2.3 Buffering against pH Changes in Biological Systems
Almost every biological process is pH-dependent; a small change in pH produces a large change in the rate of the process. This is true not only for the many reactions in which the ion is a direct participant, but also for those reactions in which there is no apparent role for ions. The enzymes that catalyze cellular reactions, and many of the molecules on which they act, contain ionizable groups with characteristic values. The protonated amino and carboxyl groups of amino acids and the phosphate groups of nucleotides, for example, function as weak acids; their ionic state is determined by the pH of the surrounding medium. (When an ionizable group is sequestered in the middle of a protein, away from the aqueous solvent, its , or apparent , can be significantly different from its in water.) As we noted above, ionic interactions are among the forces that stabilize a protein molecule and allow an enzyme to recognize and bind to its substrate.
Cells and organisms maintain a specific and constant cytosolic pH, usually near pH 7, keeping biomolecules in their optimal ionic state. In multicellular organisms, the pH of extracellular fluids is also tightly regulated. Constancy of pH is achieved primarily by biological buffers: mixtures of weak acids and their conjugate bases.
Buffers Are Mixtures of Weak Acids and Their Conjugate Bases
Buffers are aqueous systems that tend to resist changes in pH when small amounts of acid or base are added. A buffer system consists of a weak acid (the proton donor) and its conjugate base (the proton acceptor). As an example, a mixture of equal concentrations of acetic acid and acetate ion, found at the midpoint of the titration curve in Figure 2-16, is a buffer system. Notice that the titration curve of acetic acid has a relatively flat zone extending about 1 pH unit on either side of its midpoint pH of 4.76. In this zone, a given amount of or added to the system has much less effect on pH than the same amount added outside the zone. This relatively flat zone is the buffering region of the acetic acid–acetate buffer pair. At the midpoint of the buffering region, where the concentration of the proton donor (acetic acid) exactly equals that of the proton acceptor (acetate), the buffering power of the system is maximal; that is, its pH changes least on addition of or . The pH at this point in the titration curve of acetic acid is equal to its apparent . The pH of the acetate buffer system does change slightly when a small amount of or is added, but this change is very small compared with the pH change that would result if the same amount of or were added to pure water or to a solution of the salt of a strong acid and strong base, such as NaCl, which has no buffering power.
Buffering results from two reversible reaction equilibria occurring in a solution of nearly equal concentrations of a proton donor and its conjugate proton acceptor. Figure 2-18 explains how a buffer system works. Whenever or is added to a buffer, the result is a small change in the ratio of the relative concentrations of the weak acid and its anion and thus a small change in pH. The decrease in concentration of one component of the system is balanced exactly by an increase in the other. The sum of the buffer components does not change; only their ratio changes.
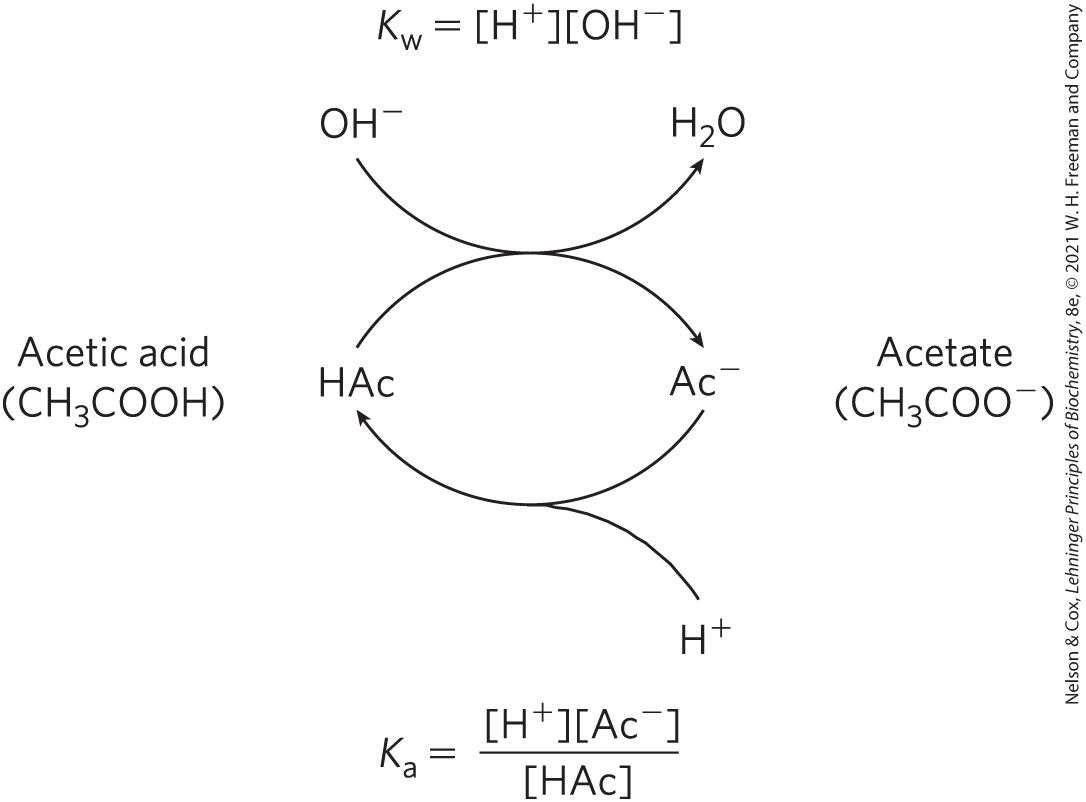
FIGURE 2-18 The acetic acid–acetate pair as a buffer system. The system is capable of absorbing either or through the reversibility of the dissociation of acetic acid. The proton donor, acetic acid (HAc), contains a reserve of bound , which can be released to neutralize an addition of to the system, forming . This happens because the product transiently exceeds . The equilibrium quickly adjusts to restore the product to (at ), thus transiently reducing the concentration of . But now the quotient is less than , so HAc dissociates further to restore equilibrium. Similarly, the conjugate base, , can react with ions added to the system; again, the two ionization reactions simultaneously come to equilibrium. Thus, a conjugate acid-base pair, such as acetic acid and acetate ion, tends to resist a change in pH when small amounts of acid or base are added. Buffering action is simply the consequence of two reversible reactions taking place simultaneously and reaching their points of equilibrium as governed by their equilibrium constants, and .
Each conjugate acid-base pair has a characteristic pH zone in which it is an effective buffer (Fig. 2-17). The pair has a of 6.86 and thus can serve as an effective buffer system between approximately pH 5.9 and pH 7.9; the pair, with a of 9.25, can act as a buffer between approximately pH 8.3 and pH 10.3.
The Henderson-Hasselbalch Equation Relates , , and Buffer Concentration
The titration curves of acetic acid, and (Fig. 2-17) have nearly identical shapes, suggesting that these curves reflect a fundamental law or relationship. This is indeed the case. The shape of the titration curve of any weak acid is described by the Henderson-Hasselbalch equation, which is important for understanding buffer action and acid-base balance in the blood and tissues of vertebrates. This equation is simply a useful way of restating the expression for the ionization constant of an acid. For the ionization of a weak acid HA, the Henderson-Hasselbalch equation can be derived as follows:
First solve for []:
Then take the negative logarithm of both sides:
Substitute pH for and for :
Now invert , which requires changing its sign, to obtain the Henderson-Hasselbalch equation:
(2-9)
This equation fits the titration curve of all weak acids and enables us to deduce some important quantitative relationships. For example, it shows why the of a weak acid is equal to the pH of the solution at the midpoint of its titration. At that point, , and
The Henderson-Hasselbalch equation also allows us (1) to calculate , given pH and the molar ratio of proton donor and acceptor; (2) to calculate pH, given and the molar ratio of proton donor and acceptor; and (3) to calculate the molar ratio of proton donor and acceptor, given pH and .
Weak Acids or Bases Buffer Cells and Tissues against pH Changes
The intracellular and extracellular fluids of multicellular organisms have a characteristic and nearly constant pH. The organism’s first line of defense against changes in internal pH is provided by buffer systems. The cytoplasm of most cells contains high concentrations of proteins, and these proteins contain many amino acids with functional groups that are weak acids or weak bases. For example, the side chain of histidine (Fig. 2-19) has a of 6.0 and thus can exist in either the protonated form or the unprotonated form near neutral pH. Proteins containing histidine residues therefore buffer effectively near neutral pH.
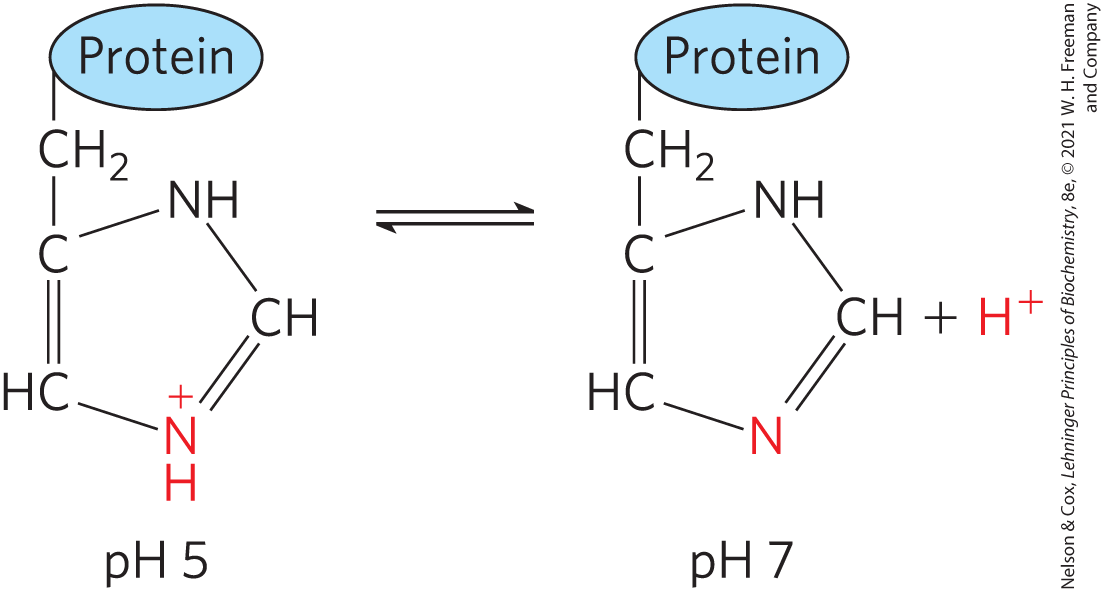
FIGURE 2-19 Ionization of histidine. The amino acid histidine, a component of proteins, is a weak acid. The of the protonated nitrogen of the side chain is 6.0.
WORKED EXAMPLE 2-4 Ionization of Histidine
Calculate the fraction of histidine that has its imidazole side chain protonated at pH 7.3. The values for histidine are , , and (see Fig. 3-12b).
SOLUTION:
The three ionizable groups in histidine have sufficiently different values (different by at least 2 pH units) that the first acid is almost completely ionized before the second acid (protonated imidazole) begins to dissociate a proton, and the second ionizes almost completely before the third begins to dissociate its proton. (With the Henderson-Hasselbalch equation, we can easily show that a weak acid goes from 1% ionized at 2 pH units below its to 99% ionized at 2 pH units above its ; see also Fig. 3-12b.) At pH 7.3, the carboxyl group of histidine is entirely deprotonated and the α-amino group is fully protonated . We can therefore assume that at pH 7.3, the only group that is partially dissociated is the imidazole group, which can be protonated (we’ll abbreviate as ) or not (His).
We use the Henderson-Hasselbalch equation:
Substituting and pH = 7.3:
This gives us the ratio of [His] to (20 to 1 in this case). We want to convert this ratio to the fraction of total histidine that is in the unprotonated form (His) at pH 7.3. That fraction is 20/21 (20 parts His per 1 part , in a total of 21 parts histidine in either form), or about 95.2%; the remainder (100% minus 95.2%) is protonated—about 5%.
Nucleotides such as ATP, as well as many metabolites of low molecular weight, contain ionizable groups that can contribute buffering power to the cytoplasm. Some highly specialized organelles and extracellular compartments have high concentrations of compounds that contribute buffering capacity: organic acids buffer the vacuoles of plant cells; ammonia buffers urine.
Two especially important biological buffers are the phosphate and bicarbonate systems. The phosphate buffer system, which acts in the cytoplasm of all cells, consists of as proton donor and as proton acceptor:
The phosphate buffer system is maximally effective at a pH close to its of 6.86 (Figs 2-15, 2-17) and thus tends to resist pH changes in the range between about 5.9 and 7.9. It is therefore an effective buffer in biological fluids; in mammals, for example, extracellular fluids and most cytoplasmic compartments have a pH in the range of 6.9 to 7.4.
WORKED EXAMPLE 2-5 Phosphate Buffers
(a) What is the pH of a mixture of and ?
SOLUTION:
We use the Henderson-Hasselbalch equation, which we’ll express here as
In this case, the acid (the species that gives up a proton) is , and the conjugate base (the species that gains a proton) is . Substituting the given concentrations of acid and conjugate base and the results in
We can roughly check this answer. When more conjugate base than acid is present, the acid is more than 50% titrated and thus the pH is above the , where the acid is exactly 50% titrated.
(b) If 1.0 mL of 10.0 m NaOH is added to a liter of the buffer prepared in (a), how much will the pH change?
SOLUTION:
A liter of the buffer contains 0.042 mol of . Adding 1.0 mL of 10.0 m NaOH (0.010 mol) would titrate an equivalent amount (0.010 mol) of to , resulting in 0.032 mol of and 0.068 mol of . The new pH is
(c) If 1.0 mL of 10.0 m NaOH is added to a liter of pure water at pH 7.0, what is the final pH? Compare this with the answer in (b).
SOLUTION:
The NaOH dissociates completely into and , giving . We can define a term pOH analogous with pH to express of a solution. The pOH is the negative logarithm of , so in our example pOH = 2.0. Given that in all solutions, pH + pOH = 14 (see Table 2-5), the pH of the solution is 12. So, an amount of NaOH that increases the pH of water from 7 to 12 increases the pH of a buffered solution, as in (b), from 7.0 to just 7.2. Such is the power of buffering!
Why is pH + pOH = 14?
Taking the negative log of both sides of the equation gives
Blood plasma is buffered in part by the bicarbonate system, consisting of carbonic acid as proton donor and bicarbonate as proton acceptor ( is the first of several equilibrium constants in the bicarbonate buffering system):
This buffer system is more complex than other conjugate acid-base pairs because one of its components, carbonic acid , is formed from dissolved (aq) carbon dioxide and water, in a reversible reaction:
Carbon dioxide is a gas under normal conditions, and dissolved in an aqueous solution is in equilibrium with in the gas (g) phase:
The pH of a bicarbonate buffer system depends on the concentrations of and , the proton donor and acceptor components. The concentration of in turn depends on the concentration of dissolved , which in turn depends on the concentration of in the gas phase, or the partial pressure of , denoted . Thus, the pH of a bicarbonate buffer exposed to a gas phase is ultimately determined by the concentration of in the aqueous phase and by in the gas phase.
The bicarbonate buffer system is an effective physiological buffer near pH 7.4, because the of blood plasma is in equilibrium with a large reserve capacity of in the air space of the lungs. As noted above, this buffer system involves three reversible equilibria, in this case between gaseous in the lungs and bicarbonate in the blood plasma (Fig. 2-20).
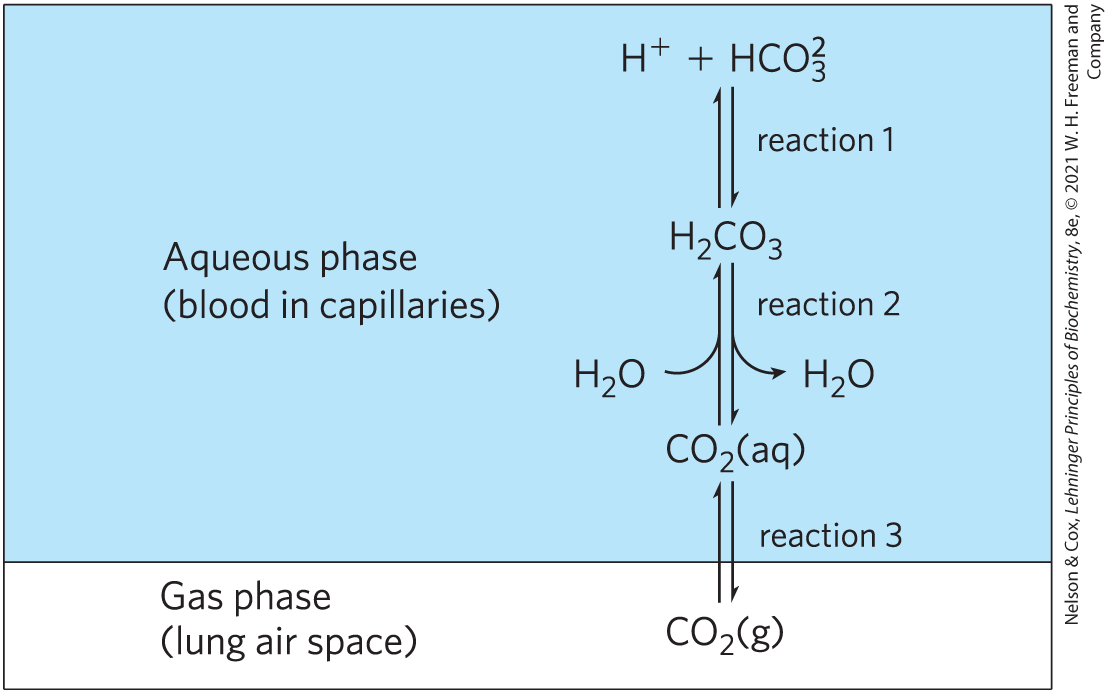
FIGURE 2-20 The bicarbonate buffer system. in the air space of the lungs is in equilibrium with the bicarbonate buffer in the blood plasma passing through the lung capillaries. Because the concentration of dissolved can be adjusted rapidly through changes in the rate of breathing, the bicarbonate buffer system of the blood is in near-equilibrium with a large potential reservoir of .
Blood can pick up , such as from the lactic acid produced in muscle tissue during vigorous exercise. Alternatively, it can lose , such as by protonation of the produced during protein catabolism. When is added to blood as it passes through the tissues, reaction 1 in Figure 2-20 proceeds toward a new equilibrium, in which [] is increased. This in turn increases [] in the blood (reaction 2) and thus increases the partial pressure of in the air space of the lungs (reaction 3); the extra is exhaled. Conversely, when is lost from the blood, the opposite events occur: more dissociates into and , and thus more from the lungs dissolves in blood plasma. The rate of respiration—that is, the rate of inhaling and exhaling—can quickly adjust these equilibria to keep the blood pH nearly constant. The rate of respiration is controlled by the brain stem, where detection of an increased blood or decreased blood pH triggers deeper and more frequent breathing.
Hyperventilation, the rapid breathing sometimes elicited by stress or anxiety, tips the normal balance of breathed in and breathed out in favor of too much breathed out, raising the blood pH to 7.45 or higher and causing alkalosis. This alkalosis can lead to dizziness, headache, weakness, and fainting. One home remedy for mild alkalosis is to breathe briefly into a paper bag. The air in the bag becomes enriched in , and inhaling this air increases the concentration in the body and blood and decreases blood pH.
At the normal pH of blood plasma (7.4), very little is present relative to and the addition of just a small amount of base ( or ) would titrate this , exhausting the buffering capacity. The important role of at ) in buffering blood plasma (pH ∼ 7.4) seems inconsistent with our earlier statement that a buffer is most effective in the range of 1 pH unit above and below its . The explanation for this apparent paradox is the large reservoir of dissolved in blood, which we refer to as . Its rapid equilibration with results in the formation of additional :
It is useful in clinical medicine to have a simple expression for blood pH in terms of , which is commonly monitored along with other blood gases. We can define a constant, , which is the equilibrium constant for the hydration of to form :
(The concentration of water is so high (55.5 m) that dissolving doesn’t change appreciably, so is made part of the constant .) Then, to take the reservoir into account, we can express as and substitute this expression for in the equation for the acid dissociation of :
Now, the overall equilibrium for dissociation of can be expressed in these terms:
We can calculate the value of the new constant, , and the corresponding apparent pK, or , from the experimentally determined values of and at :
In clinical medicine, it is common to refer to as the conjugate acid and to use the apparent, or combined, of 6.1 to simplify calculation of pH from . The concentration of dissolved is a function of , which in the lung is about 4.8 kilopascals (kPa), corresponding to . Plasma is normally about 24 mm, so is about 20, and the blood pH is 6.1 + log 20 ≈ 7.4.
Untreated Diabetes Produces Life-Threatening Acidosis
Many of the enzymes that function in the blood have evolved to have maximal activity between pH 7.35 and 7.45, the normal pH range of human blood plasma. Enzymes typically show maximal catalytic activity at a characteristic pH, called the optimum pH (Fig. 2-21). On either side of this optimum pH, catalytic activity often declines sharply. Thus, a small change in pH can make a large difference in the rate of some crucial enzyme-catalyzed reactions. Biological control of the pH of cells and body fluids is therefore of central importance in all aspects of metabolism and cellular activities, and changes in blood pH have marked physiological consequences, as we know from the alarming experiments described in Box 2-1.
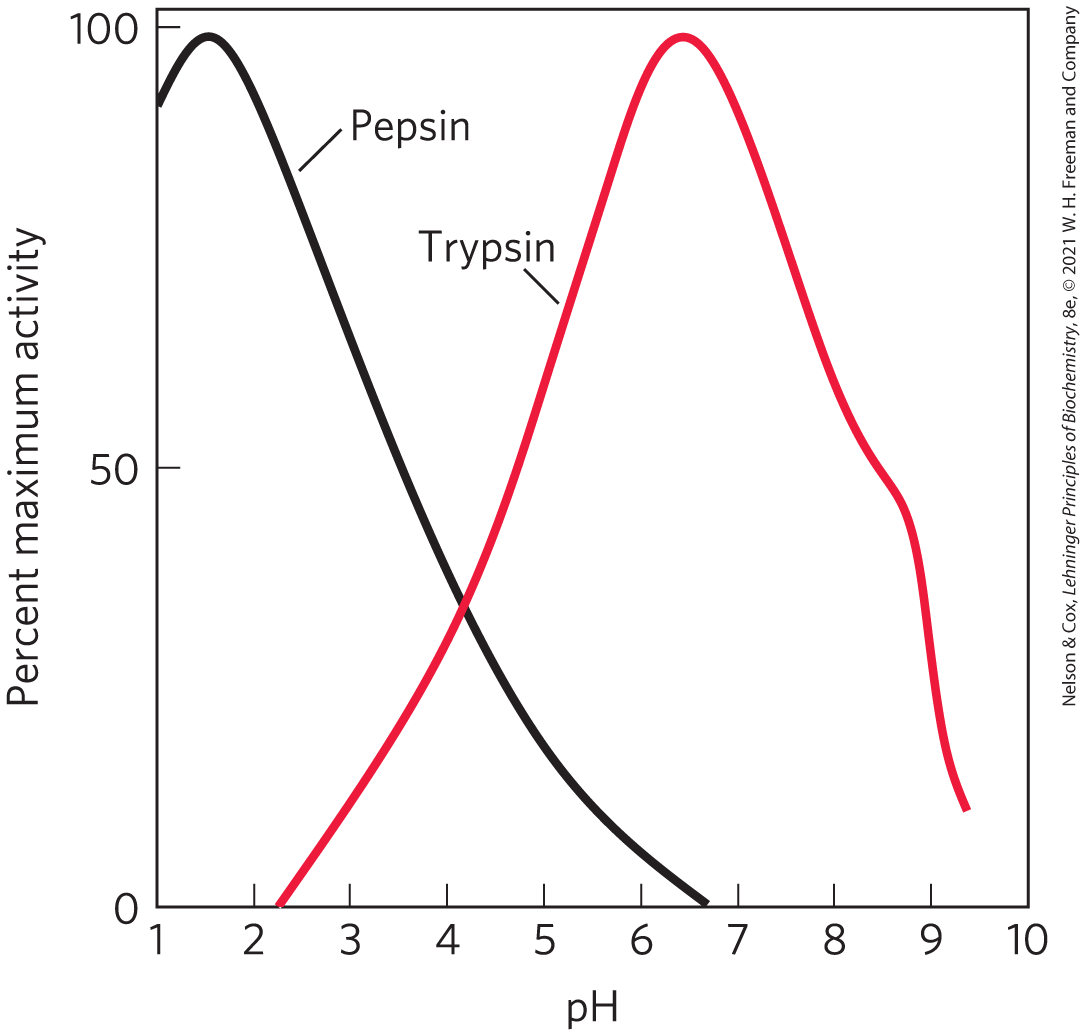
FIGURE 2-21 The pH optima of two enzymes. Pepsin is a digestive enzyme secreted into gastric juice, which has a pH of ∼1.5, allowing pepsin to act optimally. Trypsin, a digestive enzyme that acts in the small intestine, has a pH optimum that matches the neutral pH in the lumen of the small intestine.
In individuals with untreated diabetes mellitus, the lack of insulin, or insensitivity to insulin, disrupts the uptake of glucose from blood into the tissues and forces the tissues to use stored fatty acids as their primary fuel. For reasons we describe in detail later in the book (see Chapter 23), this dependence on fatty acids results in acidosis, the accumulation of high concentrations of two carboxylic acids, β-hydroxybutyric acid and acetoacetic acid (a combined blood plasma level of 90 mg/100 mL, compared with <3 mg/100 mL in control (healthy) individuals; urinary excretion of 5 g/24 h, compared with <125 mg/24 h in controls). Dissociation of these acids lowers the pH of blood plasma to less than 7.35. Severe acidosis (characterized by low blood pH) produces headache, drowsiness, nausea, vomiting, and diarrhea, followed by stupor, coma, and convulsions, presumably because, at the lower pH, some enzyme(s) do not function optimally. When a patient is found to have high blood glucose, low plasma pH, and high levels of β-hydroxybutyric acid and acetoacetic acid in blood and urine, diabetes mellitus is the likely diagnosis.
Other conditions can also produce acidosis. For example, fasting and starvation force the use of stored fatty acids as fuel, with the same consequences as for diabetes. Very heavy exertion, such as a sprint by runners or cyclists, leads to temporary accumulation of lactic acid in the blood. Kidney failure results in a diminished capacity to regulate bicarbonate levels. Lung diseases (such as emphysema, pneumonia, and asthma) reduce the capacity to dispose of the produced by fuel oxidation in the tissues, with the resulting accumulation of .
Acidosis is treated by dealing with the underlying condition—administering insulin to people with diabetes, and steroids or antibiotics to people with lung disease. Severe acidosis can be reversed by administering bicarbonate solution intravenously.
WORKED EXAMPLE 2-6 Treatment of Acidosis with Bicarbonate
Why does intravenous administration of a bicarbonate solution raise the plasma pH?
SOLUTION:
The ratio of to determines the pH of the bicarbonate buffer, according to the equation
where is directly related to , the partial pressure of . So, if is increased with no change in , the blood pH will rise.
SUMMARY 2.3 Buffering against pH Changes in Biological Systems
- A mixture of a weak acid (or base) and its salt resists changes in pH caused by the addition of or . The mixture thus functions as a buffer.
- The pH of a solution of a weak acid (or base) and its salt is given by the Henderson-Hasselbalch equation:
In cells and tissues, phosphate and bicarbonate buffer systems maintain intracellular and extracellular fluids near pH 7.4. Enzymes generally work optimally near this physiological pH.
- Medical conditions such as untreated diabetes that lower the pH of blood, causing acidosis, or raise it, causing alkalosis, can be life-threatening.
 The enzymes that catalyze cellular reactions, and many of the molecules on which they act, contain ionizable groups with characteristic values. The protonated amino and carboxyl groups of amino acids and the phosphate groups of nucleotides, for example, function as weak acids; their ionic state is determined by the pH of the surrounding medium. (When an ionizable group is sequestered in the middle of a protein, away from the aqueous solvent, its , or apparent , can be significantly different from its in water.) As we noted above, ionic interactions are among the forces that stabilize a protein molecule and allow an enzyme to recognize and bind to its substrate.
The enzymes that catalyze cellular reactions, and many of the molecules on which they act, contain ionizable groups with characteristic values. The protonated amino and carboxyl groups of amino acids and the phosphate groups of nucleotides, for example, function as weak acids; their ionic state is determined by the pH of the surrounding medium. (When an ionizable group is sequestered in the middle of a protein, away from the aqueous solvent, its , or apparent , can be significantly different from its in water.) As we noted above, ionic interactions are among the forces that stabilize a protein molecule and allow an enzyme to recognize and bind to its substrate. The shape of the titration curve of any weak acid is described by the Henderson-Hasselbalch equation, which is important for understanding buffer action and acid-base balance in the blood and tissues of vertebrates. This equation is simply a useful way of restating the expression for the ionization constant of an acid. For the ionization of a weak acid HA, the Henderson-Hasselbalch equation can be derived as follows:
The shape of the titration curve of any weak acid is described by the Henderson-Hasselbalch equation, which is important for understanding buffer action and acid-base balance in the blood and tissues of vertebrates. This equation is simply a useful way of restating the expression for the ionization constant of an acid. For the ionization of a weak acid HA, the Henderson-Hasselbalch equation can be derived as follows: The bicarbonate buffer system is an effective physiological buffer near pH 7.4, because the of blood plasma is in equilibrium with a large reserve capacity of in the air space of the lungs. As noted above, this buffer system involves three reversible equilibria, in this case between gaseous in the lungs and bicarbonate in the blood plasma (
The bicarbonate buffer system is an effective physiological buffer near pH 7.4, because the of blood plasma is in equilibrium with a large reserve capacity of in the air space of the lungs. As noted above, this buffer system involves three reversible equilibria, in this case between gaseous in the lungs and bicarbonate in the blood plasma (
 A mixture of a weak acid (or base) and its salt resists changes in pH caused by the addition of or . The mixture thus functions as a buffer.
A mixture of a weak acid (or base) and its salt resists changes in pH caused by the addition of or . The mixture thus functions as a buffer.