2.2 Ionization of Water, Weak Acids, and Weak Bases
Although many of the solvent properties of water can be explained in terms of the uncharged molecule, the small degree of ionization of water to hydrogen ions and hydroxide ions must also be taken into account. Like all reversible reactions, the ionization of water can be described by an equilibrium constant. When weak acids are dissolved in water, they contribute by ionizing; weak bases consume by becoming protonated. These processes are also governed by equilibrium constants. The total hydrogen ion concentration from all sources is experimentally measurable and is expressed as the pH of the solution. To predict the state of ionization of solutes in water, we must take into account the relevant equilibrium constants for each ionization reaction. Therefore, we now turn to a brief discussion of the ionization of water and of weak acids and bases dissolved in water.
Pure Water Is Slightly Ionized
Water molecules have a slight tendency to undergo reversible ionization to yield a hydrogen ion (a proton) and a hydroxide ion, giving the equilibrium
(2-1)
Although we commonly show the dissociation product of water as , free protons do not exist in solution; hydrogen ions formed in water are immediately hydrated to form hydronium ions . Hydrogen bonding between water molecules makes the hydration of dissociating protons virtually instantaneous:
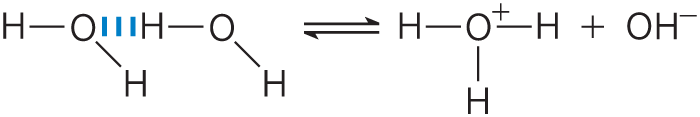
The ionization of water can be measured by its electrical conductivity; pure water carries electrical current as migrates toward the cathode and migrates toward the anode. The movement of hydronium and hydroxide ions in the electric field is extremely fast compared with that of other ions such as , , and . This high ionic mobility results from the kind of “proton hopping” shown in Figure 2-13. No individual proton moves very far through the bulk solution, but a series of proton hops between hydrogen-bonded water molecules causes the net movement of a proton over a long distance in a remarkably short time. ( also moves rapidly by proton hopping, but in the opposite direction.) As a result of the high ionic mobility of , acid-base reactions in aqueous solutions are exceptionally fast. As noted earlier, proton hopping very likely also plays a role in biological proton-transfer reactions (Fig. 2-10).
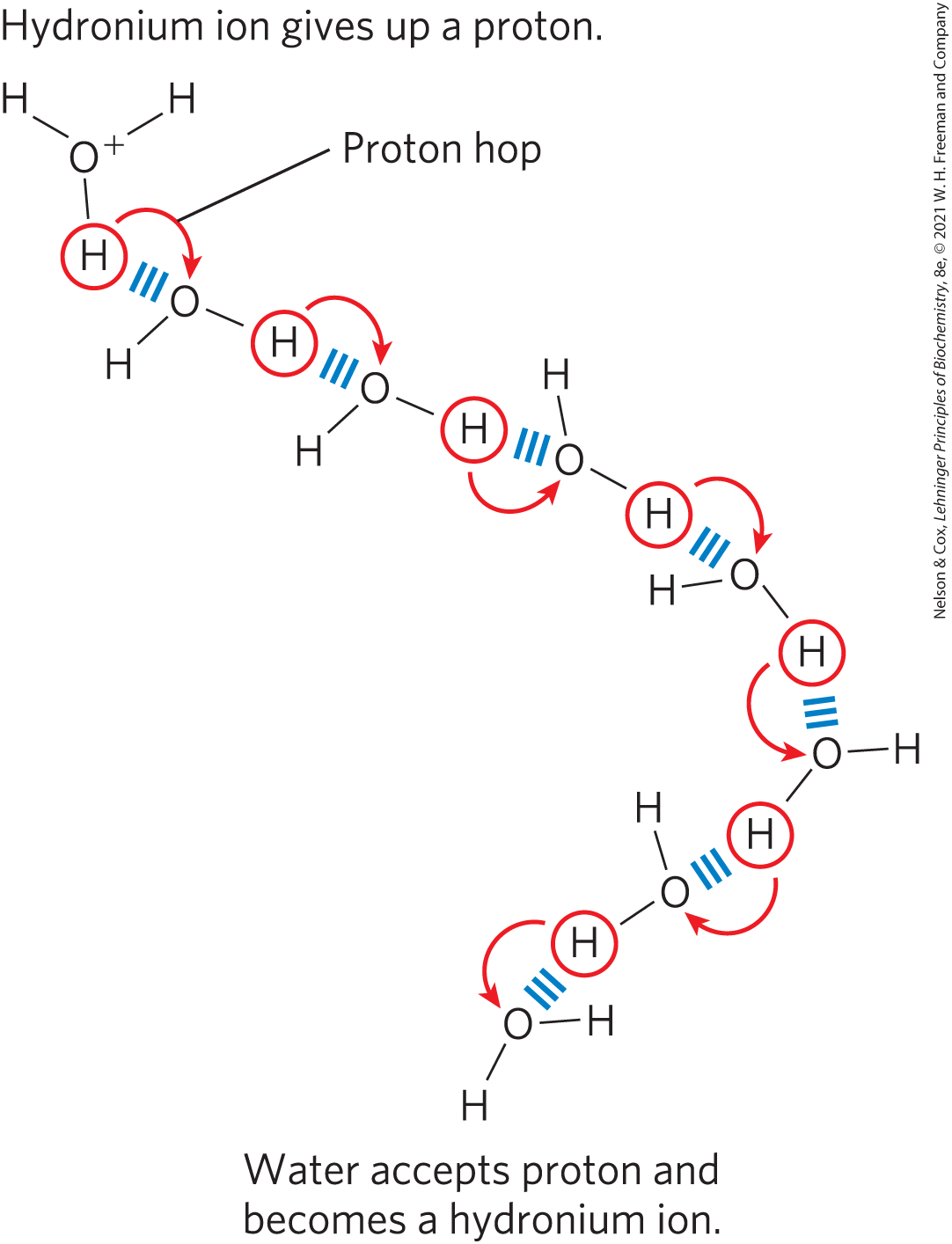
FIGURE 2-13 Proton hopping. Short “hops” of protons between a series of hydrogen-bonded water molecules result in an extremely rapid net movement of a proton over a long distance. As a hydronium ion (upper left) gives up a proton, a water molecule some distance away (bottom) acquires one, becoming a hydronium ion. Proton hopping is much faster than true diffusion and explains the remarkably high ionic mobility of ions compared with other monovalent cations such as and .
Because reversible ionization is crucial to the role of water in cellular function, we must have a means of expressing the extent of ionization of water in quantitative terms. A brief review of some properties of reversible chemical reactions shows how this can be done.
The position of equilibrium of any chemical reaction is given by its equilibrium constant, (sometimes expressed simply as K). For the generalized reaction
(2-2)
the equilibrium constant can be defined in terms of the concentrations of reactants (A and B) and products (C and D) at equilibrium:
Strictly speaking, the concentration terms should be the activities, or effective concentrations in nonideal solutions, of each species. Except in very accurate work, however, the equilibrium constant may be approximated by measuring the concentrations at equilibrium. For reasons beyond the scope of this discussion, equilibrium constants are dimensionless. Nonetheless, we have generally retained the concentration units (m) in the equilibrium expressions used in this book to remind you that molarity is the unit of concentration used in calculating .
The equilibrium constant is fixed and characteristic for any given chemical reaction at a specified temperature. It defines the composition of the final equilibrium mixture, regardless of the starting amounts of reactants and products. Conversely, we can calculate the equilibrium constant for a given reaction at a given temperature if the equilibrium concentrations of all its reactants and products are known. As we showed in Chapter 1 (p. 24), the standard free-energy change is directly related to ln .
The Ionization of Water Is Expressed by an Equilibrium Constant
The degree of ionization of water at equilibrium (Eqn 2-1) is small; at only about two of every molecules in pure water are ionized at any instant. The equilibrium constant for the reversible ionization of water is
(2-3)
In pure water at , the concentration of water is 55.5 m—grams of in 1 L divided by its gram molecular weight: (1,000 g/L)/(18.015 g/mol)—and is essentially constant in relation to the very low concentrations of and , namely . Accordingly, we can substitute 55.5 m in the equilibrium constant expression (Eqn 2-3) to yield
On rearranging, this becomes
(2-4)
where designates the product , the ion product of water at .
The value for determined by electrical-conductivity measurements of pure water, is at . Substituting this value for in Equation 2-4 gives the value of the ion product of water:
Thus the product in aqueous solutions at always equals . When there are exactly equal concentrations of and , as in pure water, the solution is said to be at neutral pH. At this pH, the concentrations of and can be calculated from the ion product of water as follows:
Solving for gives
As the ion product of water is constant, whenever is greater than must be less than , and vice versa. When is very high, as in a solution of hydrochloric acid, must be very low. From the ion product of water, we can calculate if we know , and vice versa.
WORKED EXAMPLE 2-2 Calculation of
What is the concentration of in a solution of 0.1 m NaOH? Because NaOH is a strong base, it dissociates completely into and .
SOLUTION:
We begin with the equation for the ion product of water:
With , solving for gives
WORKED EXAMPLE 2-3 Calculation of
What is the concentration of in a solution with an concentration of
SOLUTION:
We begin with the equation for the ion product of water:
With , solving for gives
In all calculations, be sure to round your answer to the correct number of significant figures, as here.
The pH Scale Designates the and Concentrations
The ion product of water, , is the basis for the pH scale (Table 2-5). It is a convenient means of designating the concentration of (and thus of ) in any aqueous solution in the range between and . The symbol p denotes “negative logarithm of.” The term pH is defined by the expression
| pH | pOHa | ||
|---|---|---|---|
0 |
14 |
||
1 |
13 |
||
2 |
12 |
||
3 |
11 |
||
4 |
10 |
||
5 |
9 |
||
6 |
8 |
||
7 |
7 |
||
8 |
6 |
||
9 |
5 |
||
10 |
4 |
||
11 |
3 |
||
12 |
2 |
||
13 |
1 |
||
14 |
|
0 |
|
aThe expression pOH is sometimes used to describe the basicity, or concentration, of a solution; pOH is defined by the expression , which is analogous to the expression for pH. Note that in all cases, pH + pOH = 14. |
|||
For a precisely neutral solution at , in which the concentration of hydrogen ions is , the pH can be calculated as follows:
Note that the concentration of must be expressed in molar (m) terms.
The value of 7 for the pH of a precisely neutral solution is not an arbitrarily chosen figure; it is derived from the absolute value of the ion product of water at , which by convenient coincidence is a round number. Solutions having a pH greater than 7 are alkaline or basic; the concentration of is greater than that of . Conversely, solutions having a pH less than 7 are acidic.
Keep in mind that the pH scale is logarithmic, not arithmetic. To say that two solutions differ in pH by 1 pH unit means that one solution has ten times the concentration of the other, but it does not tell us the absolute magnitude of the difference. Figure 2-14 gives the pH values of some common aqueous fluids. A cola drink (pH 3.0) or red wine (pH 3.7) has an concentration approximately 10,000 times that of blood (pH 7.4).
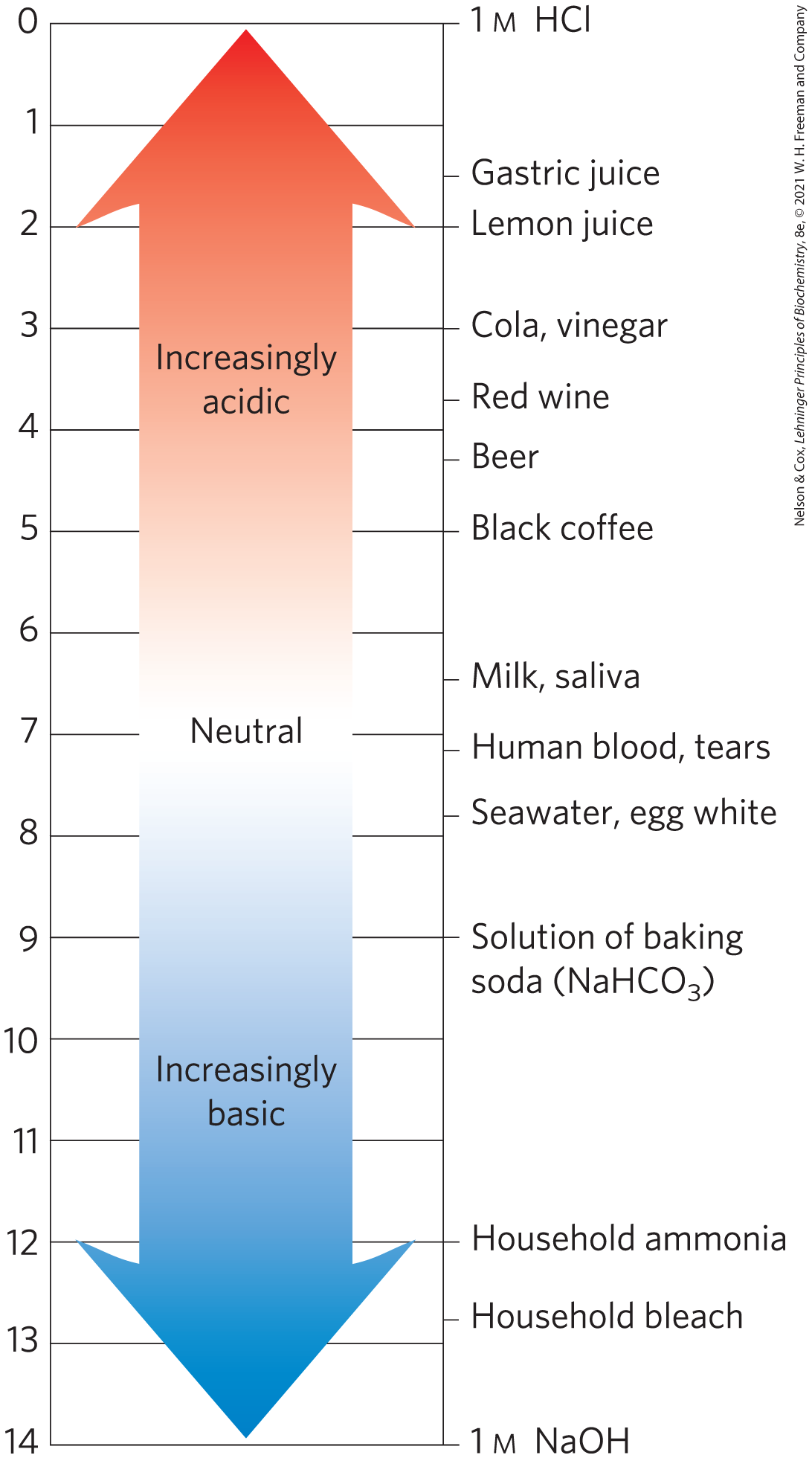
FIGURE 2-14 The pH of some aqueous fluids.
Accurate determinations of pH in the chemical or clinical laboratory are made with a glass electrode that is selectively sensitive to concentration but insensitive to , , and other cations. In a pH meter, the signal from the glass electrode placed in a test solution is amplified and compared with the signal generated by a solution of accurately known pH.
Measurement of pH is one of the most important and frequently used procedures in biochemistry. The pH affects the structure and activity of biological macromolecules, so a small change in pH can cause a large change in the structure and function of a protein. Measurements of the pH of blood and urine are commonly used in medical diagnoses. The pH of the blood plasma of people with severe, uncontrolled diabetes, for example, is often below the normal value of 7.4; this condition is called acidosis (described in more detail below). In certain other diseases the pH of the blood is higher than normal, a condition known as alkalosis. Extreme acidosis or alkalosis can be life-threatening (see Box 2-1).
Weak Acids and Bases Have Characteristic Acid Dissociation Constants
Hydrochloric, sulfuric, and nitric acids, commonly called strong acids, are completely ionized in dilute aqueous solutions; the strong bases NaOH and KOH are also completely ionized. Of more interest to biochemists is the behavior of weak acids and bases—those not completely ionized when dissolved in water. These are ubiquitous in biological systems and play important roles in metabolism and its regulation. The behavior of aqueous solutions of weak acids and bases is best understood if we first define some terms.
Acids (in the Brønsted-Lowry definition) are proton donors, and bases are proton acceptors. When a proton donor such as acetic acid loses a proton, it becomes the corresponding proton acceptor, in this case the acetate anion . A proton donor and its corresponding proton acceptor make up a conjugate acid-base pair (Fig. 2-15), related by the reversible reaction
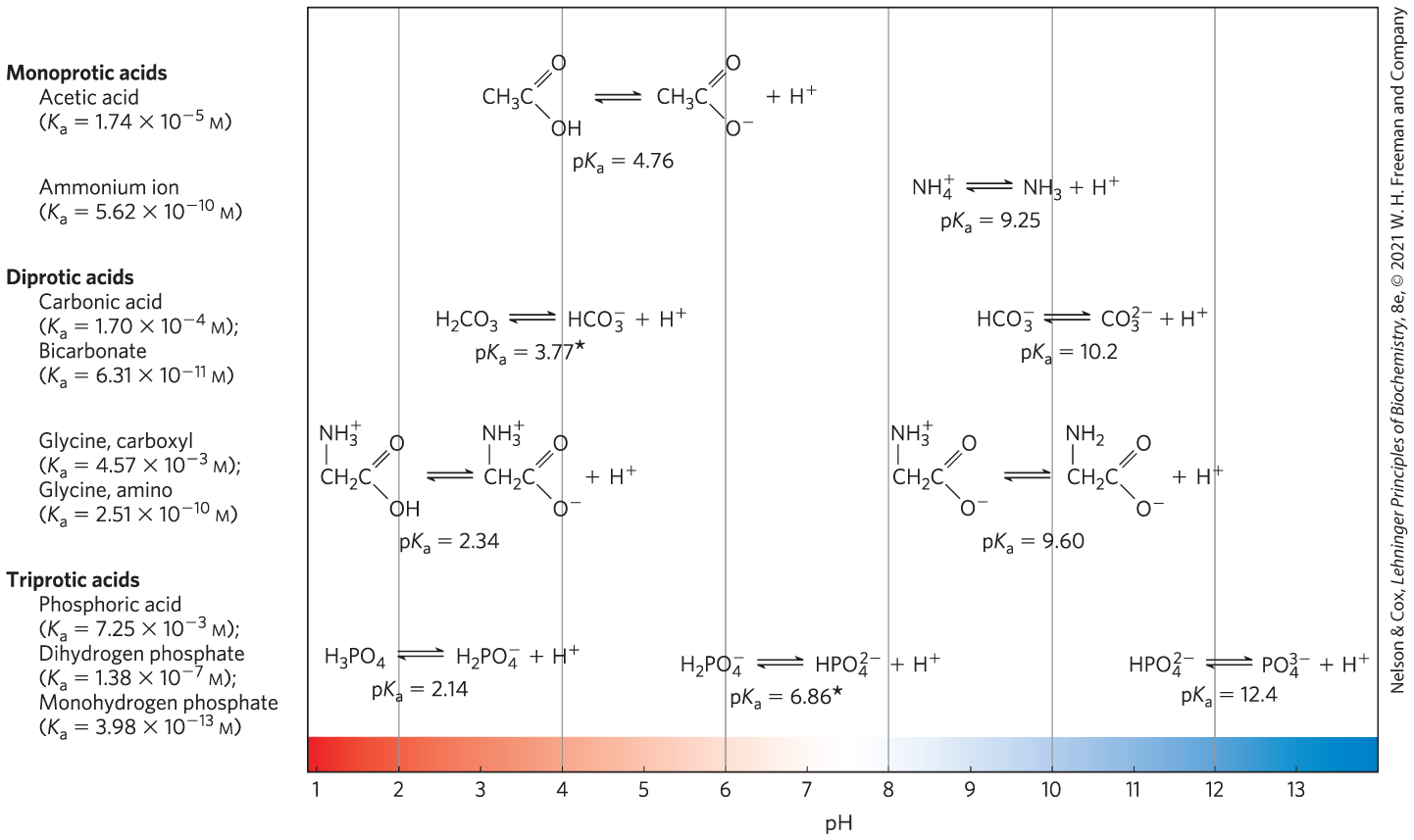
FIGURE 2-15 Conjugate acid-base pairs consist of a proton donor and a proton acceptor. Some compounds, such as acetic acid and ammonium ion, are monoprotic: they can give up only one proton. Others are diprotic (carbonic acid and glycine) or triprotic (phosphoric acid). The dissociation reactions for each pair are shown where they occur along a pH gradient. The equilibrium or dissociation constant (K) and its negative logarithm, the , are shown for each reaction. *For an explanation of apparent discrepancies in values for carbonic acid , see p. 63; and for dihydrogen phosphate , see p. 58.
Each acid has a characteristic tendency to lose its proton in an aqueous solution. The stronger the acid, the greater its tendency to lose its proton. The tendency of any acid (HA) to lose a proton and form its conjugate base is defined by the equilibrium constant for the reversible reaction
for which
Equilibrium constants for ionization reactions are usually called ionization constants or acid dissociation constants, often designated . The dissociation constants of some acids are given in Figure 2-15. Stronger acids, such as phosphoric and carbonic acids, have larger ionization constants; weaker acids, such as monohydrogen phosphate , have smaller ionization constants.
Also included in Figure 2-15 are values of , which is analogous to pH and is defined by the equation
The stronger the tendency to dissociate a proton, the stronger the acid and the lower its . As we shall now see, the of any weak acid can be determined experimentally.
Titration Curves Reveal the of Weak Acids
Titration can be used to determine the amount of an acid in a given solution. A measured volume of the acid is titrated with a solution of a strong base, usually sodium hydroxide (NaOH), of known concentration. The NaOH is added in small increments until the acid is consumed (neutralized), as determined with an indicator dye or a pH meter. The concentration of the acid in the original solution can be calculated from the volume and concentration of NaOH added. The amounts of acid and base in titrations are often expressed in terms of equivalents, where one equivalent is the amount of a substance that will react with, or supply, one mole of hydrogen ions in an acid-base reaction. Recall that for monoprotic acids such as HCl, 1 mol = 1 equivalent; for diprotic acids such as , 1 mol = 2 equivalents.
A plot of pH against the amount of NaOH added (a titration curve) reveals the of the weak acid. Consider the titration of a 0.1 m solution of acetic acid with 0.1 m NaOH at (Fig. 2-16). Two reversible equilibria are involved in the process (here, for simplicity, acetic acid is denoted HAc):
(2-5)
(2-6)
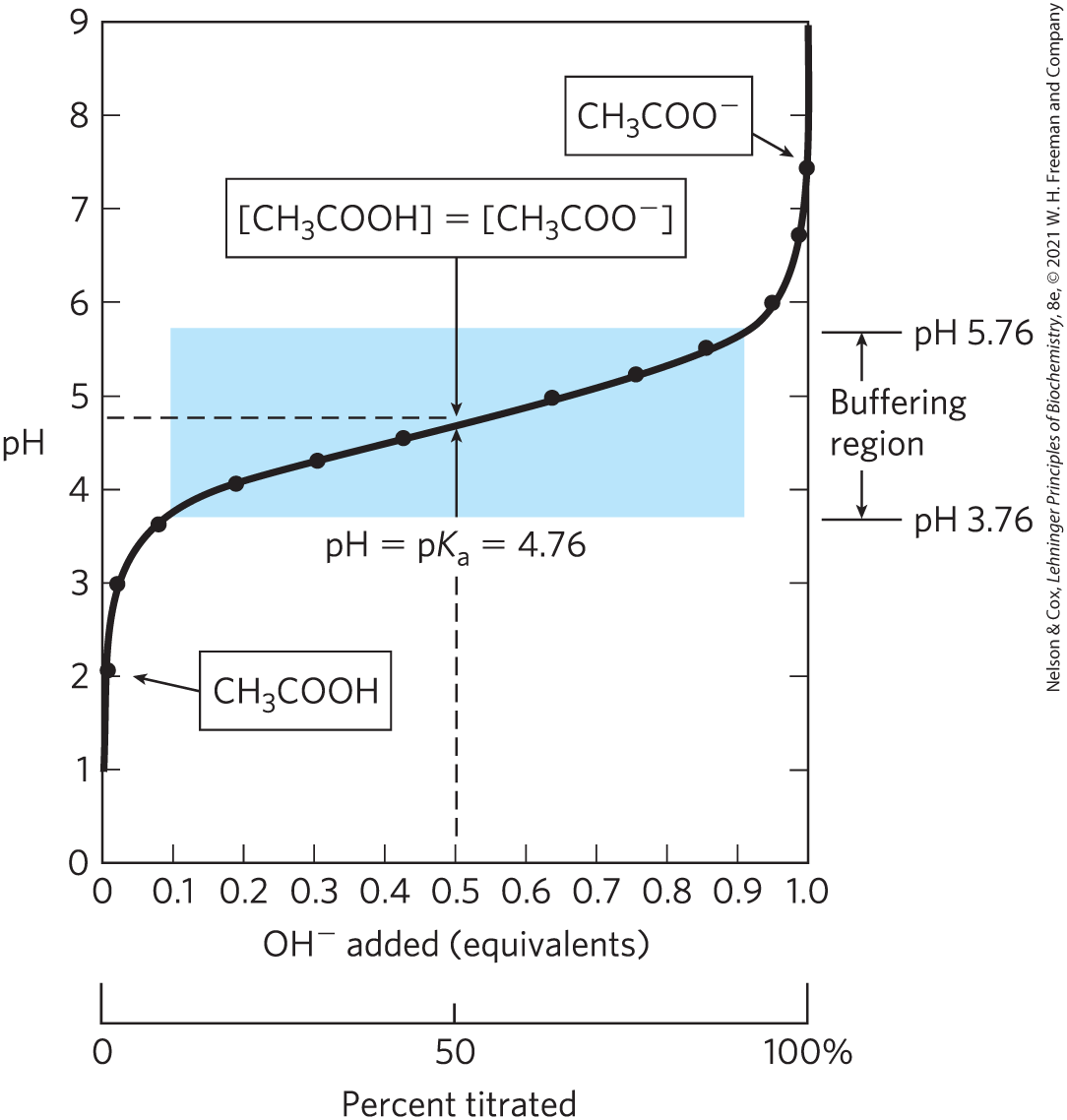
FIGURE 2-16 The titration curve of acetic acid. After addition of each increment of NaOH to the acetic acid solution, the pH of the mixture is measured. This value is plotted against the amount of NaOH added, expressed as a fraction of the total NaOH required to convert all the acetic acid to its deprotonated form, acetate (). The points so obtained yield the titration curve. Shown in the boxes are the predominant ionic forms at the points designated. At the midpoint of the titration, the concentrations of the proton donor and the proton acceptor are equal, and the pH is numerically equal to the . The shaded zone is the useful region of buffering power, generally between 10% and 90% titration of the weak acid.
The equilibria must simultaneously conform to their characteristic equilibrium constants, which are, respectively,
(2-7)
(2-8)
At the beginning of the titration, before any NaOH is added, the acetic acid is already slightly ionized, to an extent that can be calculated from its ionization constant (Eqn 2-8).
As NaOH is gradually introduced, the added combines with the free in the solution to form , to an extent that satisfies the equilibrium relationship in Equation 2-7. As free is removed, HAc dissociates further to satisfy its own equilibrium constant (Eqn 2-8). The net result as the titration proceeds is that more and more HAc ionizes, forming , as the NaOH is added. At the midpoint of the titration, at which exactly 0.5 equivalent of NaOH has been added per equivalent of the acid, one-half of the original acetic acid has undergone dissociation, so that the concentration of the proton donor, [HAc], now equals that of the proton acceptor, . At this midpoint a very important relationship holds: the pH of the equimolar solution of acetic acid and acetate is exactly equal to the of acetic acid (; Figs 2-15, 2-16). The basis for this relationship, which holds for all weak acids, will soon become clear.
As the titration is continued by adding further increments of NaOH, the remaining nondissociated acetic acid is gradually converted into acetate. The end point of the titration occurs at about pH 7.0: all the acetic acid has lost its protons to , to form and acetate. Throughout the titration the two equilibria (Eqns 2-5, 2-6) coexist, each always conforming to its equilibrium constant.
Figure 2-17 compares the titration curves of three weak acids with very different ionization constants: acetic acid (); dihydrogen phosphate, ; and ammonium ion, (). Although the titration curves of these acids have the same shape, they are displaced along the pH axis because the three acids have different strengths. Acetic acid, with the highest (lowest ) of the three, is the strongest of the three weak acids (loses its proton most readily); it is already half dissociated at pH 4.76. Dihydrogen phosphate loses a proton less readily, being half dissociated at pH 6.86. Ammonium ion is the weakest acid of the three and does not become half dissociated until pH 9.25. The titration curve of these weak acids shows graphically that a weak acid and its anion—a conjugate acid-base pair—can act as a buffer, as we describe in the next section.

FIGURE 2-17 Comparison of the titration curves of three weak acids. Shown here are the titration curves for , , and . The predominant ionic forms at designated points in the titration are given in boxes. The regions of buffering capacity are indicated at the right. Conjugate acid-base pairs are effective buffers between approximately 10% and 90% neutralization of the proton-donor species.
Like all equilibrium constants, and are defined for specific conditions of concentration (components at 1 m) and temperature . Concentrated buffer solutions do not show ideal behavior. For example, the of dihydrogen phosphate is sometimes given as 7.2, sometimes as 6.86. The higher value (the apparent ) is not corrected for the effects of buffer concentration, and is defined at a temperature of . The value of 6.86 is corrected for buffer concentration and measured at physiological temperature , and is probably a closer approximation to the relevant value of in warm-blooded animals. We therefore use the value for dihydrogen phosphate throughout this book.
SUMMARY 2.2 Ionization of Water, Weak Acids, and Weak Bases
- Pure water ionizes slightly, forming equal numbers of hydrogen ions (hydronium ions, ) and hydroxide ions.
- The extent of ionization is described by an equilibrium constant, from which the ion product of water, , is derived. At ,
- The pH of an aqueous solution reflects, on a logarithmic scale, the concentration of hydrogen ions:
Weak acids partially ionize to release a hydrogen ion, thus lowering the pH of the aqueous solution. Weak bases accept a hydrogen ion, increasing the pH. The extent of these processes is characteristic of each particular weak acid or base and is expressed as an acid dissociation constant:
The expresses, on a logarithmic scale, the relative strength of a weak acid or base:
The stronger the acid, the smaller its ; the stronger the base, the larger the of its conjugate acid. The can be determined experimentally; it is the pH at the midpoint of the titration curve.
 As the ion product of water is constant, whenever is greater than must be less than , and vice versa. When is very high, as in a solution of hydrochloric acid, must be very low. From the ion product of water, we can calculate if we know , and vice versa.
As the ion product of water is constant, whenever is greater than must be less than , and vice versa. When is very high, as in a solution of hydrochloric acid, must be very low. From the ion product of water, we can calculate if we know , and vice versa. Measurement of pH is one of the most important and frequently used procedures in biochemistry. The pH affects the structure and activity of biological macromolecules, so a small change in pH can cause a large change in the structure and function of a protein. Measurements of the pH of blood and urine are commonly used in medical diagnoses. The pH of the blood plasma of people with severe, uncontrolled diabetes, for example, is often below the normal value of 7.4; this condition is called
Measurement of pH is one of the most important and frequently used procedures in biochemistry. The pH affects the structure and activity of biological macromolecules, so a small change in pH can cause a large change in the structure and function of a protein. Measurements of the pH of blood and urine are commonly used in medical diagnoses. The pH of the blood plasma of people with severe, uncontrolled diabetes, for example, is often below the normal value of 7.4; this condition is called 
 The tendency of any acid (HA) to lose a proton and form its conjugate base is defined by the equilibrium constant for the reversible reaction
The tendency of any acid (HA) to lose a proton and form its conjugate base is defined by the equilibrium constant for the reversible reaction Pure water ionizes slightly, forming equal numbers of hydrogen ions (hydronium ions, ) and hydroxide ions.
Pure water ionizes slightly, forming equal numbers of hydrogen ions (hydronium ions, ) and hydroxide ions.