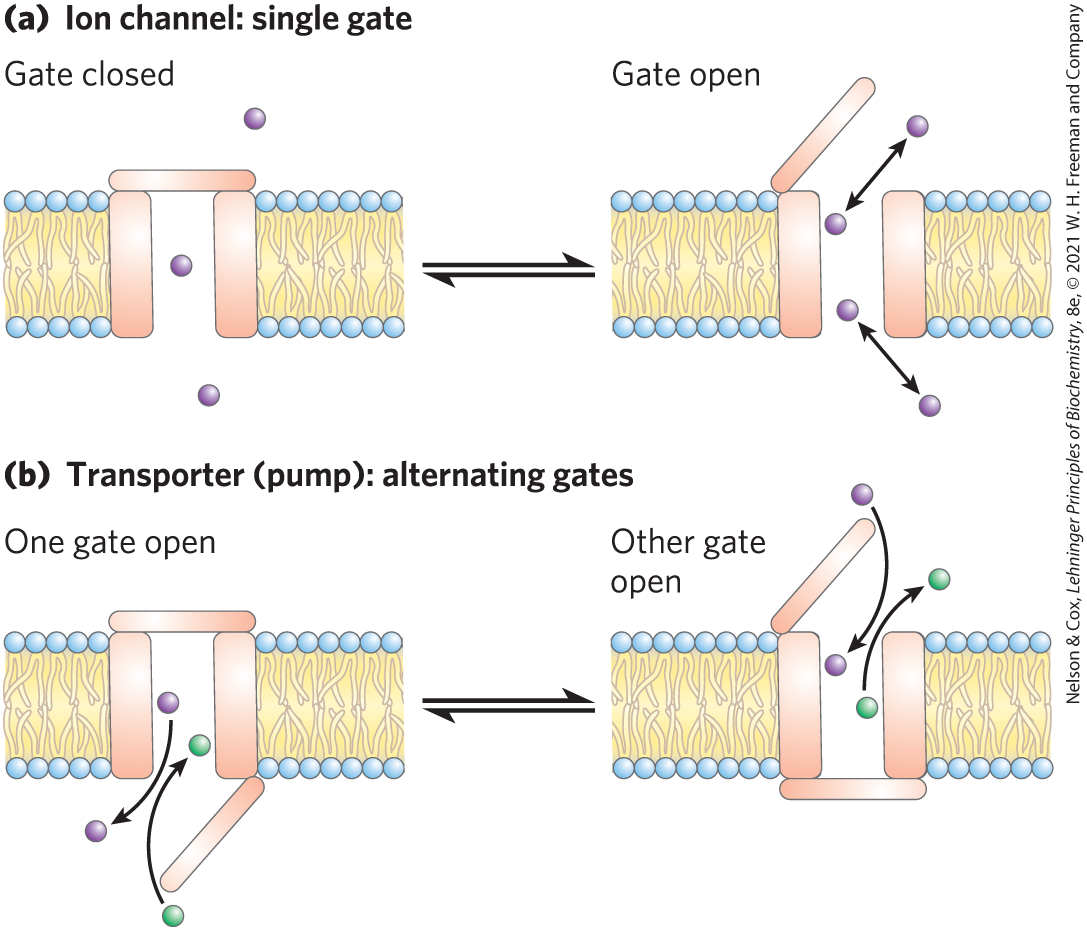
FIGURE 11-30 Differences between channels and transporters. (a) Ion channels have a transmembrane pore that is either open or closed, depending on the position of the single gate. When the gate is open, ions move through at a rate limited only by the maximum rate of diffusion. (b) Transporters have two gates, and both are never open at the same time. Movement of a substrate (an ion or a small molecule) through the membrane is therefore limited by the time needed for one gate to open and close (on one side of the membrane) and the second gate to open. Rates of movement through ion channels can be orders of magnitude greater than rates through transporters, but channels simply allow the ion to flow down the electrochemical gradient, whereas active transporters (pumps) can move a substrate against its concentration gradient. [Information from D. C. Gadsby, Nat. Rev. Mol. Cell Biol. 10:344, 2009, Fig. 1.]
Both transporters and ion channels constitute large families of proteins, defined not only by their primary sequences but also by their secondary structures. We next consider some well-studied representatives of the main transporter and channel families. You will also encounter some of these in Chapter 12 when we discuss transmembrane signaling, and some in later chapters in the context of the metabolic pathways in which they participate.
The Glucose Transporter of Erythrocytes Mediates Passive Transport
Energy-yielding metabolism in erythrocytes depends on a constant supply of glucose from the blood plasma, where the glucose concentration is maintained at about 4.5 to 5 mm. Glucose enters the erythrocyte by passive transport via a specific glucose transporter called GLUT1, at a rate about 50,000 times greater than it could cross the membrane unassisted.
The process of glucose transport can be described by analogy with an enzymatic reaction in which the “substrate” is glucose outside the cell , the “product” is glucose inside the cell , and the “enzyme” is the transporter, T. When the initial rate of glucose uptake is measured as a function of external glucose concentration (Fig. 11-31), the resulting plot is hyperbolic: at high external glucose concentrations, the rate of uptake approaches . Formally, such a transport process can be described by the set of equations
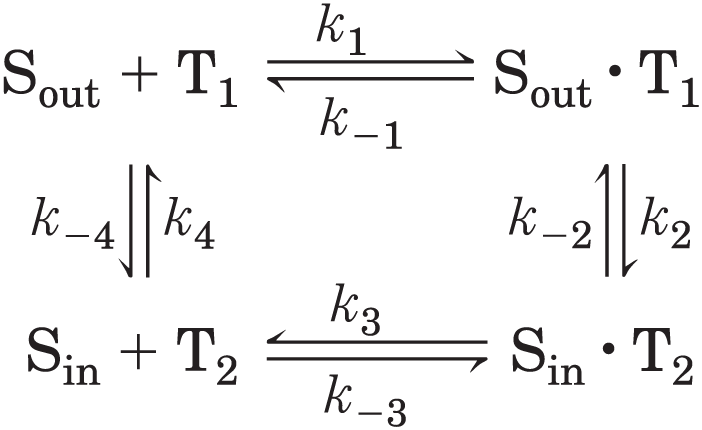
in which , and so forth are the forward and reverse rate constants for each step; is the transporter conformation in which the glucose-binding site faces outward (in contact with blood plasma), and is the conformation in which it faces inward. Given that every step in this sequence is reversible, the transporter is, in principle, equally able to move glucose into or out of the cell. As for enzyme assays, this transporter assay measures the initial rate of uptake, when the product concentration (glucose concentration inside the cell) is zero, while the substrate concentration (glucose on the outside) is varied. In the living cell, GLUT1 accelerates the movement of glucose down its concentration gradient, which normally means into the cell. Glucose that enters a cell is generally metabolized immediately, and the intracellular glucose concentration is thereby kept low relative to its concentration in the blood.
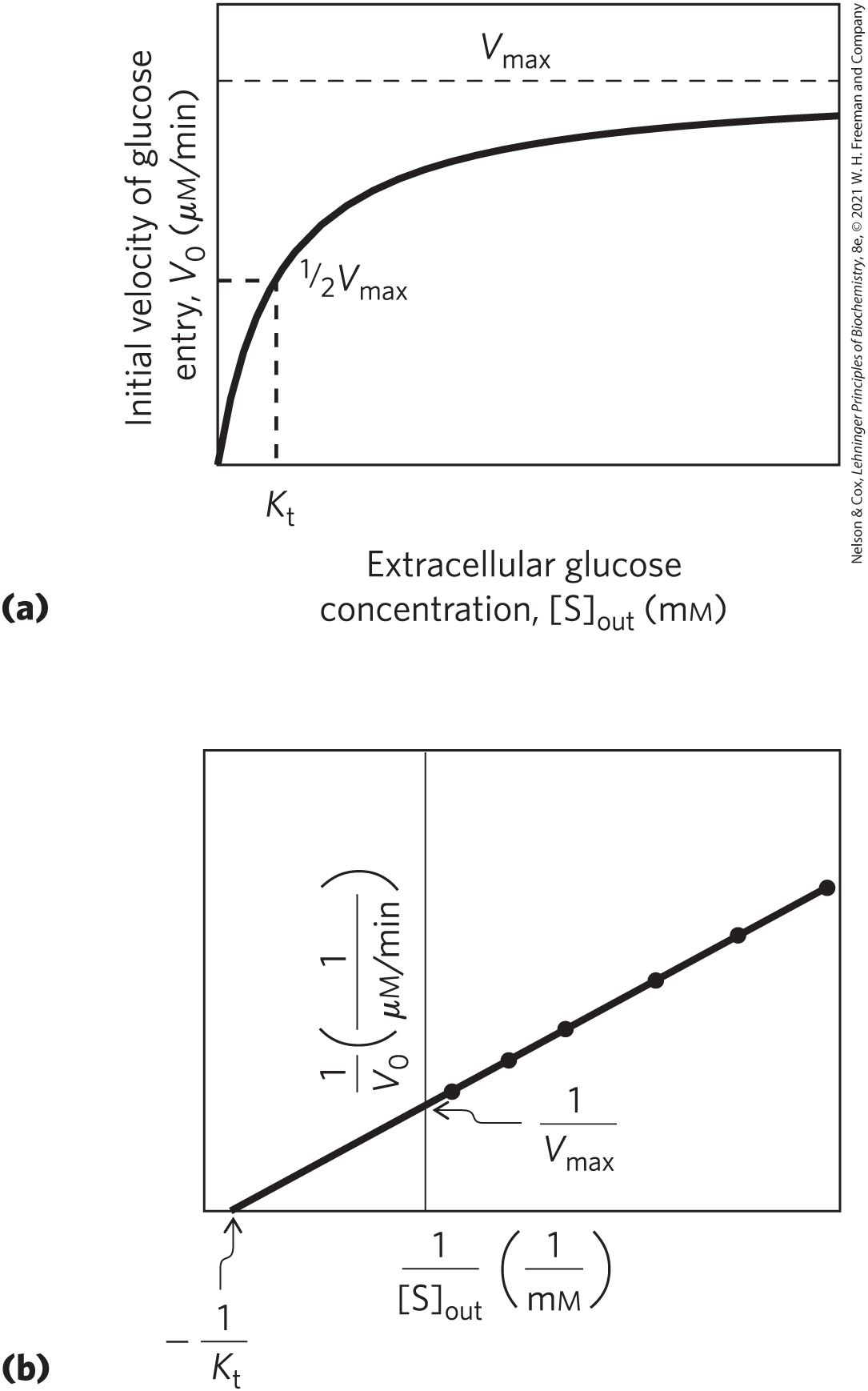
FIGURE 11-31 Kinetics of glucose transport into erythrocytes. (a) The initial rate of glucose entry into an erythrocyte, , depends on the initial concentration of glucose on the outside, . (b) Double-reciprocal plot of the data in (a). The kinetics of passive transport is analogous to the kinetics of an enzyme-catalyzed reaction. (Compare these plots with Fig. 6-12 and 6-14.) is analogous to , the Michaelis constant.
The rate equations for glucose transport can be derived exactly as for enzyme-catalyzed reactions (Chapter 6), yielding an expression analogous to the Michaelis-Menten equation
(11-1)
in which is the initial velocity of accumulation of glucose inside the cell when its concentration in the surrounding medium is , and is a constant analogous to the Michaelis constant, a combination of rate constants that is characteristic of each transport system. This equation describes the initial velocity, the rate observed when . As is the case for enzyme-catalyzed reactions, the slope-intercept form of the equation describes a linear plot of against , from which we can obtain values of and (Fig. 11-31b). When , the rate of uptake is ; the transport process is half-saturated. The concentration of glucose in blood, as noted above, is 4.5 to 5 mm, which is close enough to the to ensure that GLUT1 is half-saturated with substrate and operates near one-half .
Because no chemical bonds are made or broken in the conversion of to , neither “substrate” nor “product” is intrinsically more stable, and the process of entry is therefore fully reversible. As approaches , the rates of entry and exit become equal. Such a system is therefore incapable of accumulating glucose within a cell at concentrations above that in the surrounding medium; it simply equilibrates glucose on the two sides of the membrane much faster than would occur in the absence of a specific transporter. GLUT1 is specific for d-glucose, with a of about 6 mm. For the close analogs d-mannose and d-galactose, which differ only in the position of one hydroxyl group, the values of are 20 mm and 30 mm, respectively, and for l-glucose, exceeds 3,000 mm. Thus, GLUT1 shows the three hallmarks of passive transport: high rates of diffusion down a concentration gradient, saturability, and stereospecificity.
GLUT1 is an integral protein with 12 hydrophobic segments, each forming a membrane-spanning helix (Fig. 11-32a). The helices that line the transmembrane path for glucose are amphipathic; for each helix, the residues along one side are predominantly nonpolar, and those on the other side are mainly polar. This amphipathic structure is evident in a helical wheel diagram (Fig. 11-32b). A cluster of amphipathic helices are arranged so that their polar sides face each other and line a hydrophilic pore through which glucose can pass (Fig. 11-32c), while their hydrophobic sides interact with the surrounding membrane lipids such that the hydrophobic effect stabilizes the entire transporter structure.
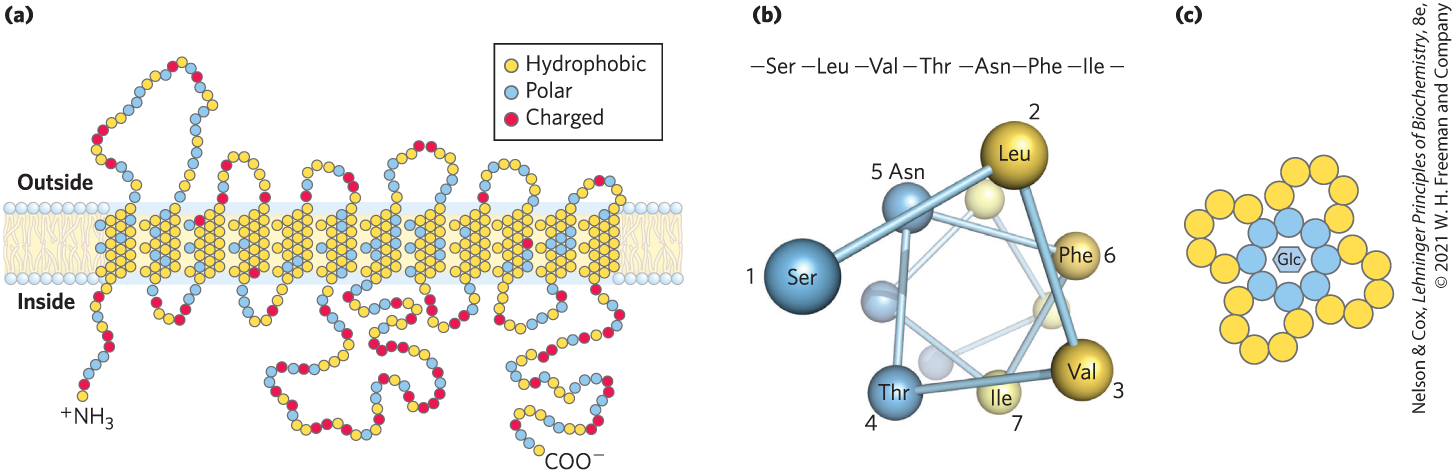
FIGURE 11-32 Membrane topology of the glucose transporter GLUT1. (a) Transmembrane helices are represented here as oblique (angled) rows of three or four amino acid residues, each row depicting one turn of the α helix. Of the 12 helices, 9 contain three or more polar or charged residues (blue or red), often separated by several hydrophobic residues (yellow). (b) A helical wheel diagram shows the distribution of polar and nonpolar residues on the surface of a helical segment. The helix is diagrammed as though observed along its axis from the amino terminus. Adjacent residues in the linear sequence are connected, and each residue is placed around the wheel in the position it occupies in the helix; recall that 3.6 residues are required to make one complete turn of the α helix. In this example, the polar residues (blue) are on one side of the helix, and the hydrophobic residues (yellow) are on the other. This is, by definition, an amphipathic helix. (c) Side-by-side association of amphipathic helices, each with its polar face oriented toward the central cavity, produces a transmembrane channel lined with polar (and charged) residues, available for interaction with glucose. [Information from (a, c) M. Mueckler, Eur. J. Biochem. 219:713, 1994.]
Structural studies of mammalian GLUT1 and other GLUT transporters suggest that the protein cycles through a series of conformational changes, interconverting a form with its glucose-binding site accessible only from the extracellular side, through a form in which the bound glucose is sequestered and inaccessible from either side, to a form with the glucose-binding site open only to the intracellular side (Fig. 11-33).
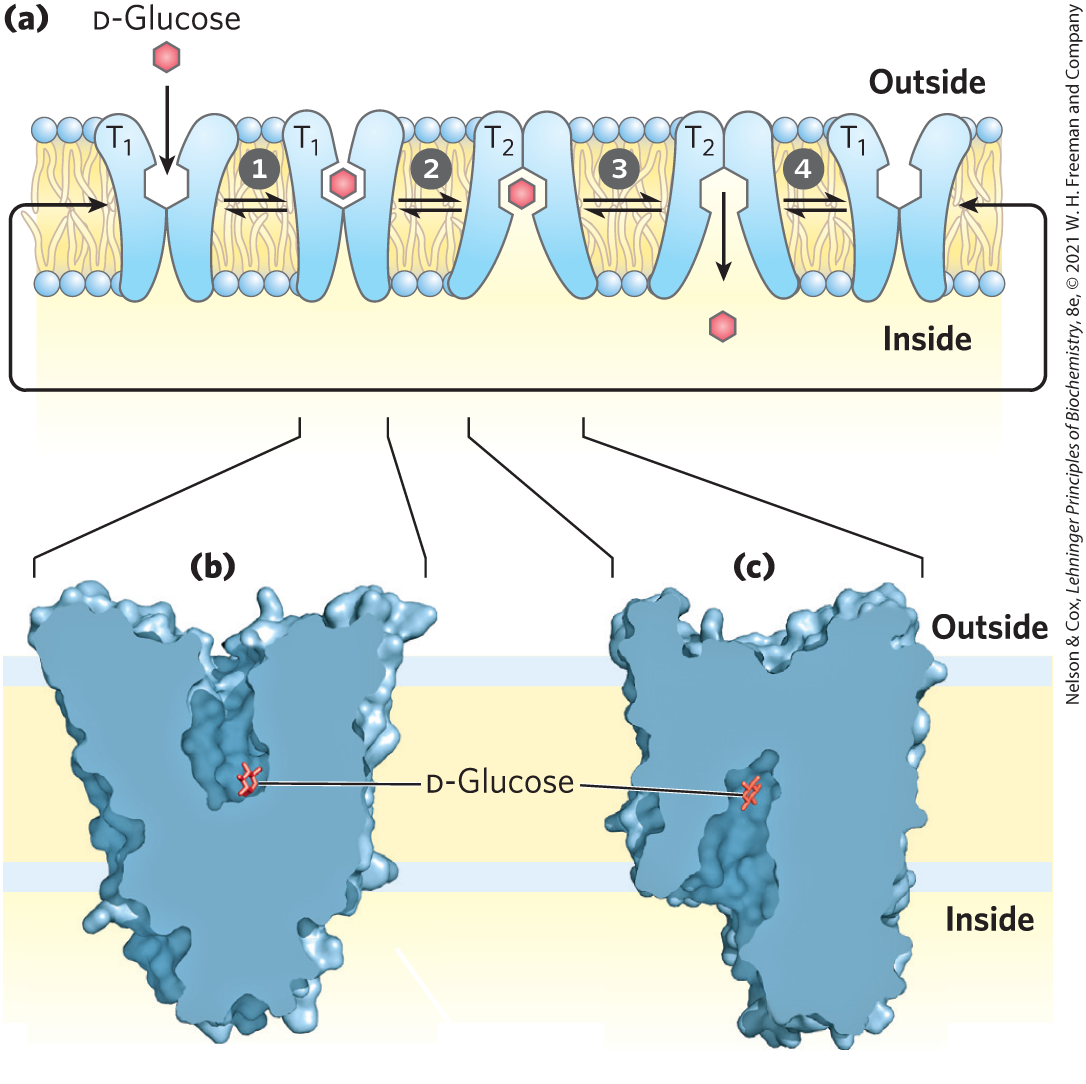
FIGURE 11-33 Model of glucose transport into erythrocytes by GLUT1. (a) The transporter exists in two extreme conformations: , with the glucose-binding site exposed on the outer surface of the plasma membrane, and , with the binding site exposed on the inner surface. Glucose transport occurs in four steps. Glucose in blood plasma binds to a stereospecific site on ; this lowers the activation energy for a conformational change from • to • , effecting transmembrane passage of the glucose. Glucose is released from into the cytoplasm, and the transporter returns to the conformation, ready to transport another glucose molecule. Between the forms and , there is an intermediate form (not shown here) in which glucose is sequestered within the transporter, with access to neither side. The structures of (b) human GLUT3 in the conformation and (c) human GLUT1 in the conformation, determined by x-ray crystallography, support the model shown in (a). [Data from (b) PDB ID 4ZWC, D. Deng et al., Nature 526:391, 2015; (c) PDB ID 4PYP, D. Deng et al., Nature 510:121, 2014.]
Twelve passive glucose transporters are encoded in the human genome, each with its unique kinetic properties, patterns of tissue distribution, and function (Table 11-1). GLUT1, in addition to supplying glucose to erythrocytes, also transports glucose across the blood-brain barrier, supplying the glucose that is essential for normal brain metabolism. The very rare individuals with defects in GLUT1 have a variety of brain-related symptoms, including seizures, movement and language disorders, and developmental delays. Standard care for such individuals includes a ketogenic diet, which provides the ketones that can serve as an alternative energy source for the brain. In the liver, GLUT2 transports glucose out of hepatocytes when liver glycogen is broken down to replenish blood glucose. GLUT2 has a large and can therefore respond to increased levels of intracellular glucose (produced by glycogen breakdown) by increasing outward transport. Skeletal and heart muscle and adipose tissue have yet another glucose transporter, GLUT4 , which is distinguished by its response to insulin: its activity increases when insulin signals a high blood glucose concentration, thus increasing the rate of glucose uptake into muscle and adipose tissue. Box 11-1 describes the effect of insulin on this transporter.
| Transporter | Tissue(s) where expressed | Role/characteristicsa | |
|---|---|---|---|
| GLUT1 | Erythrocytes, blood-brain barrier, placenta, most tissues at a low level | 3 | Basal glucose uptake; defective in De Vivo disease |
| GLUT2 | Liver, pancreatic islets, intestine, kidney | 17 | In liver and kidney, removal of excess glucose from blood; in pancreas, regulation of insulin release |
| GLUT3 | Brain (neuron), testis (sperm) | 1.4 | Basal glucose uptake; high turnover number |
| GLUT4 | Muscle, fat, heart | 5 | Activity increased by insulin |
| GLUT5 | Intestine (primarily), testis, kidney | 6b | Primarily fructose transport |
| GLUT6 | Spleen, leukocytes, brain | > 5 | Possibly no transporter function |
| GLUT7 | Small intestine, colon, testis, prostate | 0.3 | — |
| GLUT8 | Testis, sperm acrosome | ~2 | — |
| GLUT9 | Liver, kidney, intestine, lung, placenta | 0.6 | Urate and glucose transporter in liver, kidney |
| GLUT10 | Heart, lung, brain, liver, muscle, pancreas, placenta, kidney | 0.3c | Glucose and galactose transporter |
| GLUT11 | Heart, skeletal muscle | 0.16 | Glucose and fructose transporter |
| GLUT12 | Skeletal muscle, heart, prostate, placenta | — | — |
|
Information on localization from M. Mueckler and B. Thorens, Mol. Aspects Med. 34:121, 2013. values for glucose from R. Augustin, IUBMB Life 62:315, 2010. aDash indicates role uncertain. b for fructose. c for 2-deoxyglucose. |
|||
The Chloride-Bicarbonate Exchanger Catalyzes Electroneutral Cotransport of Anions across the Plasma Membrane
The erythrocyte contains another passive transport system, an anion exchanger that is essential in transport to the lungs from tissues such as skeletal muscle and liver. Waste released from respiring tissues into the blood plasma enters the erythrocyte, where it is converted to bicarbonate by the enzyme carbonic anhydrase. (Recall that is the primary buffer of blood pH; see Fig. 2-20.) The reenters the blood plasma for transport to the lungs (Fig. 11-34). Because is much more soluble in blood plasma than is , this roundabout route increases the capacity of the blood to carry carbon dioxide from the tissues to the lungs. In the lungs, reenters the erythrocyte and is converted to , which is eventually released into the lung space and exhaled. To be effective, this shuttle requires very rapid movement of across the erythrocyte membrane. As described in Chapter 5 (pp. 160–161), there is a second mechanism for moving from tissue to lung, involving reversible binding of to hemoglobin.)
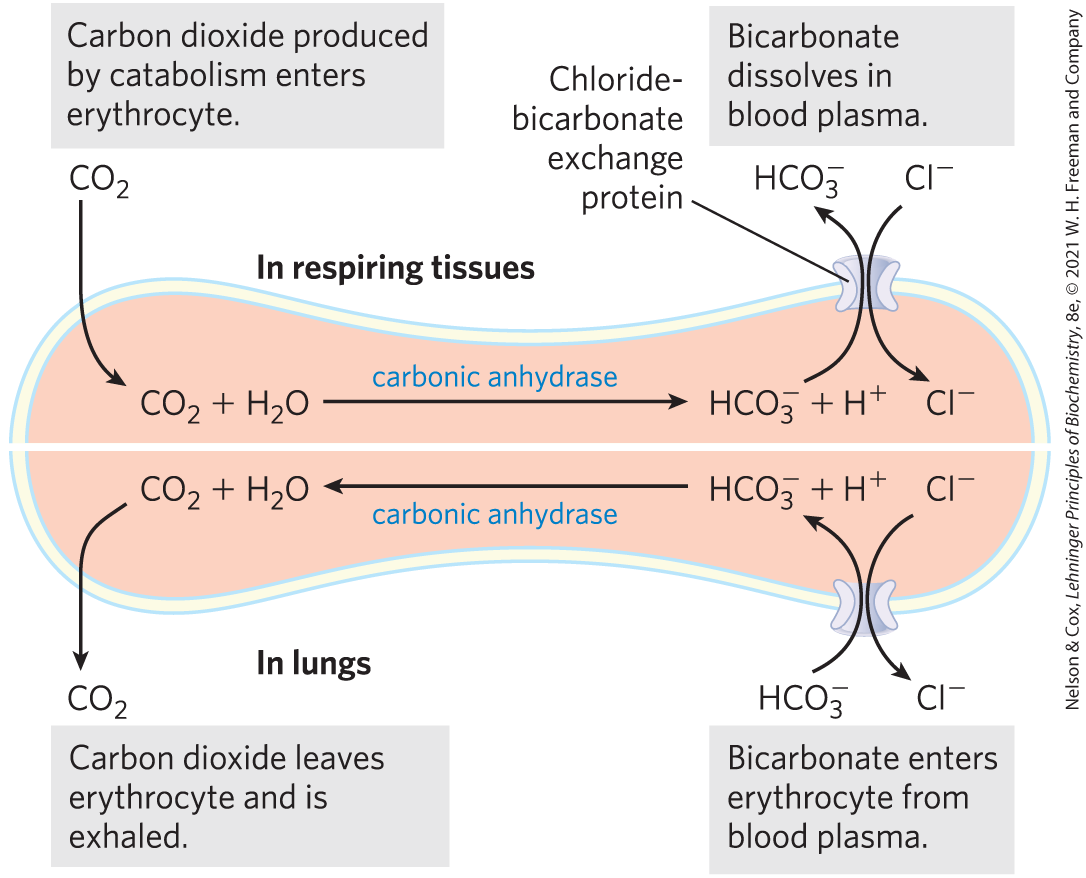
FIGURE 11-34 Chloride-bicarbonate exchanger of the erythrocyte membrane. This cotransport system allows the entry and exit of without changing the membrane potential. Its role is to increase the -carrying capacity of the blood. The top half of the figure illustrates the events that take place in respiring tissues; the bottom half illustrates the events in the lungs.
An erythrocyte is shown from the side as a thin cell that is wider on the left and right sides. It is divided in middle by a horizontal line. The top half is labeled in respiring tissues and the bottom half is labeled in lungs. C O 2 is shown entering the cell accompanied by text reading, Carbon dioxide produced by metabolism enters erythrocyte. Within the cell, C O 2 plus H 2 O is shown next to an arrow pointing right below carbonic anhydrase to show that the reaction yields H C O 3 minus plus H plus. C l minus is shown nearby. A transporter labeled chloride-bicarbonate exchange protein is shown in the membrane above these products. Arrows show that H C O 3 minus leaves the cell and C l minus enters the cell. Text above H C O 3 minus outside of the cell, reads, bicarbonate dissolves in blood plasma. A similar reaction is shown in the bottom half of the cell except that the arrow points to the left above carbonic anhydrase. An arrow shows that C O 2 on the left side of this equation moves across the membrane and into the lungs, where accompanying text reads, carbon dioxide leaves erythrocyte and is exhaled. A transporter is shown beneath the reactants on the right. Arrows show that H C O 3 minus enters the cell and C l minus leaves the cell through this transporter. Accompanying text reads, bicarbonate enters erythrocyte from blood plasma.
The chloride-bicarbonate exchanger, also called the anion exchange (AE) protein, increases the rate of transport across the erythrocyte membrane more than a millionfold. Like the glucose transporter, it is a dimeric integral protein that spans the membrane 14 times. This protein mediates the simultaneous movement of two anions: for every ion that moves in one direction, one ion moves in the opposite direction, with no net transfer of charge: the exchange is electroneutral. The coupling of and movements is obligatory; in the absence of chloride, bicarbonate transport stops. In this respect, the anion exchanger is typical of those systems, called cotransport systems, that simultaneously carry two solutes across a membrane (Fig. 11-35). When, as in this case, the two substrates move in opposite directions, the process is antiport. In symport, two substrates are moved simultaneously in the same direction. Transporters that carry only one substrate, such as the erythrocyte glucose transporter, are known as uniport systems.
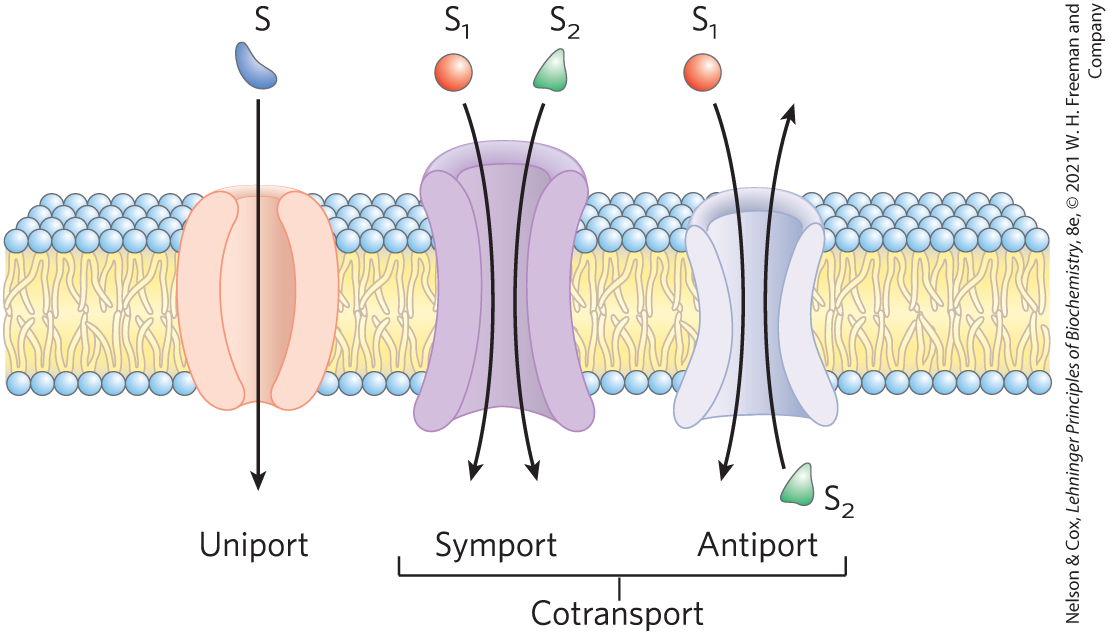
FIGURE 11-35 Three general classes of transport systems. Transporters differ in the number of solutes (substrates) transported and the direction in which each solute moves. Examples of all three types of transporter are discussed in the text. Note that this classification tells us nothing about whether these are energy-requiring (active transport) or energy-independent (passive transport) processes.
The light red transporter labeled uniport has crescent-shape sides that are closer together at the top and bottom, producing an oval channel. A crescent-shaped blue molecule labeled S is shown above and an arrow points down from it through the channel. The transporters labeled symport and antiport are joined by a bracket labeled cotransport. Both have crescent-shaped sides that are closer together in the center and curve apart at the top and bottom of the channel. The dark purple symport channel is larger. A red sphere labeled S subscript 1 and a green irregular triangle labeled S subscript 2 are shown above the symport channel. Arrows point from the red sphere and green triangle through the channel. The light purple antiport channel has a similar red sphere labeled S subscript 1 above the channel with an arrow pointing down through the channel and a green triangle below the channel with an arrow pointing up through the channel.
Active Transport Results in Solute Movement against a Concentration or Electrochemical Gradient
In passive transport, the transported species always moves down its electrochemical gradient and is not accumulated above the equilibrium concentration. Active transport, by contrast, results in the accumulation of a solute above the equilibrium point. Active transport is essential when cells function in an environment in which key substrates are present outside the cell only at very low concentrations. For example, the bacterium E. coli can grow in a medium containing only 1 μm (inorganic phosphate), but the cell must maintain internal levels in the millimolar range. Worked Example 11-2 describes another such situation, which requires cells to pump outward across the plasma membrane. Active transport is thermodynamically unfavorable (endergonic) and takes place only when coupled, directly or indirectly, to an exergonic process such as the absorption of sunlight, an oxidation reaction, the breakdown of ATP, or the concomitant flow of some other chemical species down its electrochemical gradient. In primary active transport, solute accumulation is coupled directly to an exergonic chemical reaction, such as conversion of ATP to (Fig. 11-36). Secondary active transport occurs when endergonic (uphill) transport of one solute is coupled to the exergonic (downhill) flow of a different solute that was originally pumped uphill by primary active transport.
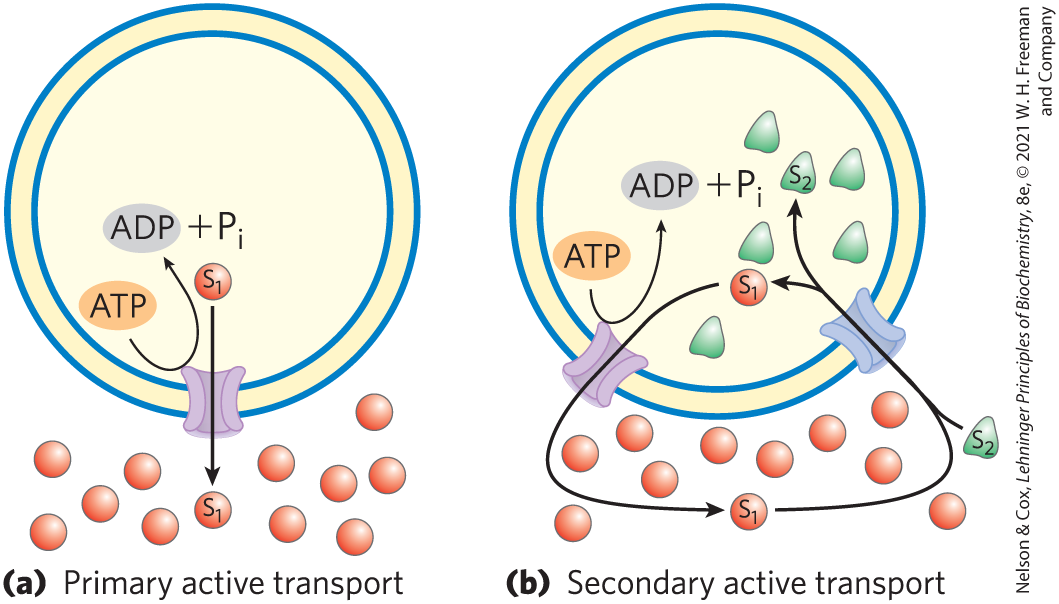
FIGURE 11-36 Two types of active transport. (a) In primary active transport, the energy released by ATP hydrolysis drives solute movement against an electrochemical gradient. (b) In secondary active transport, a gradient of an ion (designated ; often ) has been established by primary active transport. Movement of down its electrochemical gradient now provides the energy to drive cotransport of a second solute, , against its electrochemical gradient.
Both parts show a circular cell. Part a is labeled primary active transport. The cell has a single transporter at the bottom of the cell. A light red sphere labeled S subscript 1 is inside the cell with an arrow pointing through the transporter to a similar light red sphere below the cell surrounded by 11 similar light red spheres. An arrow from A T P curves across the upper left of the transporter inside the cell to produce A D P plus P subscript i. Part b is labeled secondary active transporters. Two transporters are present, one at the lower left and one at the lower right. A light red sphere labeled S subscript 1 within the cell has an arrow pointing through the transporter to the lower left, which resembles the transporter in part a, and circling around to S subscript 1 below the center of the cell and surrounded by 10 similar light red spheres. An arrow from A T P curves across the upper left of the transporter inside the cell to produce A D P plus P subscript i. A small roughly triangular green molecule labeled S subscript 2 to the lower right of the cell has an arrow pointing through the second transporter to a similar molecule inside of the cell. There are five additional similar green molecules inside of the cell and none outside of the cell.
The amount of energy needed for the transport of a solute against a gradient can be calculated from the initial concentration gradient. The general equation for the free-energy change in the chemical process that converts substrate (S) to product (P) is
(11-2)
where is the standard free-energy change, R is the gas constant (8.315 J/mol • K), and T is the absolute temperature. When the “reaction” is simply transport of a solute from a region where its concentration is to a region where its concentration is , no bonds are made or broken and is zero. The free-energy change for transport, , is then
(11-3)
If there is, say, a 10-fold difference in concentration between two compartments, the cost of moving 1 mol of an uncharged solute at 25 °C uphill across a membrane separating the compartments is
Equation 11-3 holds for all uncharged solutes.
WORKED EXAMPLE 11-1 Energy Cost of Pumping an Uncharged Solute
Calculate the energy cost (free-energy change) of pumping an uncharged solute against a -fold concentration gradient at 25 °C.
SOLUTION:
Begin with Equation 11-3. Substitute for (), 8.315 J/mol • K for R, and 298 K for T:
When the solute is an ion, its movement without an accompanying counterion results in the endergonic separation of positive and negative charges, producing an electrical potential; such a transport process is said to be electrogenic. The energetic cost of moving an ion depends on the electrochemical potential (Fig. 11-25), the sum of the chemical and electrical gradients:
(11-4)
where Z is the charge on the ion, F is the Faraday constant (96,480 J/V • mol), and Δψ is the transmembrane electrical potential (in volts). Eukaryotic cells typically have plasma membrane potentials of about 0.05 V (with the inside negative relative to the outside), so the second term on the right side of Equation 11-4 can make a significant contribution to the total free-energy change for transporting an ion. Most cells maintain more than a 10-fold difference in ion concentrations across their plasma or intracellular membranes, and for many cells and tissues active transport is therefore a major energy-consuming process.
WORKED EXAMPLE 11-2 Energy Cost of Pumping a Charged Solute
Calculate the energy cost (free-energy change) of pumping from the cytosol, where its concentration is about m, to the extracellular fluid, where its concentration is about 1.0 mm. Assume a temperature of 37 °C (body temperature in a mammal) and a standard transmembrane potential of 50 mV (inside negative) for the plasma membrane.
SOLUTION:
This is a case in which energy must be expended to counter two forces acting on the ion being transported: the membrane potential and the concentration difference across the membrane. These forces are expressed in the two terms on the right side of Equation 11-4:
in which the first term describes the chemical gradient and the second describes the electrical potential.
In Equation 11-4, substitute 8.315 J/mol • K for R, 310 K for T, for , for (the charge on a ion) for Z, 96,500 J/V • mol for F, and 0.050 V for Δψ. Note that the transmembrane potential is 50 mV (inside negative), so the change in potential when an ion moves from inside to outside is 50 mV.
The mechanism of active transport is of fundamental importance in biology. As we shall see in Chapters 19 and 20, ATP is formed in mitochondria and chloroplasts by a mechanism that is essentially ATP-driven ion transport operating in reverse. The energy made available by the spontaneous flow of protons across a membrane is calculable from Equation 11-4; remember that ΔG for flow down an electrochemical gradient has a negative value, and ΔG for transport of ions against an electrochemical gradient has a positive value.
P-Type ATPases Undergo Phosphorylation during Their Catalytic Cycles
The family of active transporters called P-type ATPases are cation transporters that are reversibly phosphorylated by ATP (thus the name P-type) as part of the transport cycle. Phosphorylation forces a conformational change that is central to movement of the cation across the membrane. The human genome encodes at least 70 P-type ATPases that share similarities in amino acid sequence and topology, especially near the Asp residue that undergoes phosphorylation. All are integral proteins with 8 or 10 predicted membrane-spanning regions in a single polypeptide, and all are sensitive to inhibition by the transition-state analog vanadate, which mimics phosphate when under nucleophilic attack by a water molecule.
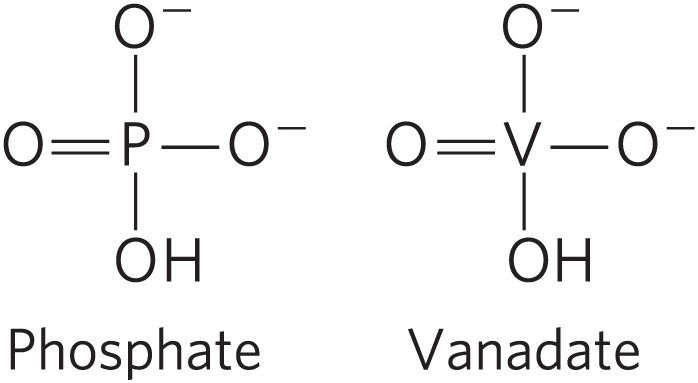
The P-type ATPases are widespread in eukaryotes and bacteria. The ATPase of animal cells (an antiporter for and ions) and the plasma membrane ATPase of plants and fungi set the transmembrane electrochemical potential in cells by establishing ion gradients across the plasma membrane. These gradients provide the driving force for secondary active transport and are also the basis for electrical signaling in neurons. In animal tissues, the sarcoplasmic/endoplasmic reticulum ATPase (SERCA) pump and the plasma membrane ATPase pump, which are uniporters for ions, together maintain the cytosolic level of below 1 µm. The SERCA pump moves from the cytosol into the lumen of the sarcoplasmic reticulum. Parietal cells in the lining of the mammalian stomach have a P-type ATPase that pumps and out of the cells and into the stomach, thereby acidifying the stomach contents. Lipid flippases, as we noted earlier, are structurally and functionally related to P-type transporters. Bacteria and eukaryotes use P-type ATPases to pump toxic heavy metal ions such as and out of cells.
All P-type pumps have similar structures (Fig. 11-37) and similar mechanisms. The mechanism postulated for P-type ATPases takes into account the large conformational changes and the phosphorylation-dephosphorylation of the critical Asp residue in the P (phosphorylation) domain that is known to occur during a catalytic cycle. For the SERCA pump (Fig. 11-38), each catalytic cycle moves two ions across the membrane and converts an ATP to ADP and . The role of ATP binding and phosphoryl transfer to the enzyme is to bring about the interconversion of two conformations, E1 and E2, of the transporter. In the E1 conformation, the two -binding sites are exposed on the cytosolic side of the ER or sarcoplasmic reticulum and bind with high affinity. ATP binding and Asp phosphorylation drive a conformational change from E1 to E2 that exposes the -binding sites to the lumen and greatly reduces their affinity for , which releases the ions into the lumen. By this mechanism, the energy released by hydrolysis of ATP during one phosphorylation-dephosphorylation cycle drives across the membrane against a large electrochemical gradient.
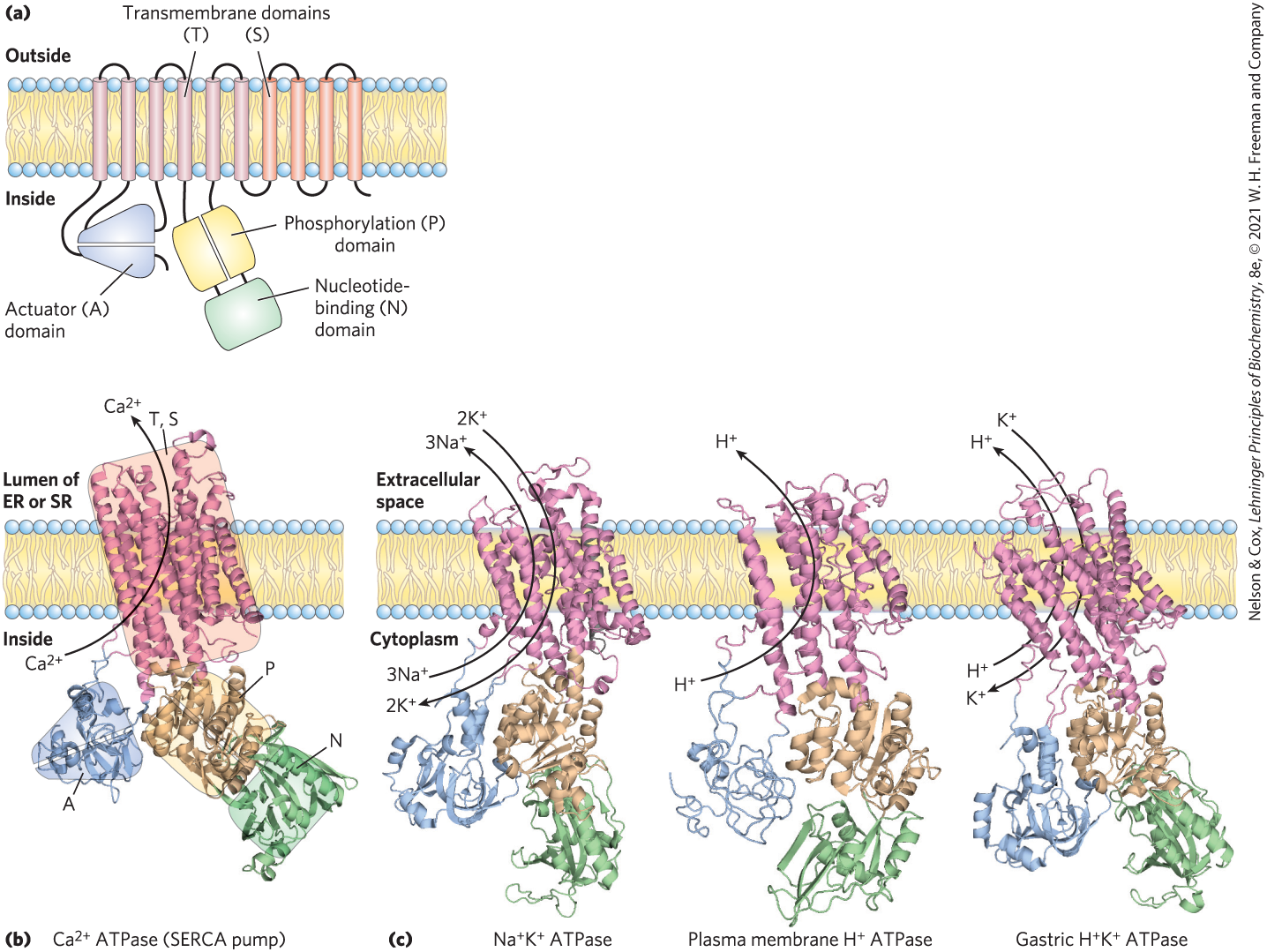
FIGURE 11-37 The general structure of the P-type ATPases. (a) P-type ATPases have three cytoplasmic domains (A, N, and P) and two transmembrane domains (T and S) consisting of multiple helices. The N (nucleotide-binding) domain binds ATP and and has protein kinase activity that phosphorylates a specific Asp residue in the P (phosphorylation) domain of all P-type ATPases. The A (actuator) domain has protein phosphatase activity and removes the phosphoryl group from the Asp residue with each catalytic cycle of the pump. A transport (T) domain with six transmembrane helices includes the ion-transporting structure, and four more transmembrane helices make up the support (S) domain, which provides physical support to the transport domain and may have other specialized functions in certain P-type ATPases. The binding sites for the ions to be transported are near the middle of the membrane, 40 to 50 Å from the phosphorylated Asp residue — thus, Asp phosphorylation-dephosphorylation does not directly affect ion binding. The A domain communicates movements of the N and P domains to the ion-binding sites. (b) A ribbon representation of the ATPase (SERCA pump). ATP binds to the N domain, and the ions to be transported bind to the T domain. (c) Other P-type ATPases have domain structures, and presumably mechanisms, like those of the SERCA pump; shown here are ATPase, the plasma membrane ATPase, and the gastric ATPase. [(a) Information from M. Bublitz et al., Curr. Opin. Struct. Biol. 20:431, 2010, Fig. 1. Data from (b) PDB ID 1SU4, C. Toyoshima et al., Nature 405:647, 2000; (c) ATPase, PDB ID 3KDP, J. Preben Morth et al., Nature 450:1043, 2007; ATPase, PDB ID 3B8C, B. P. Pedersen et al., Nature 450:1111, 2007; ATPase, PDB ID 3IXZ, K. Abe et al., EMBO J. 28:1637, 2009, and PDB ID 3B8E, J. Preben Morth et al., Nature 450:1043, 2007.]
Part a shows a horizontal piece of membrane with the area above labeled outside and the area below labeled inside. A light blue triangular structure labeled actuator open parenthesis A close parenthesis domain has two halves and points toward the left. A short thread runs into the lower right of this structure. A thread runs from the lower left of this structure to a vertical pink cylinder in the membrane above. Six pink cylinders are labeled open parenthesis T close parenthesis transmembrane domain. A thread emerges from the top of the first cylinder and enters the top of the second cylinder, from which a thread emerges from the bottom to join with the upper left side of the A domain. A thread emerges from the upper right side of the A domain and runs up to the bottom of the third pink cylinder, from which a thread runs to the top of the fourth pink cylinder. The fourth cylinder has a thread down to the upper left of a rectangular yellow structure with two halves labeled phosphorylation open parenthesis P close parenthesis domain. A thread extends from the lower left of the P domain into the top left of a green rectangle labeled nucleotide-binding open parenthesis N close parenthesis domain. A second thread emerges from the upper right of the N domain and enters the lower right of the P domain. Another thread emerges from the upper right of the P domain and runs to the bottom of the fifth cylinder. A thread runs from the top of the fifth cylinder to the top of the sixth cylinder. A thread from the bottom of the sixth cylinder enters the bottom of an orange vertical cylinder that is the first of four vertical cylinders on the right side of the membrane labeled transmembrane domain open parenthesis S close parenthesis. The first orange cylinder has a thread connecting it to the top of the second orange cylinder, which has a thread from its bottom to the bottom of the third orange cylinder, which has a thread from its top to the top of the fourth cylinder, which has a short thread at its bottom. Part b is labeled C a 2 plus A T P ase open parenthesis S E R C A close parenthesis pump. It has a horizontal piece of membrane with the area above labeled lumen of E R or S R and the area below labeled as inside. It has a ribbon structure overlaid by shaded shapes from part a to show the locations of the domains. Blue ribbons fit into the two halves of the blue triangle labeled A, which is connected to diagonal helices within an orange diagonal rectangle that crosses the membrane from upper left to lower right. The rectangle is labeled T, S. C a 2 plus below the membrane has a curved arrow through the pink rectangle to C a 2 plus above the membrane. Beneath the rectangle, there are yellow helices in a roughly rectangular structure overlaid with two halves of a yellow rectangle labeled P that joins with green helices that fit into a smaller rectangle labeled N to its lower right. Part c shows three ribbon structures that each cross a horizontal piece of membrane with the area above labeled extracellular space and the region below labeled cytoplasm. All of the structures are similar and resemble the ribbon structure in part a. N a plus K plus A T P ase has 3 N a plus below the membrane that has a curved arrow through the pink rectangle to 3 N a plus above the membrane. K plus above the membrane has a curved arrow through the pink rectangle to 2 K plus inside the cell. The plasma membrane H plus A T P ase has H plus below the membrane with a curved arrow to H plus above the membrane. Gastric H plus K plus A T P ase has a curved arrow from H plus below the membrane to H plus above the membrane and a curved arrow from K plus above the membrane to K plus below the membrane.
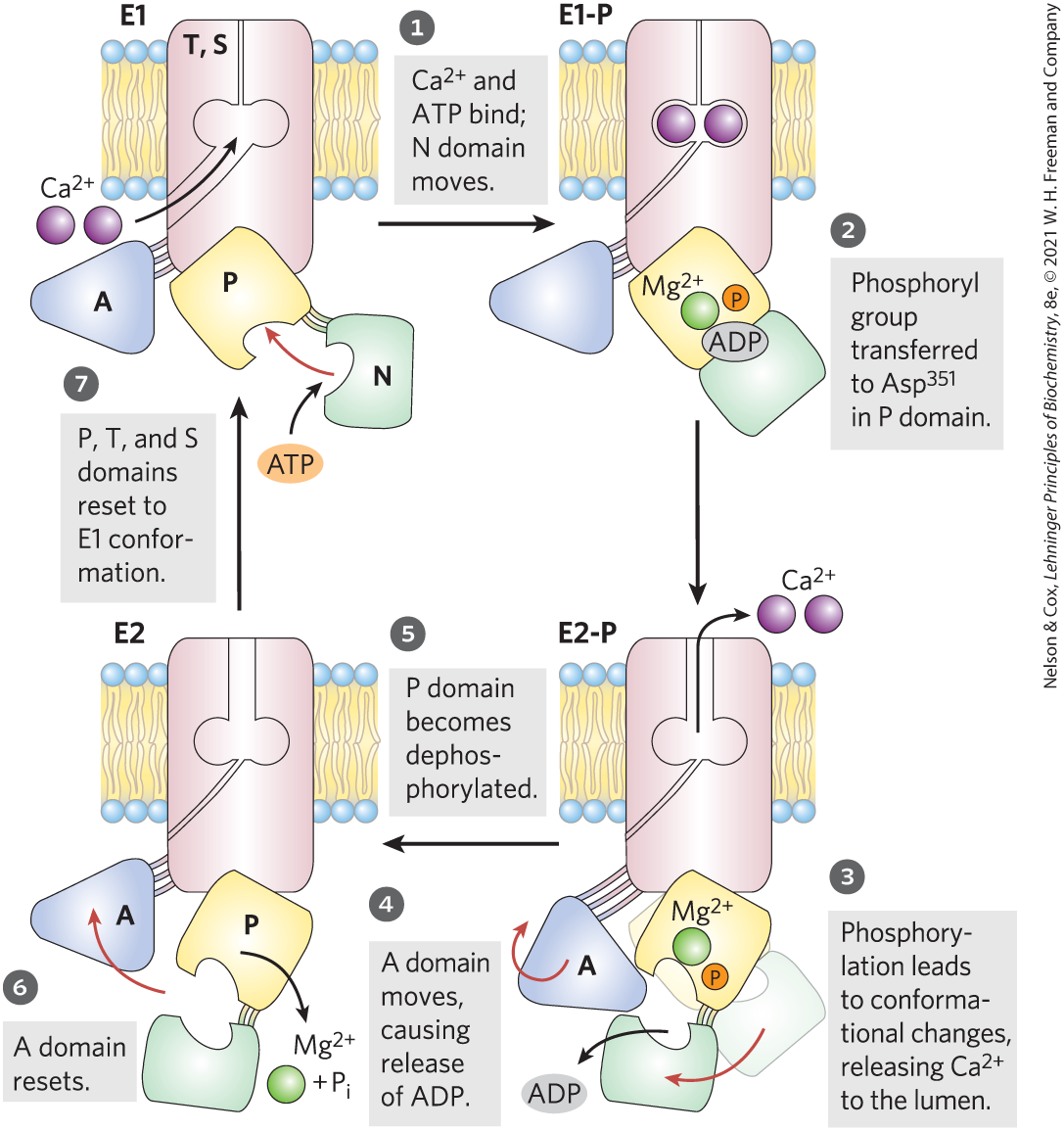
FIGURE 11-38 Postulated mechanism of the SERCA pump. The transport cycle begins with the protein in the E1 conformation, with the -binding sites facing the cytosol. Two ions bind, then ATP binds to the transporter and phosphorylates , forming E1-P. Phosphorylation favors the second conformation, E2-P, in which the -binding sites, now with a reduced affinity for , are accessible on the other side of the membrane (the lumen or extracellular space), and the released diffuses away. ADP is released, and E2-P is dephosphorylated. The A domain resets and the protein returns to the E1 conformation for another round of transport. [Information from W. Kühlbrandt, Nat. Rev. Mol. Cell Biol. 5:282, 2004.]
A horizontal piece of membrane at the upper left contains a transporter labeled E 1. The membrane has a pink vertical rectangle embedded in it labeled T, S. A narrow vertical line runs from the top of the transporter to its center, where it widens to circular regions to the left and right from which a wider channel opens to the lower left. Two purple spheres labeled C a 2 plus are shown near the entrance to this channel below the membrane. A blue triangle labeled A is connected by three strands to the lower left of the pink vertical rectangle, which also has a yellow diamond labeled A overlapping its bottom. The yellow square has a curved opening in its lower right side and two strands around its right vertex connect to the upper left of a green rectangle labeled N that has a similar curved opening on its left side. Step 1: C a 2 plus and A T P bind; N domain moves. The structure is labeled E 1-P. The two purple calcium ions are shown within the circular openings in the pink rectangle and the channel to the lower left has become narrow. The yellow diamond and green rectangle have come together and their curved openings now contain a single gray oval labeled A D P that has a green sphere to its upper left inside the yellow diamond labeled M g 2 plus. A small orange circle labeled P is also present inside the yellow diamond. Step 2: Phosphoryl group transferred to A s p superscript 351 in P domain. An arrow points down to a similar structure labeled E 2 P. An arrow points from the central opening in the pink rectangle to 2 C a 2 plus spheres above the transporter. The yellow diamond now has a curved opening to its lower left with M g 2 plus and P still present near it. Two strands connect it to the green rectangle below, which has its curved opening facing upward. A pale outline of the yellow diamond and green rectangle shows their previous location without A T P with a red arrow pointing from the faded green rectangle to the new green rectangle. An arrow points from the curved opening in the new green rectangle to a gray oval labeled A D P. The blue triangle labeled A is oriented so that it has a single vertex to the right in between the lower left side of the yellow diamond and the upper left side of the green rectangle with the threads connecting it to the pink rectangle in the membrane now connected to its upper left. A red curved arrow shows it moving clockwise. Two steps are shown. Step 3: Phosphorylation leads to conformational changes, releasing C a 2 plus to the lumen. Step 4: A domain moves, causing release of A D P. Step 5 leads to another illustration on the left and reads: P domain becomes dephosphorylated. This yields a similar structure labeled E 2. C a 2 plus is no longer present. The yellow diamond has an arrow pointing right to the green sphere labeled M g 2 plus plus P I nearby. A red arrow points from the region between the lower left of the yellow diamond and the upper left of the green square into A. Step 6: A domain resets. Step 5 leads back to the starting illustration and reads: P, T, and S domains reset to E 1 conformation.
A variation on this basic mechanism is seen in the ATPase of the plasma membrane. This cotransporter couples phosphorylation-dephosphorylation of the critical Asp residue to the simultaneous movement of both and against their electrochemical gradients. The ATPase is responsible for maintaining low and high concentrations in the cell relative to the extracellular fluid (Fig. 11-39). For each molecule of ATP converted to ADP and , the transporter moves two ions inward and three ions outward across the plasma membrane. Cotransport is therefore electrogenic, creating a net separation of charge across the membrane; in animals, this produces the membrane potential of –50 to –70 mV (inside negative relative to outside) that is characteristic of most cells and is essential to the conduction of action potentials in neurons. The central role of the ATPase is reflected in the energy invested in this single reaction: about 25% of the total energy consumption of a human at rest.
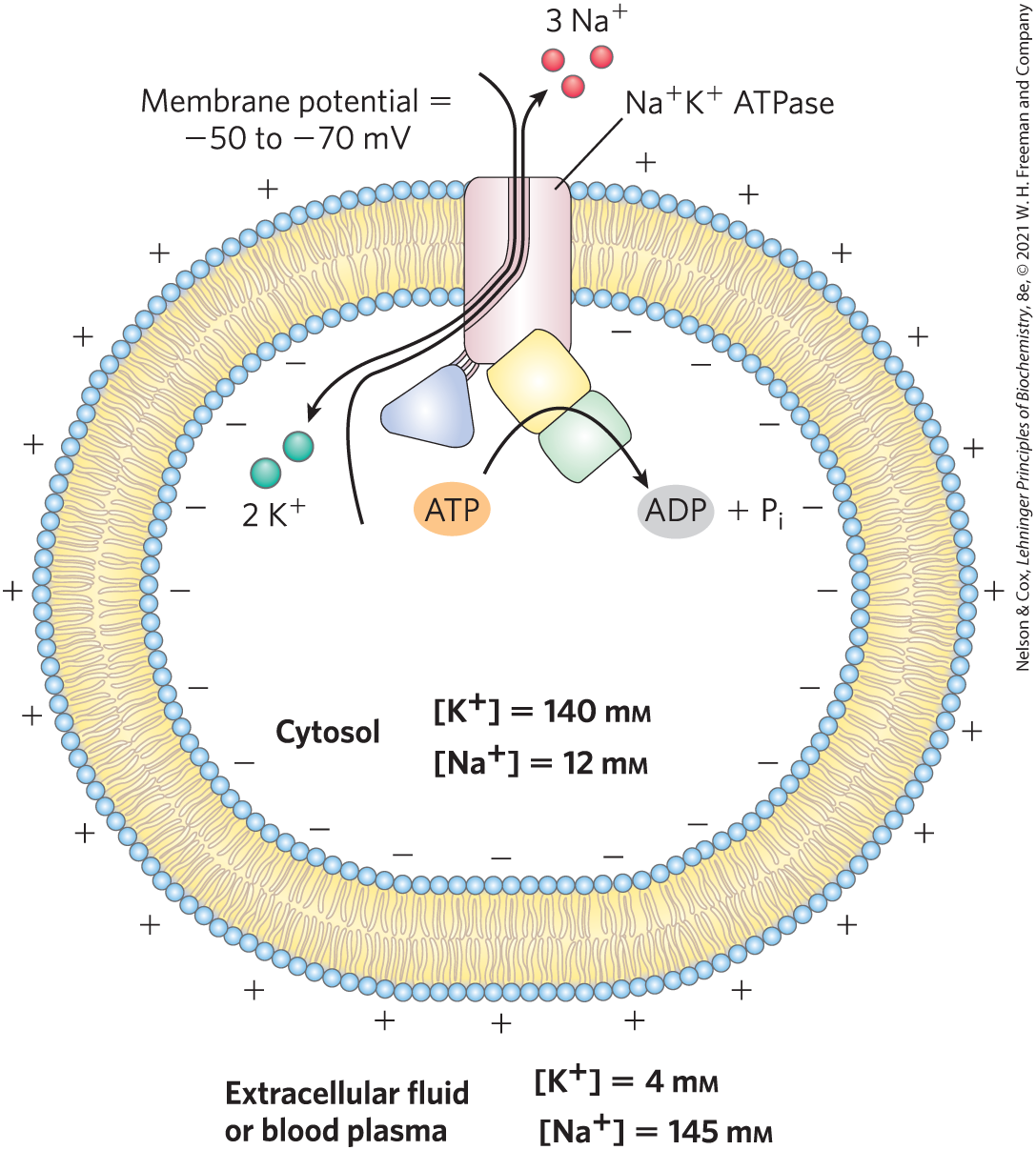
FIGURE 11-39 Role of the ATPase in animal cells. This active transport system is primarily responsible for setting and maintaining the intracellular concentrations of and in animal cells and for generating the membrane potential. It does this by moving three ions out of the cell for every two ions it moves in. The electrical potential across the plasma membrane is central to electrical signaling in neurons, and the gradient of is used to drive the uphill cotransport of solutes in many cell types.
The cell is shown as a circular piece of membrane with an N a plus K plus A T P ase embedded in the top with the interior labeled cytosol and the exterior labeled extracellular fluid or blood plasma. The transporter has a pink vertical rectangle that cross the membrane, a channel that runs vertically from the top to the center and then angles down to open to the lower left, a blue triangle to the lower left of the pink rectangle and connected by three threads, and a yellow diamond overlapping the lower right of the pink rectangle that is adjacent to a green rectangle. An arrow runs from above the cell, through the channel in the pink rectangle, and into the cell to 2 green spheres labeled 2 K plus. An arrow runs from inside the cell, through the channel in the pink rectangle, and out of the cell to three pink spheres labeled 3 N a plus. The outside of the membrane is lined by plus signs and the interior of the membrane is lined by minus signs. Text at the upper left reads, membrane potential equals negative 50 to negative 70 m V. Text inside the cell reads, open bracket K plus close bracket equals 140 m M; open bracket N a plus close bracket equals 12 n M. Text outside the cell reads, open bracket K plus close bracket equals 4 m M; open bracket N a plus close bracket equals 145 n M.
V-Type and F-Type ATPases Are ATP-Driven Proton Pumps
V-type ATPases, a class of proton-transporting ATPases, are responsible for acidifying intracellular compartments in many organisms (thus, V for vacuolar). Proton pumps of this type maintain the vacuoles of fungi and higher plants at a pH between 3 and 6, well below that of the surrounding cytosol (pH 7.5). V-type ATPases are also responsible for the acidification of lysosomes, endosomes, the Golgi complex, and secretory vesicles in animal cells. All V-type ATPases have a similar complex structure, with an integral (transmembrane) domain that serves as a proton channel and a peripheral domain that contains the ATP-binding site and the ATPase activity (Fig. 11-40a). The structure is similar to that of the well-characterized F-type ATPases.
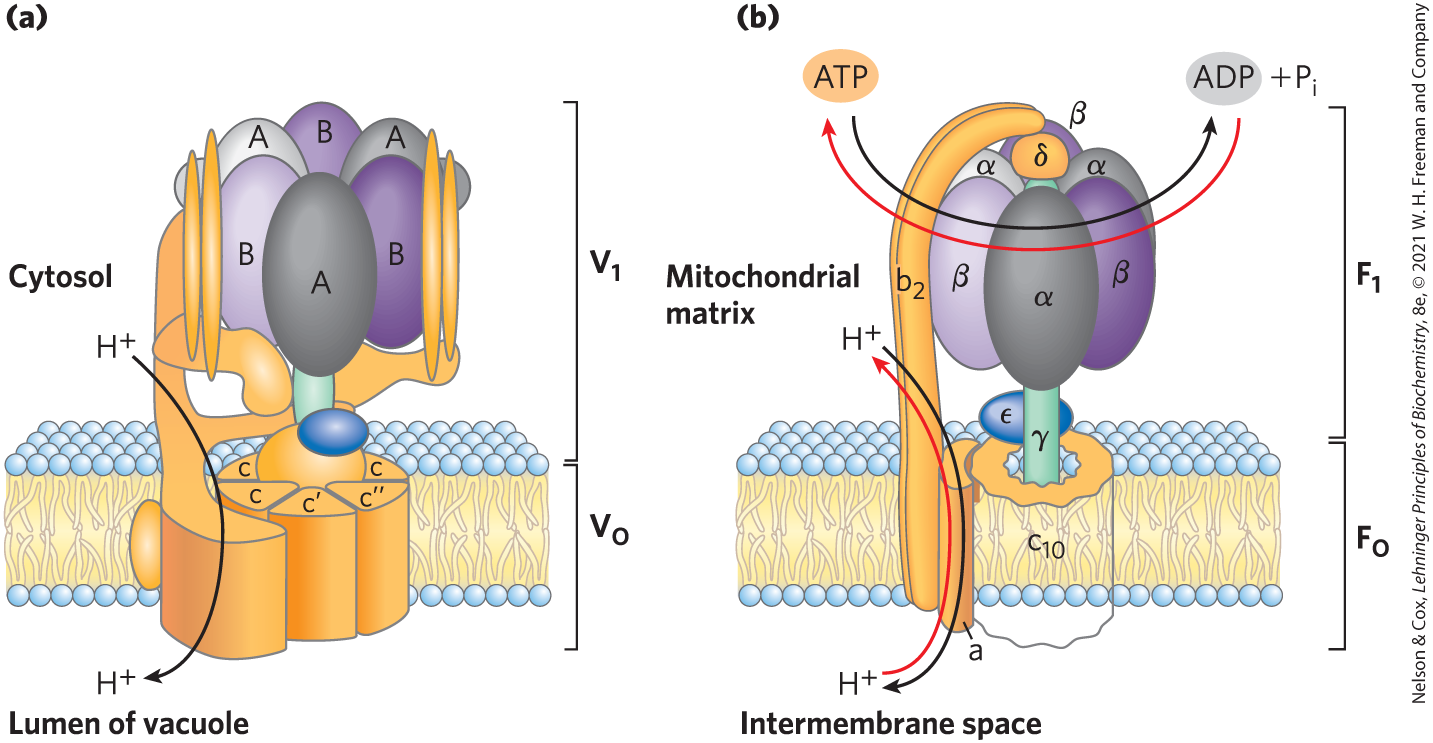
FIGURE 11-40 Two proton pumps with similar structures. (a) The ATPase uses ATP to pump protons into vacuoles and lysosomes, creating their low internal pH. It has an integral (membrane-embedded) domain, , that includes multiple identical c subunits, and a peripheral domain that projects into the cytosol and contains the ATP-hydrolyzing sites, on three identical B subunits (purple). (b) The ATPase/ATP synthase of mitochondria has an integral domain, , with multiple copies of the c subunit, and a peripheral domain, , consisting of three α subunits, three β subunits, and a central shaft joined to the integral domain. and provide transmembrane channels through which protons are pumped as ATP is hydrolyzed on the β subunits of (B subunits of ). An ATP-driven proton transporter also can catalyze ATP synthesis (red arrows) as protons flow down their electrochemical gradient. This is the central reaction in the processes of oxidative phosphorylation and photophosphorylation.
Part a shows a structure embedded in a horizontal piece of membrane with the region above labeled cytosol and the region below labeled lumen of vacuole. A circular disc-shaped structure is made up of triangular cylinders around a spherical center that extends above the membrane. The piece at the six o’clock position is labeled C prime, the piece at the lower right is labeled C two prime, and the other three are labeled C. A crescent-shaped piece to the left has a small oval to its left and curves up two split above the membrane into a vertical piece and a horizontal piece. The vertical piece is overlaid by the left side of a structure that runs horizontally and then bends down to contact the horizontal piece before it runs behind a light blue oval that joins the top of the sphere in the center of the disc-shaped structure. There is a small blue oval to the upper right of this sphere. The vertical piece along the left side has a thin oval to its right next to a longer thin oval that contacts a large vertical light purple oval labeled B that is part of a ring of vertical ovals. Clockwise, these spheres are a light gray A with a small protrusion to its upper left, a purple B, a gray A with a protrusion to the right, a dark purple B with a long thin oval to its right next to a shorter thin oval, and a dark gray A in the front. The two thin ovals to the right of the dark purple B overlap the right end of a horizontal piece that is curved as it runs below the dark purple B oval and that runs behind the vertical light blue oval above the central sphere. An arrow curves from H plus in the cytosol across the vertical arm at the left side of the structure, then down to the left of the disc-shaped structure to H plus in the lumen of the vacuole. The part of the structure above the membrane is labeled V subscript 1 and the part within the membrane is labeled V subscript 0. Part b shows a structure in a horizontal piece of membrane with the area above labeled mitochondrial matrix and the area below labeled intermembrane space. It has a light blue vertical cylinder in the membrane labeled C subscript 10 with a rod labeled Greek letter gamma extending out from it and with a blue oval labeled Greek letter epsilon on its rear top side, behind the light blue vertical cylinder. A crescent shaped piece labeled lowercase A fits against the left side of the cylinder and also runs across the membrane. To the left of this crescent, there are two long strands labeled lowercase B subscript 2 that run through and above the membrane before curving right over the top of the structure, right above a small sphere labeled Greek letter delta above a ring of ovals with the light blue vertical cylinder labeled Greek letter gamma in its center. The light purple oval labeled Greek letter beta is between the strand labeled lowercase B subscript 2 and the central light blue vertical cylinder. Clockwise from this oval, the other ovals are light gray Greek letter alpha, purple Greek letter beta, gray Greek letter alpha, dark purple Greek letter beta, and dark gray Greek letter alpha. The part of the structure above the membrane is labeled V subscript 1 and the part within the membrane is labeled V subscript 0. A T P at the upper left has a black arrow that runs across the strands and ring of ovals to the upper right to A D P plus P I and a red arrow shows the reverse reaction. H plus above the membrane has a black arrow that runs across the lowercase b subscript 2 strands and crescent-shaped piece lowercase a to end below the membrane in the intermembrane space. A red arrow shows the reverse reaction.
F-type ATPase transporters catalyze the uphill transmembrane passage of protons, driven by ATP hydrolysis. The “F-type” designation derives from the identification of these ATPases as energy-coupling factors. The integral membrane protein complex (Fig. 11-40b; subscript “o” denotes its inhibition by the drug oligomycin) provides a transmembrane pathway for protons, and the peripheral protein (subscript “1” indicating that this was the first of several factors isolated from mitochondria) uses the energy of ATP to drive protons uphill (into a region of higher concentration). The organization of proton-pumping transporters must have developed very early in evolution. Bacteria such as E. coli use an ATPase complex in their plasma membrane to pump protons outward, and archaea have a closely homologous proton pump, the ATPase.
Like all enzymes, F-type ATPases catalyze their reactions in both directions. Therefore, a sufficiently large proton gradient can supply the energy to drive the reverse reaction, ATP synthesis (Fig. 11-40b). When functioning in this direction, the F-type ATPases are more appropriately named ATP synthases. ATP synthases are central to ATP production in mitochondria during oxidative phosphorylation and in chloroplasts during photophosphorylation, as well as in bacteria and archaea. The proton gradient needed to drive ATP synthesis is produced by other types of proton pumps powered by substrate oxidation or sunlight. We provide a detailed description of these processes in Chapters 19 and 20.
ABC Transporters Use ATP to Drive the Active Transport of a Wide Variety of Substrates
ABC transporters constitute a large family of ATP-driven transporters that pump amino acids, peptides, proteins, metal ions, various lipids, bile salts, and many hydrophobic compounds, including drugs, across a membrane against a concentration gradient. Many ABC transporters are located in the plasma membrane, but some are also found in the ER and in the membranes of mitochondria and lysosomes. All members of this family have two ATP-binding domains (“cassettes”) that give the family its name — ATP-binding cassette transporters — and two transmembrane domains, each containing six transmembrane helices. In some cases, all these domains are in a single, long polypeptide; other ABC transporters have two subunits, each contributing a nucleotide-binding domain (NBD) and a domain with six transmembrane helices. The transport mechanism is believed to involve two forms of the transporter, one with its substrate-binding site facing the outside of the cell, the other open to substrate on the inside (Fig. 11-41). Substrates move across the membrane when the two forms interconvert, driven by ATP hydrolysis. The NBDs of all ABC proteins are similar in sequence and presumably in three-dimensional structure. They constitute the conserved molecular motor that can be coupled to a wide variety of transmembrane domains, each capable of pumping one specific substrate across a membrane. When coupled this way, the ATP-driven motor moves solutes against a concentration gradient, with a stoichiometry of about one ATP hydrolyzed per molecule of substrate transported.
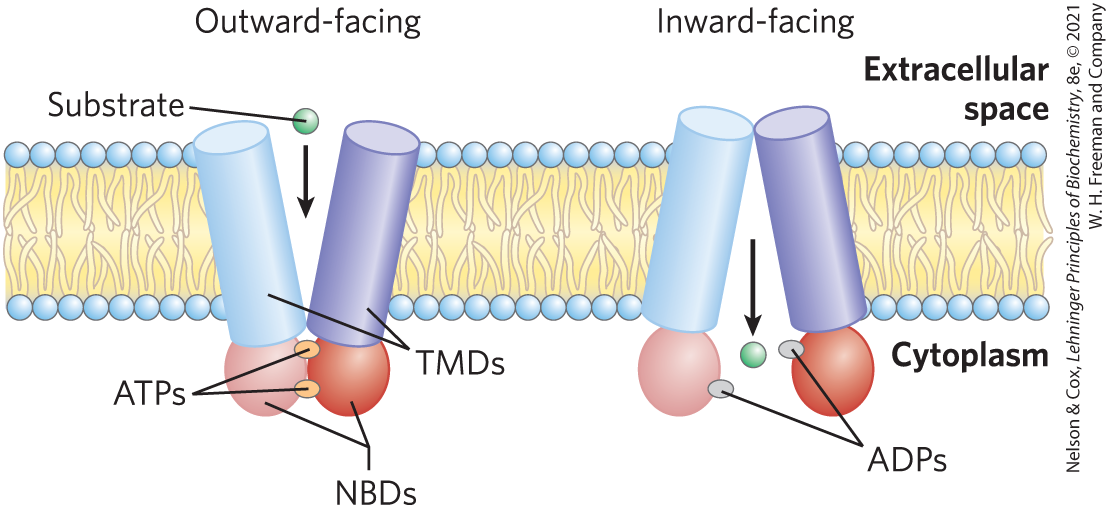
FIGURE 11-41 ABC transporters. The protein has two homologous halves, each with a six-helix transmembrane domain (TMD; blue), and a cytoplasmic nucleotide-binding domain (NBD; red). In the mechanism proposed for the coupling of ATP hydrolysis to transport, with ATP bound to the NBD sites, substrate binds to the transporter on the cytoplasmic side. Upon substrate binding and ATP hydrolysis to ADP, a conformational change exposes the substrate to the outside surface and lowers the transporter’s affinity for its substrate; substrate diffuses away from the transporter into the extracellular space. This mechanism for the coupling of ATP hydrolysis to transport is based on structures of a number of ABC transporters crystallized under different conditions. Compare this process with the model of glucose transport in Figure 11-33.
A horizontal piece of membrane is shown with the area above labeled extracellular space and the area below labeled cytoplasm. On the left, labeled outward-facing, a light blue cylinder extends at a slight diagonal to meet a dark blue cylinder that runs diagonally in the opposite direction. This creates an opening at the top, where the cylinders are farther apart, but no opening at the bottom, where they are close together. The cylinders are labeled T M Ds. The light blue cylinder has a very light red sphere beneath and the dark blue sphere has a darker light red sphere beneath. These are labeled N B Ds and they are connected by two ovals labeled A T Ps. A green sphere labeled substrate is shown just above the membrane at the opening between the two cylinders. On the left, labeled inward-facing, a light blue cylinder and a dark blue cylinder are in contact at the top but angle away in opposite directions to produce an opening below. The light blue cylinder on the left has a very light red sphere beneath it and the dark blue cylinder has a darker light red sphere beneath. Each light red sphere has a gray oval attached labeled, A D Ps. A green substrate molecule is shown just beneath the membrane in the opening between the two cylinders. An arrow points down from the top of the space between the cylinders to the substrate.
The human genome contains at least 48 genes that encode ABC transporters; a number of these are presented in Table 11-2. Some of these transporters have very high specificity for a single substrate; others are more promiscuous, able to transport drugs that cells presumably did not encounter during their evolution. Many ABC transporters are involved in maintaining the composition of the lipid bilayer, such as the floppases that move membrane lipids from one leaflet of the bilayer to the other. Many others are needed to move sterols, sterol derivatives, and fatty acids into the bloodstream for transport throughout the body. For example, the cellular machinery for exporting excess cholesterol includes an ABC transporter (see Fig. 21-47). Mutations in the genes that encode some of these proteins contribute to genetic diseases, including liver failure, retinal degeneration, and Tangier disease. The cystic fibrosis transmembrane conductance regulator protein (CFTR) of the plasma membrane is an interesting case of an ABC protein that is an ion channel (for ), regulated by ATP hydrolysis, but without the pumping function characteristic of an active transporter (Box 11-2).
| Gene(s) | Role/characteristics | Text reference |
|---|---|---|
| ABCA1 | Reverse cholesterol transport; defect causes Tangier disease | Fig. 21-47 |
| ABCA4 | Only in visual receptors, recycling of all-trans-retinal | Fig. 12-19 |
| ABCB1 | Multidrug resistance P-glycoprotein 1; transport across blood-brain barrier | — |
| ABCB4 | Multidrug resistance; transport of phosphatidylcholine in bile | — |
| ABCB11 | Transports bile salts out of hepatocytes | Fig. 17-1 |
| ABCC6 | Sulfonylurea receptor; targeted by the drug glipizide in type 2 diabetes | Fig. 23-27 |
| ABCG2 | Breast cancer resistance protein (BCRP); major exporter of anticancer drugs | p. 396 |
| ABCC7 | CFTR ( channel); defect causes cystic fibrosis | Box 11-2 |
Box 11-2 MEDICINE
A Defective Ion Channel in Cystic Fibrosis
Cystic fibrosis (CF) is a serious hereditary disease. In the United States, the frequency of CF is about 1 in 3,200 live births, and 1% to 4% (depending on ethnicity) are carriers, having one defective copy of the gene and one normal copy. Only individuals with two defective copies show the severe symptoms of the disease: obstruction of the gastrointestinal and respiratory tracts, commonly leading to bacterial infection of the airways.
The defective gene underlying CF was discovered in 1989. It encodes a membrane protein called cystic fibrosis transmembrane conductance regulator, or CFTR. This protein has two segments, each containing six transmembrane helices, two nucleotide-binding domains (NBDs), and a regulatory region that connects them (Fig. 1). CFTR is therefore very similar to other ABC transporter proteins, except that it functions as an ion channel (for ), not as a pump. The channel conducts across the plasma membrane when both NBDs have bound ATP, and it closes when the ATP on one of the NBDs is broken down to ADP and . The channel is further regulated by phosphorylation of several Ser residues in the regulatory domain, catalyzed by cAMP-dependent protein kinase. When the regulatory domain is not phosphorylated, the channel is closed.
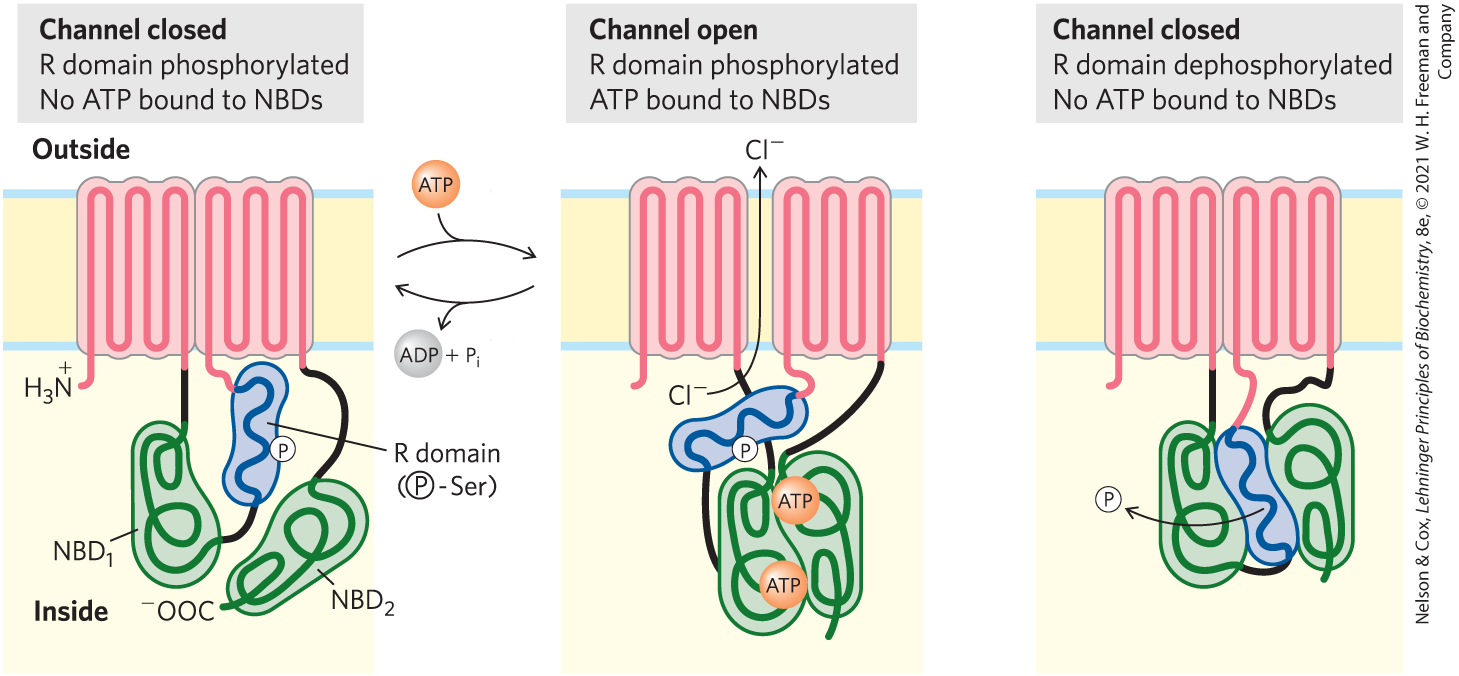
FIGURE 1 Three states of the CFTR protein. The protein has two segments, each with six transmembrane helices, and three functionally significant domains extend from the cytoplasmic surface: and (green) are nucleotide-binding domains that bind ATP, and the regulatory R domain (blue) is the site of phosphorylation by cAMP-dependent protein kinase. When this R domain is phosphorylated but no ATP is bound to the NBDs (left), the channel is closed. The binding of ATP opens the channel (middle) until the bound ATP is hydrolyzed. When the R domain is unphosphorylated (right), it binds the NBD domains and prevents ATP binding and channel opening. CFTR is a typical ABC transporter in all but two respects: most ABC transporters lack the regulatory domain, and CFTR acts as an ion channel (for ), not as a typical transporter.
The left-hand illustration is labeled channel closed: R domain phosphorylated; no A T P bound to N B Ds. A horizontal piece of membrane has the area above labeled outside and the area below labeled inside. Two adjacent vertical pink rectangles are present in the membrane. At the lower left, a thread ending at N H 3 with a positive charge on N runs into the left-hand pink square and runs up, then down, then up, then down, then up, then down to join a small black thread that joins to a green thread just before it enters a irregular green oval labeled N B D subscript 1 that is narrower on top and wider on the bottom. The green thread forms two loops, then emerges to meet a black thread that joins to a blue thread inside a blue structure that resembles a rectangle with rounded edges and a slight “S” shape. The blue thread bends a few times and then emerges at the upper left of the blue structure to join a pink structure that runs into the right-hand vertical rectangle, where it runs up, then down, then up, then down, then up, then down to leave the pink rectangle at the lower right and meet a black thread that runs down to a second irregular green oval labeled N B D subscript 2. The green thread levels the second green oval at its lower left to end at C O O minus. A circle labeled P is on the right side of the blue structure, where it meets the blue thread inside. The blue structure is labeled R domain open parenthesis P in a circle bonded to S e r close parenthesis. A sphere labeled A T P joins with an arrow pointing right to the center illustration and the accompanying left-pointing arrow has an arrow showing the departure of A D P plus P i. The center illustration is labeled channel open: R domain phosphorylated A T P bound to N B Ds. In this illustration, the two pink rectangles have moved apart show there is a vertical channel between them. C l minus below the membrane has an arrow pointing through the channel to C l minus above the membrane. The blue structure has moved so that it is beneath the channel and more horizontal than previously. Beneath the blue structure, the two green structures have joined together and each has a circle labeled A T P next to the place that they meet. The right-hand illustration is labeled channel closed: R domain dephosphorylated; no A T P bonded to N B Ds. The two pink rectangles are adjacent again. The green structures are on either side of the blue structure and all fit closely together. P in a circle is shown to the left with an arrow showing that it came from the blue structure.
The mutation responsible for CF in up to 90% of cases results in deletion of a Phe residue at position 508 (a mutation denoted F508del). The mutant protein folds incorrectly, causing it to be degraded in proteasomes. As a result, movement is reduced across the plasma membranes of epithelial cells that line the airways, digestive tract, exocrine glands (pancreas, sweat glands), bile ducts, and vas deferens. Less-common mutations, such as G551D ( changed to Asp), lead to production of CFTR that is correctly folded and inserted into the membrane but is defective in transfer.
Diminished export of in individuals with CF is accompanied by diminished export of water from cells, causing the mucus on cell surfaces to become dehydrated, thick, and excessively sticky. In normal circumstances, cilia on the epithelial cells lining the inner surface of the lungs constantly sweep away bacteria that settle in this mucus, but the thick mucus in individuals with CF hinders this process, providing a haven in the lungs for pathogenic bacteria. Frequent infections by bacteria such as Staphylococcus aureus and Pseudomonas aeruginosa cause progressive damage to the lungs and reduce respiratory efficiency, eventually resulting in death due to inadequate lung function.
Advances in therapy have raised the average life expectancy for people who have CF from just 10 years in 1960 to more than 40 years today. CFTR potentiators such as ivacaftor (VX-770) increase the function of the mutant G551D protein that is properly folded and in place in the plasma membrane. For individuals with the folding defect, F508del, CFTR correctors improve the processing and delivery of the mutant protein to the cell surface; a combination of potentiator and corrector drugs is more effective than the corrector drug alone for these patients (Fig. 2). In 2019, clinicals trials of a combination of three drugs (ivacaftor, tezacaftor, and elexacaftor), acting as both potentiators and correctors, showed dramatic improvements in individuals with the most common mutation (F508del).
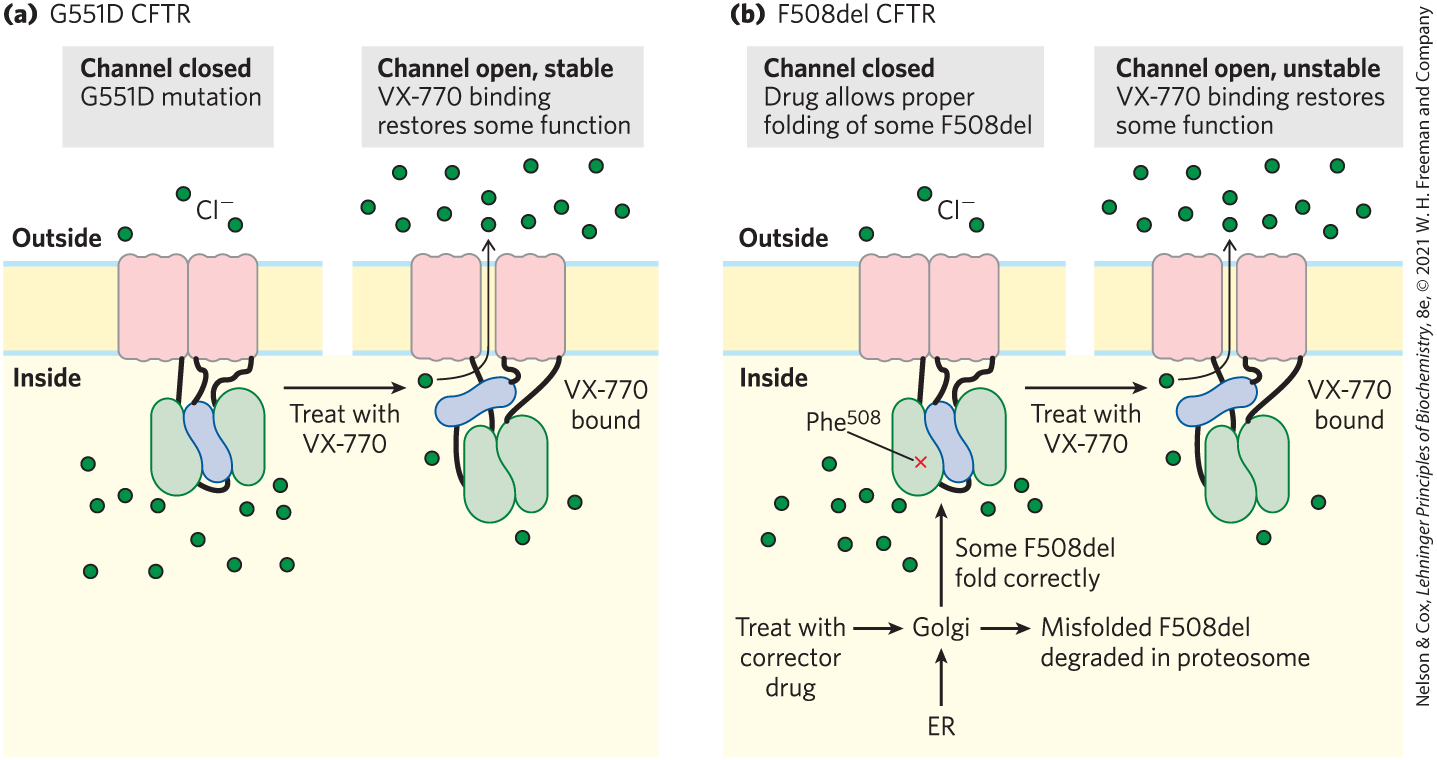
FIGURE 2 (a) The CFTR mutation G551D (replacement of with Asp) results in a protein that is inserted into the membrane correctly but is defective as a channel. Addition of the potentiator drug VX-770 (ivacaftor) restores partial function to the channel. (b) The more common mutation F508del (deletion of ) prevents proper folding of CFTR, causing it to be degraded in proteasomes. In the presence of a corrector drug, folding and membrane insertion can take place; addition of the potentiator drug results in partial restoration of channel activity. The channel is unstable and is degraded over time. [Information from J. P. Clancy, Sci. Transl. Med. 6:1, 2014.]
Both parts show two pieces of horizontal plasma membrane that each contains a different state of the C F T R protein. The area above the membrane is labeled outside and the area below the membrane is labeled inside. Part a shows G 551 D C F T R. Text above the left-hand side reads, channel closed: G 551 D mutation. Two pink vertical rectangles are adjacent within the membrane with no space between them. Three green spheres labeled C l minus are present above the membrane and 12 green spheres are present below the membrane. A black thread from the bottom right of the left-hand pink rectangle runs down to the top of a green structure that is roughly oval but wider at the bottom than at the top. A black thread runs from the bottom of the green structure to an adjacent “S”-shaped blue structure that fits tightly against the right side of the green structure. A black thread extends up from this blue structure to the lower left of the right-hand pink rectangle, which has a black thread extending down from its lower right to the upper left of a green structure that resembles the first green structure except that it is wider at the top than at the bottom. The two green structures fit around the blue structure between them. An arrow pointing right is labeled, treat with V X-770. Text above the right-hand side reads, channel open, stable: V X-770 binding restores some function. The right-hand structure shows a narrow vertical space between the two pink rectangles, representing a channel. The two green structures fit together and the blue structure is placed almost horizontally above them. Adjacent text reads, V X-770 bound. There are four green sphere below the membrane and an arrow from one runs through the channel to point at a green sphere among many green spheres above the membrane. Part b shows F 508 del C F T R. Text above the left side reads, channel closed: drug allows proper folding of some F 508 del. The left-hand illustration is similar to the left-hand illustration in part a except that there is a red “X” labeled P h e superscript 503 on the left-hand green structure and additional steps are shown below. E R and treat with corrector drug both point to Golgi. An arrow points right from Golgi to text reading, misfolded F 508 del degraded in proteasome. An arrow pointing up to the green structures around the blue structure reads, some F 508 del fold correctly. An arrow pointing right is labeled, treat with V X-770. Text above the right-hand side reads, channel open, unstable: V X-770 binding restores some function. The illustration is similar to the right-hand illustration in part a.
One human ABC transporter with very broad substrate specificity is the multidrug transporter (MDR1), encoded by the ABCB1 gene. MDR1 in the placental membrane and in the blood-brain barrier ejects toxic compounds that would damage the fetus or the brain. But it is also responsible for the striking resistance of certain tumors to some generally effective antitumor drugs. For example, MDR1 pumps the chemotherapeutic drugs doxorubicin and vinblastine out of cells, thus preventing their accumulation within a tumor and blocking their therapeutic effects. Overexpression of MDR1 is often associated with treatment failure in cancers of the liver, kidney, and colon. A related ABC transporter, BCRP (breast cancer resistance protein, encoded by the ABCG2 gene), is overexpressed in breast cancer cells, also conferring resistance to anticancer drugs. Highly selective inhibitors of these multidrug transporters are expected to enhance the effectiveness of antitumor drugs and are the objects of current drug discovery and design.
ABC transporters are also present in simpler animals and in plants and microorganisms. Yeast has 31 genes that encode ABC transporters, Drosophila has 56, and E. coli has 80, representing 2% of its entire genome. ABC transporters that are used by E. coli and other bacteria to import essentials such as vitamin are the presumed evolutionary precursors of the MDRs of animal cells. The presence of ABC transporters that confer antibiotic resistance in pathogenic microbes (Pseudomonas aeruginosa, Staphylococcus aureus, Candida albicans, Neisseria gonorrhoeae, and Plasmodium falciparum) is a serious public health concern and makes these transporters attractive targets for drug design.
Ion Gradients Provide the Energy for Secondary Active Transport
The ion gradients formed by primary transport of or can, in turn, provide the driving force for cotransport of other solutes. Many cell types have transport systems that couple the spontaneous, downhill flow of these ions to the simultaneous uphill pumping of another ion, sugar, or amino acid.
In intestinal epithelial cells, glucose and certain amino acids are accumulated by symport with , down the gradient established by the ATPase of the plasma membrane (Fig. 11-42). The apical surface of the intestinal epithelial cell (the surface that faces the intestinal contents) is covered with microvilli — long, thin projections of the plasma membrane that greatly increase the surface area exposed to the intestinal contents. The -glucose symporter in the apical plasma membrane takes up glucose from the intestine in a process driven by the downhill flow of :
The energy required for this process comes from two sources: the greater concentration of outside than inside the cell (the chemical potential) and the membrane (electrical) potential, which is inside negative and therefore draws inward. The strong thermodynamic tendency for to move into the cell provides the energy needed for the transport of glucose into the cell, against its concentration gradient. An ion gradient created and sustained by energy-dependent ion pumping serves as the potential energy for cotransport of another species against its concentration gradient.
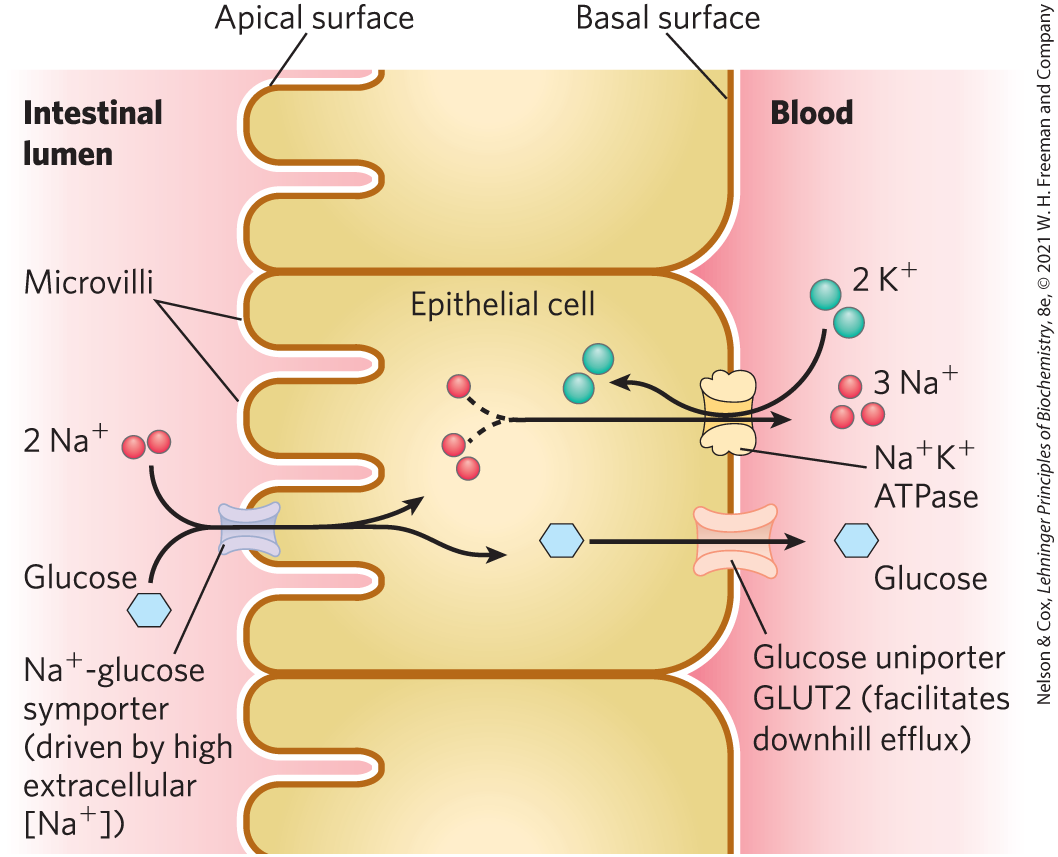
FIGURE 11-42 Glucose transport in intestinal epithelial cells. Glucose is cotransported with across the apical plasma membrane into the epithelial cell. It moves through the cell to the basal surface, where it passes into the blood via GLUT2, a passive glucose uniporter. The ATPase continues to pump outward to maintain the gradient that drives glucose uptake.
A vertical row of epithelial cells shows protrusions to the left from the side labeled apical surface and smooth rounded right sides, labeled basal surface. The protrusions are labeled microvilli. The area to the left of the cells is labeled intestinal lumen and the area to the right of the cells is labeled blood. On the left, in the intestinal lumen, arrows from two red spheres labeled 2 N a plus join with an arrow from a blue hexagon labeled glucose to pass through a transporter at the end of one villus labeled N a plus-glucose symporter open parenthesis driven by high extracellular open bracket N a plus close bracket close parenthesis. Two different transporters are shown on the right side of the same cell. Three N a plus ions move through the top transporter to the blood and 2 green spheres labeled 2 K plus move through the same transporter into the cell. An arrow points from the blue hexagon representing glucose inside the cell through the bottom transporter, labeled glucose uniporter G L U T 2 open parenthesis facilitates downhill efflux close parenthesis to a glucose molecule outside of the cell.
WORKED EXAMPLE 11-3 Energetics of Pumping by Symport
Calculate the maximum ratio that can be achieved by the plasma membrane -glucose symporter of an epithelial cell when is 12 mm, is 145 mm, the membrane potential is −50 mV (inside negative), and the temperature is 37 °C.
SOLUTION:
Using Equation 11-4 (p. 392), we can calculate the energy inherent in an electrochemical gradient — that is, the cost of moving one ion up this gradient:
We then substitute standard values for R, T, and F; the given values for [] (expressed as molar concentrations); +1 for Z (because has a positive charge); and 0.050 V for Δψ. Note that the membrane potential is −50 mV (inside negative), so the change in potential when an ion moves from inside to outside is 50 mV.
When reenters the cell, it releases the electrochemical potential created by pumping it out; ΔG for reentry is −11.2 kJ/mol of . This is the potential energy per mole of that is available to pump glucose. Given that two ions pass down their electrochemical gradient and into the cell for each glucose carried in by symport, the energy available to pump 1 mol of glucose is . We can now calculate the maximum concentration ratio of glucose that can be achieved by this pump (from Eqn 11-3, p. 392):
Rearranging, then substituting the values of , R, and T, gives
Thus, the cotransporter can pump glucose inward until its concentration inside the epithelial cell is about 6,000 times the concentration outside (in the intestine). (This is the maximum theoretical ratio, assuming a perfectly efficient coupling of reentry and glucose uptake.)
As glucose molecules are pumped from the intestine into the epithelial cell at the apical surface, glucose is simultaneously moved from the cell into the blood by passive transport through a glucose transporter (GLUT2) in the basal surface (Fig. 11-42). The crucial role of in symport and antiport systems such as this requires the continued outward pumping of to maintain the transmembrane gradient.
In the kidney, a different -glucose symporter (SGLT2) is the target of drugs used to treat type 2 diabetes. Gliflozins are specific inhibitors of this -glucose symporter. They lower blood glucose by inhibiting glucose reabsorption in the kidney, thus preventing the damaging effects of elevated blood glucose. Glucose not reabsorbed in the kidney is cleared in the urine. This class of drugs, taken orally in combination with diet and exercise, lowers blood glucose significantly in individuals with type 2 diabetes.
Because of the essential role of ion gradients in active transport and energy conservation, compounds that collapse ion gradients across cellular membranes are effective poisons, and those that are specific for infectious microorganisms can serve as antibiotics. One such substance is valinomycin, a small cyclic peptide that neutralizes the charge by surrounding the ion with six carbonyl oxygens (Fig. 11-43). The hydrophobic peptide then acts as a shuttle, carrying across the membrane down its concentration gradient and deflating that gradient. Compounds that shuttle ions across membranes in this way are called ionophores (“ion bearers”). Both valinomycin and monensin (a -carrying ionophore) are antibiotics; they kill microbial cells by disrupting secondary transport processes and energy-conserving reactions. Monensin is widely used as an antifungal and antiparasitic agent.
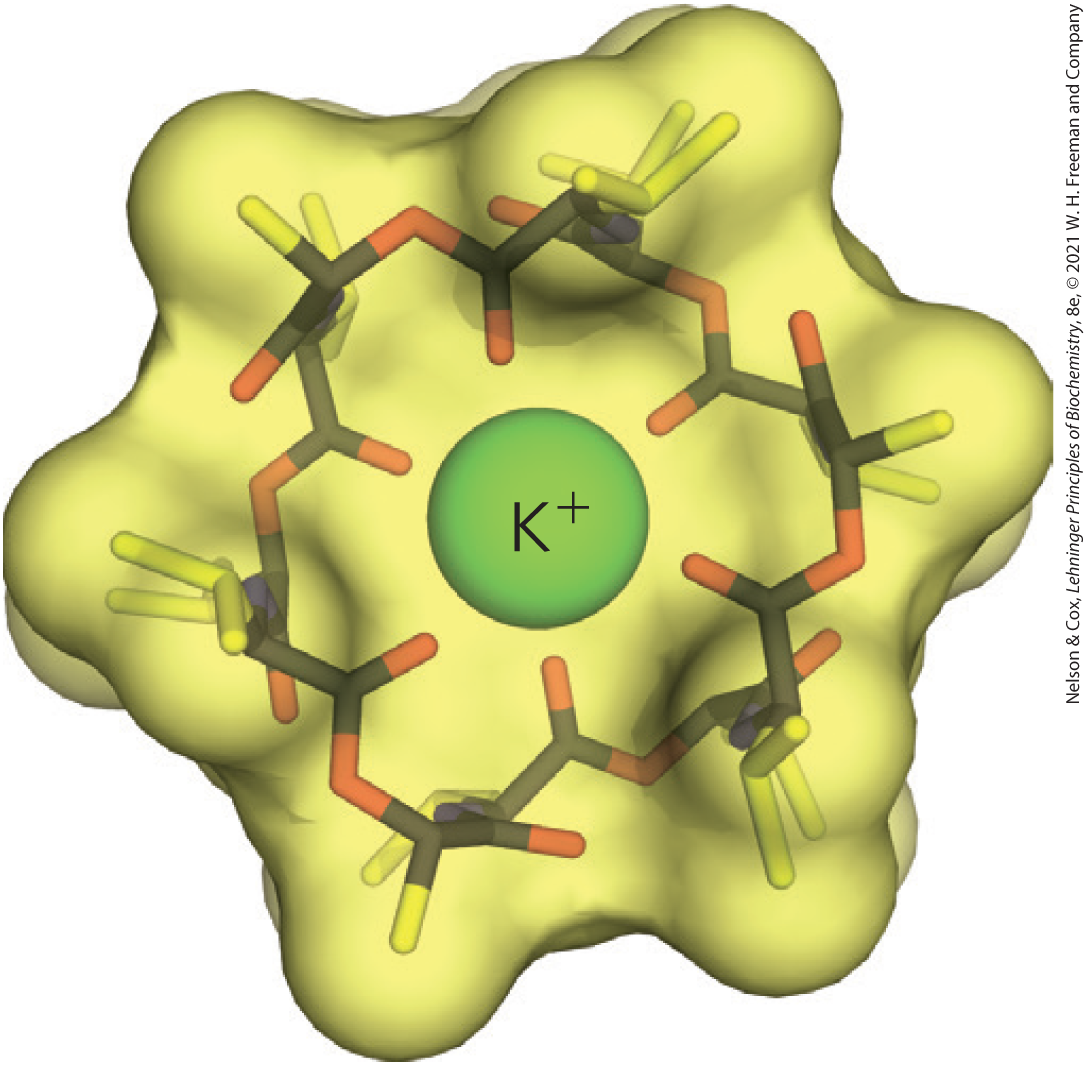
FIGURE 11-43 Valinomycin, a peptide ionophore that binds . The central ion is surrounded by inward-facing polar and charged amino acid side chains, and the outside surface is covered with nonpolar side chains that make the whole structure hydrophobic enough to diffuse through the lipid bilayer, carrying down its concentration gradient. The resulting dissipation of the transmembrane ion gradient kills microbial cells, making valinomycin a potent antibiotic. [Information from P. Barak and E. A. Nater, http://virtual-museum.soils.wisc.edu, and K. Neupert-Laves and M. Dobler, Helv. Chim. Acta 58:432, 1975.]
A large green sphere labeled K plus is in the center surrounded by a symmetrical ring structure. The repeating units of a structure has orange and black components that extends red pieces toward K plus and has yellow pieces extending out into rounded bulges in the overall molecule. The three-dimensional structure is rounded with spherical protrusions on the sides and above at six evenly spaced locations where these yellow pieces are located.
Aquaporins Form Hydrophilic Transmembrane Channels for the Passage of Water
A family of integral membrane proteins, the aquaporins (AQPs), provide channels for rapid movement of water molecules across all plasma membranes. Aquaporins are found in all organisms, and multiple aquaporin genes are generally present, encoding similar but not identical proteins. Eleven aquaporins are known in mammals, each with a specific location and role (Table 11-3). The exocrine glands that produce sweat, saliva, and tears secrete water through aquaporins. Erythrocytes, which swell or shrink rapidly in response to abrupt changes in extracellular osmolarity as blood travels through the renal medulla, have a high density of aquaporin in their plasma membrane ( copies of AQP1 per cell). Seven aquaporins play roles in urine production and water retention in the nephron (the functional unit of the kidney). Each renal AQP has a specific location in the nephron, and each has specific properties and regulatory features. For example, AQP2 in the epithelial cells of the renal collecting duct is regulated by vasopressin (also called antidiuretic hormone): more water is reabsorbed from the duct into the kidney tissues when the vasopressin level is high. Mutant mice with no AQP2 gene have greater urine output (polyuria) and more dilute urine, the result of the proximal tubule becoming less permeable to water. In humans, genetically defective AQPs are known to be responsible for a variety of diseases.
| Aquaporin | Permeant (permeability) | Tissue distribution | Primary subcellular distributiona |
|---|---|---|---|
| AQP0 | Water (low) | Lens | Plasma membrane |
| AQP1 | Water (high) | Erythrocyte, kidney, lung, vascular endothelium, brain, eye | Plasma membrane |
| AQP2 | Water (high) | Kidney, vas deferens | Apical plasma membrane, intracellular vesicles |
| AQP3 | Water (high), glycerol (high), urea (moderate) | Kidney, skin, lung, eye, colon | Basolateral plasma membrane |
| AQP4 | Water (high) | Brain, muscle, kidney, lung, stomach, small intestine | Basolateral plasma membrane |
| AQP5 | Water (high) | Salivary gland, lacrimal gland, sweat gland, lung, cornea | Apical plasma membrane |
| AQP6 | Water (low), anions | Kidney | Intracellular vesicles |
| AQP7 | Water (high), glycerol (high), urea (high) | Adipose tissue, kidney, testis | Plasma membrane |
| AQP8b | Water (high) | Testis, kidney, liver, pancreas, small intestine, colon | Plasma membrane, intracellular vesicles |
| AQP9 | Water (low), glycerol (high), urea (high) | Liver, leukocyte, brain, testis | Plasma membrane |
| AQP10 | Water (low), glycerol (high), urea (high) | Small intestine | Intracellular vesicles |
|
Information from L. S. King et al., Nat. Rev. Mol. Cell Biol. 5:688, 2004. aThe apical plasma membrane faces the lumen of the gland or tissue; the basolateral plasma membrane is along the sides and base of the cell, not facing the lumen of the gland or tissue. bAQP8 might also be permeated by urea. |
|||
Water molecules flow through an AQP1 channel at a rate of about . For comparison, the highest known turnover number for an enzyme is that for catalase, , and many enzymes have turnover numbers between 1 and (see Table 6-7). The low activation energy for passage of water through aquaporin channels suggests that water moves through the channels in a continuous stream, in the direction dictated by the osmotic gradient. (For a discussion of osmosis, see p. 52.) Aquaporins do not allow passage of protons (hydronium ions, ), which would collapse membrane electrochemical gradients.
Ion-Selective Channels Allow Rapid Movement of Ions across Membranes
Ion-selective channels — first recognized in neurons and now known to be present in the plasma membranes of all cells, as well as in the intracellular membranes of eukaryotes — provide another mechanism for moving inorganic ions across membranes. Ion channels, together with ion pumps such as the ATPase, determine a plasma membrane’s permeability to specific ions and regulate the cytosolic concentration of ions and the membrane potential. In neurons, very rapid changes in the activity of ion channels cause the changes in membrane potential (action potentials) that carry signals from one end of a neuron to the other. In myocytes, rapid opening of channels in the sarcoplasmic reticulum releases the that triggers muscle contraction. We discuss the signaling functions of ion channels in Chapter 12. Here we describe the structural basis for ion-channel function, using as our example a well-studied channel.
Ion channels are distinct from ion transporters in at least three ways. First, the rate of flux through channels can be orders of magnitude greater than the turnover number for a transporter — to ions/s for an ion channel, approaching the theoretical maximum for unrestricted diffusion. By contrast, the turnover rate of the ATPase is about 100 . Second, ion channels are not saturable: rates do not approach a maximum at high substrate concentration. Third, they are gated in response to some type of cellular event. In ligand-gated channels (which are generally oligomeric), binding of an extracellular or intracellular small molecule forces an allosteric transition in the protein, which opens or closes the channel. In voltage-gated ion channels, a change in transmembrane electrical potential causes a charged protein domain to move relative to the membrane, opening or closing the channel. Both types of gating can be very fast. A channel typically opens in a fraction of a millisecond and may remain open for only milliseconds, making these molecular devices effective for very fast signal transmission in the nervous system.
Because a single ion channel typically remains open for only a few milliseconds, monitoring this process is beyond the limit of most biochemical measurements. Ion fluxes must therefore be measured electrically, either as changes in (in the millivolt range) or as electric current I (in the microampere or picoampere range), using microelectrodes and appropriate amplifiers. In patch-clamping, very small currents are measured through a tiny region of the membrane surface containing only one or a few ion-channel molecules (Fig. 11-44). The researcher can measure the size and duration of the current that flows during one opening of an ion channel and can determine how often a channel opens and how that frequency is affected by membrane potential, regulatory ligands, toxins, and other agents. Patch-clamp studies have revealed that as many as ions can move through a single ion channel in 1 ms. Such an ion flux represents a huge amplification of the initial signal, which may be just one or two signaling molecules (neurotransmitters, for example).
FIGURE 11-44 Electrical measurements of ion-channel function. The “activity” of an ion channel is estimated by measuring the flow of ions through it, using the patch-clamp technique. A finely drawn-out pipette (micropipette) is pressed against the cell surface, and negative pressure in the pipette forms a pressure seal between pipette and membrane. As the pipette is pulled away from the cell, it pulls off a tiny patch of membrane (which may contain one or a few ion channels). After placing the pipette and attached patch in an aqueous solution, the researcher can measure channel activity as the electric current that flows between the contents of the pipette and the aqueous solution. In practice, a circuit is set up that “clamps” the transmembrane potential at a given value and measures the current that must flow to maintain this voltage. With highly sensitive detectors, researchers can measure the current flowing through a single ion channel, typically a few picoamperes. The trace shows the current through a single acetylcholine receptor channel as a function of time (in milliseconds), revealing how fast the channel opens and closes, how frequently it opens, and how long it stays open. Downward deflection represents channel opening. Clamping the at different values permits determination of the effect of membrane potential on these parameters of channel function. [Information from V. Witzemann et al., Proc. Natl. Acad. Sci. USA 93:13,286, 1996.]
A circle shows a piece of membrane being pushed into a “U” shape by the two sides of the tip of micropipette. A channel is at the base of the “U”, where the membrane has been maximally pulled up into the micropipette. Text below reads, micropipette applied tightly to plasma membrane. An arrow points right to a similar illustration in which the membrane outside of the micropipette has been removed, leaving only the small rounded piece with the channel. Text below reads, patch of membrane pulled from cell. An arrow points down to a dish below and is accompanied by text reading, path of membrane, still attached to micropipette tip, placed in aqueous solution. A long, pen-like device labeled micropipette extends down to a small blue line labeled membrane patch. Two wires run from a rectangle on the left to small circles labeled electrodes, one in a different part of the dish of aqueous solution and the other halfway down the micropipette. The rectangle to which the wires run shows a graph with a dotted horizontal line running across it near the bottom. The vertical axis of the graph is labeled 2 p A and the horizontal axis is labeled 50 m s. A line begins at three-quarters of the height of the vertical axis at the left side of the graph and runs horizontally except for 10 times that it descends almost vertically to meet the dotted horizontal line and then rises rapidly back up. There are three of these downward deflections close together between the start of the line and one-quarter of the way across the horizontal axis, two between this location and halfway across the horizontal axis, a space with no deflections, and then six deflections on the right side of the graph. All data are approximate.
The Structure of a Channel Reveals the Basis for Its Specificity
The structure of a potassium channel from the bacterium Streptomyces lividans provides important insight into the way ion channels work. This bacterial ion channel is related in sequence to all other known channels and serves as the prototype for such channels, including the voltage-gated channel of neurons. Among the members of this protein family, the similarities in sequence are greatest in the “pore region,” which contains the ion selectivity filter that allows (radius 1.33 Å) to pass times more readily than (radius 0.95 Å) — at a rate (about ions/s) approaching the theoretical limit for unrestricted diffusion.
The channel consists of four identical subunits that span the membrane and form a cone within a cone surrounding the ion channel, with the wide end of the double cone facing the extracellular space (Fig. 11-45a). Each subunit has two transmembrane α helices and a third, shorter helix that contributes to the pore region. The outer cone is formed by one of the transmembrane helices of each subunit. The inner cone, formed by the other four transmembrane helices, surrounds the ion channel and cradles the ion selectivity filter. Viewed perpendicular to the plane of the membrane, the central channel is seen to be just wide enough to accommodate an unhydrated metal ion such as potassium (Fig. 11-45b).
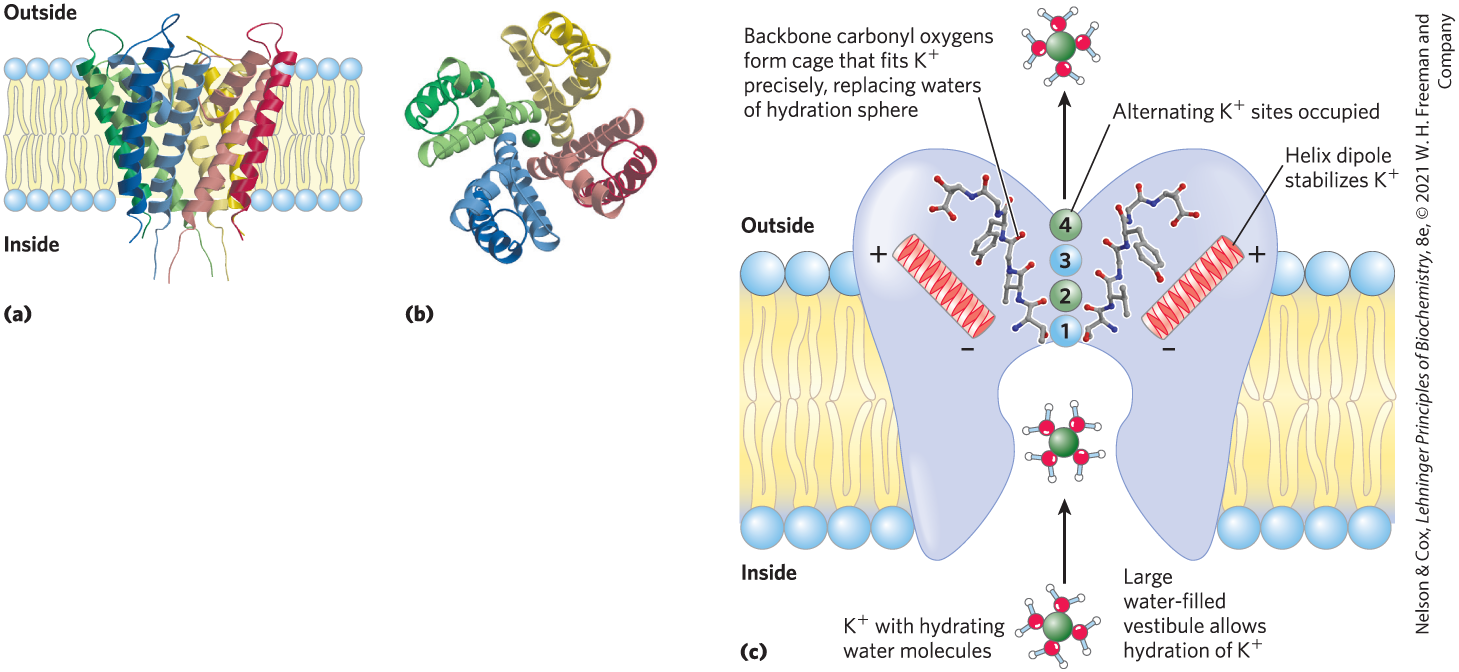
FIGURE 11-45 The channel of Streptomyces lividans. (a) Viewed in the plane of the membrane, the channel consists of eight transmembrane helices (two from each of four identical subunits), forming a cone with its wide end toward the extracellular space. The inner helices of the cone (lighter colored) line the transmembrane channel, and the outer helices interact with the lipid bilayer. Short segments of each subunit converge in the open end of the cone to make a selectivity filter. (b) This view, perpendicular to the plane of the membrane, shows the four subunits arranged around a central channel just wide enough for a single ion to pass. (c) Diagram of a channel in cross section, showing the structural features critical to function. ions go through the channel in pairs, first in sites 1 and 3, then in sites 2 and 4. Carbonyl oxygens (red) of the peptide backbone in the selectivity filter protrude into the channel, interacting with and stabilizing the ions that are passing through. [Data from (a, b) PDB ID 1BL8, D. A. Doyle et al., Science 280:69, 1998; (c) G. Yellen, Nature 419:35, 2002, and PDB ID 1J95, M. Zhou et al., Nature 411:657, 2001.]
Part a shows a horizontal piece of membrane with the area above the membrane labeled outside and the area below the membrane labeled inside. There are eight helices visible within the membrane. The helices form a roughly triangular structure that is wider at the top and narrower at the bottom. Two blue helices run almost vertically through the membrane, angling only slightly to the right, with the lighter helix to the right of the darker helix. Two green helices angle from the bottom center to the upper left, with the lighter one to the right of the darker one. The lighter helix is joined with a smaller piece that runs to its right along its upper half. A light red helix runs from the bottom center to the upper right and has a smaller piece that runs to its left along its upper half. It is joined to a darker red helix to its right. Two yellow helices begin below the red helices and run to the upper rear center. The light yellow helix is to the left of the dark yellow helix. Part b shows a green sphere with four evenly spaced pairs of helices around it. The yellow helices are to the upper right, the red helices are to the lower right, the blue helices are to the lower left, and the green helices are to the upper left. Each set of helices has a dark helix running along a light helix and the light helices have smaller subunits that run almost perpendicular to the other helices of the same color. Part c shows a piece of membrane with the area above labeled outside and the area below labeled inside. A large protein is present in the middle that has spherical lobes extending to the upper right and upper left and an open rounded region in the middle that opens to the inside of the cell below. A light red cylindrical helix runs from just to the upper left of the rounded open region, where it is labeled minus, to the upper left side just above the upper layer of phosphate heads of the membrane and below the upper rounded region, where it is labeled plus. A similar light red helix runs from above the upper left of the rounded open region to the upper right and is labeled, helix dipole stabilizes K plus. A ball-and stick model is shown that begins at the top center of the rounded open region, just to the left of a blue sphere labeled 1 to the left of a similar ball-and-stick model. The ball-and-stick model runs up toward the left and shows a series of amino acids with red oxygen atoms extending to the upper right at regular intervals. Accompanying text reads, backbone carbonyl oxygens form cage that fits K plus precisely, replacing waters of hydration sphere. The ball-and-stick model to the right of the sphere labeled 1 is similar, with O atoms extending toward the upper left as it runs up to the right. The blue sphere numbered 1 is part of a vertical chain of spheres that runs to the upper surface of the protein. The sequence if blue 1, green 2, blue 3, green 4. The top sphere, numbered 4, has accompanying text reading, alternating K plus sites occupied. A large green K plus ion is shown with four water molecules around it that all have O facing K plus. Accompanying text reads, K plus with hydrating water molecules. An arrow points up from this K plus with four water molecules to a similar K plus with four water molecules in the open rounded region in the middle of the protein. Accompanying text reads, large water-filled vestibule allows hydration of K plus. An arrow points up from sphere 4 at the top of the protein to another K plus with four water molecules above.
Both the ion specificity and the high flux through the channel are understandable from what we know of the channel’s structure (Fig. 11-45c). At the inner and outer plasma membrane surfaces, the entryways to the channel have several negatively charged amino acid residues, which presumably increase the local concentration of cations such as and . The ion path through the membrane begins (on the inner surface) as a wide, water-filled channel in which the ion can retain its hydration sphere. Further stabilization is provided by the short helices in the pore region of each subunit, with the partial negative charges of their electric dipoles pointed at in the channel. About two-thirds of the way through the membrane, this channel narrows in the region of the selectivity filter, forcing the ion to give up its hydrating water molecules. Carbonyl oxygen atoms in the backbone of the selectivity filter replace the water molecules in the hydration sphere, forming a series of perfect coordination shells through which the moves. This favorable interaction with the filter is not possible for , which is too small to make contact with all the potential oxygen ligands. The preferential stabilization of is the basis for the ion selectivity of the filter, and mutations that change residues in this part of the protein eliminate the channel’s ion selectivity. The -binding sites of the filter are flexible enough to collapse to fit any that enters the channel, and this conformational change closes the channel.
There are four potential -binding sites along the selectivity filter, each composed of an oxygen “cage” that provides ligands for the ions (Fig. 11-45c). In the crystal structure, two ions are visible within the selectivity filter, about 7.5 Å apart, and two water molecules occupy the unfilled positions. ions pass through the filter in single file; their mutual electrostatic repulsion probably just balances the interaction of each ion with the selectivity filter and keeps them moving. Movement of the two ions is concerted: first they occupy positions 1 and 3, then they hop to positions 2 and 4. The energetic difference between these two configurations (1, 3 and 2, 4) is very small; energetically, the selectivity pore is not a series of hills and valleys but a flat surface, which is ideal for rapid ion movement through the channel. The structure of the channel seems to have been optimized during evolution to give maximal flow rates and high specificity.
SUMMARY 11.3 Solute Transport across Membranes
- Some transporters simply facilitate passive diffusion of a solute across the membrane, from a higher concentration to a lower concentration. Others transport solutes against an electrochemical gradient; this requires a source of metabolic energy.
- Transporters move solutes across a membrane one or a few at a time, providing a binding site on each side of the membrane. The binding sites alternate between being accessible from the outside and from the inside. Ion channels provide a path across the membrane, which is either open or closed. When open, the channel allows the movement of large numbers of solute ion across the membrane at nearly the speed of unhindered diffusion.
- A family of glucose transporters in humans includes the passive transporter GLUT1, which is saturated at normal levels of glucose in the blood. GLUT1 facilitates movement of glucose from blood into erythrocytes.
- The chloride-bicarbonate exchanger of erythrocytes exchanges one ion for one ion across the erythrocyte plasma membrane, mediating the uptake of in the tissues and its release in the lungs.
- Active transporters use energy to pump solutes against an electrochemical gradient.
- P-type ATPases, including the ATPase of the plasma membrane and the transporters of the sarcoplasmic/endoplasmic reticulum, couple phosphorylation and dephosphorylation of the transporter to alternate exposure of solute binding sites on the inside and the outside of the membrane. In animal cells, the ATPase maintains the differences in cytosolic and extracellular concentrations of and , and the resulting gradient is used as the energy source for a variety of secondary active transport processes.
- V-type and F-type ATPases are active transporters that couple ATP cleavage to the uphill transport of ions. The same mechanism, working in reverse, allows the synthesis of ATP, driven by movement of protons down their electrochemical gradient.
- ABC transporters carry a variety of substrates (including many drugs) out of cells, using ATP as the energy source. The ATP-using domain is conserved in many ABC transporters, and it is coupled with various transmembrane domains that give substrate specificity.
- Some active cotransporters use the energy in an ion gradient generated catabolically to move a solute uphill. The -glucose cotransporter of the kidney and intestine is such a transporter.
- Water moves across membranes through aquaporins. Some aquaporins are regulated; some also transport glycerol or urea.
- Ion channels provide hydrophilic pores through which select ions can diffuse, moving down their electrical or chemical concentration gradients. Ion channels are unsaturable, have very high flux rates, and are highly specific for one ion.
- Structural studies of channels reveal the mechanism that allows great discrimination between and other ions like . The polar transmembrane passage precisely fits the ion, but allows neither larger ions nor smaller ions to pass.
 A few nonpolar compounds can dissolve in the lipid bilayer and cross a membrane unassisted, but for any polar compound or ion, a specific membrane protein carrier is essential. Approximately 2,000 genes in the human genome encode proteins that function in transporting solutes across membranes. In some cases, a membrane protein simply facilitates the diffusion of a solute down its concentration gradient; but transport can also occur against a gradient of concentration, electrical potential, or both, and in these cases, as we shall see, the transport process requires energy. Ions may also diffuse across membranes via ion channels formed by proteins, or they may be carried across by ionophores, small molecules that mask the charge of ions and allow them to diffuse through the lipid bilayer.
A few nonpolar compounds can dissolve in the lipid bilayer and cross a membrane unassisted, but for any polar compound or ion, a specific membrane protein carrier is essential. Approximately 2,000 genes in the human genome encode proteins that function in transporting solutes across membranes. In some cases, a membrane protein simply facilitates the diffusion of a solute down its concentration gradient; but transport can also occur against a gradient of concentration, electrical potential, or both, and in these cases, as we shall see, the transport process requires energy. Ions may also diffuse across membranes via ion channels formed by proteins, or they may be carried across by ionophores, small molecules that mask the charge of ions and allow them to diffuse through the lipid bilayer.  Glucose in blood plasma binds to a stereospecific site on ; this lowers the activation energy for
Glucose in blood plasma binds to a stereospecific site on ; this lowers the activation energy for  a conformational change from • to • , effecting transmembrane passage of the glucose.
a conformational change from • to • , effecting transmembrane passage of the glucose.  Glucose is released from into the cytoplasm, and
Glucose is released from into the cytoplasm, and  the transporter returns to the conformation, ready to transport another glucose molecule. Between the forms and , there is an intermediate form (not shown here) in which glucose is sequestered within the transporter, with access to neither side. The structures of (b) human GLUT3 in the conformation and (c) human GLUT1 in the conformation, determined by x-ray crystallography, support the model shown in (a). [Data from (b) PDB ID 4ZWC, D. Deng et al., Nature 526:391, 2015; (c) PDB ID 4PYP, D. Deng et al., Nature 510:121, 2014.]
the transporter returns to the conformation, ready to transport another glucose molecule. Between the forms and , there is an intermediate form (not shown here) in which glucose is sequestered within the transporter, with access to neither side. The structures of (b) human GLUT3 in the conformation and (c) human GLUT1 in the conformation, determined by x-ray crystallography, support the model shown in (a). [Data from (b) PDB ID 4ZWC, D. Deng et al., Nature 526:391, 2015; (c) PDB ID 4PYP, D. Deng et al., Nature 510:121, 2014.] Twelve passive glucose transporters are encoded in the human genome, each with its unique kinetic properties, patterns of tissue distribution, and function (
Twelve passive glucose transporters are encoded in the human genome, each with its unique kinetic properties, patterns of tissue distribution, and function (
 E2-P is dephosphorylated.
E2-P is dephosphorylated.  The A domain resets and
The A domain resets and  the protein returns to the E1 conformation for another round of transport. [Information from W. Kühlbrandt, Nat. Rev. Mol. Cell Biol. 5:282, 2004.]
the protein returns to the E1 conformation for another round of transport. [Information from W. Kühlbrandt, Nat. Rev. Mol. Cell Biol. 5:282, 2004.] Some transporters simply facilitate passive diffusion of a solute across the membrane, from a higher concentration to a lower concentration. Others transport solutes against an electrochemical gradient; this requires a source of metabolic energy.
Some transporters simply facilitate passive diffusion of a solute across the membrane, from a higher concentration to a lower concentration. Others transport solutes against an electrochemical gradient; this requires a source of metabolic energy.