Chapter 1 THE FOUNDATIONS OF BIOCHEMISTRY
About 14 billion years ago, the universe arose as a cataclysmic explosion of hot, energy-rich subatomic particles. Within seconds, the simplest elements (hydrogen and helium) were formed. As the universe expanded and cooled, material condensed under the influence of gravity to form stars. Some stars became enormous and then exploded as supernovae, releasing the energy needed to fuse simpler atomic nuclei into the more complex elements. Atoms and molecules formed swirling masses of dust particles, and their accumulation led eventually to the formation of rocks, planetoids, and planets. Thus were produced, over billions of years, Earth itself and the chemical elements found on Earth today. About 4 billion years ago, life arose on Earth — simple microorganisms with the ability to extract energy from chemical compounds and, later, from sunlight, which they used to make a vast array of more complex biomolecules from the simple elements and compounds on the Earth’s surface. We and all other living organisms are made of stardust.
Biochemistry asks how the remarkable properties of living organisms arise from thousands of different biomolecules. When these molecules are isolated and examined individually, they conform to all the physical and chemical laws that describe the behavior of inanimate matter — as do all the processes occurring in living organisms. The study of biochemistry shows how the collections of inanimate molecules that constitute living organisms interact to maintain and perpetuate life governed solely by the same physical and chemical laws that govern the nonliving universe.
In each chapter of this book, we organize our discussion around central principles or issues in biochemistry. In this chapter, we consider the features that define a living organism, and we develop these principles:
Cells are the fundamental unit of life. Although they vary in complexity and can be highly specialized for their environment or function within a multicellular organism, they share remarkable similarities.
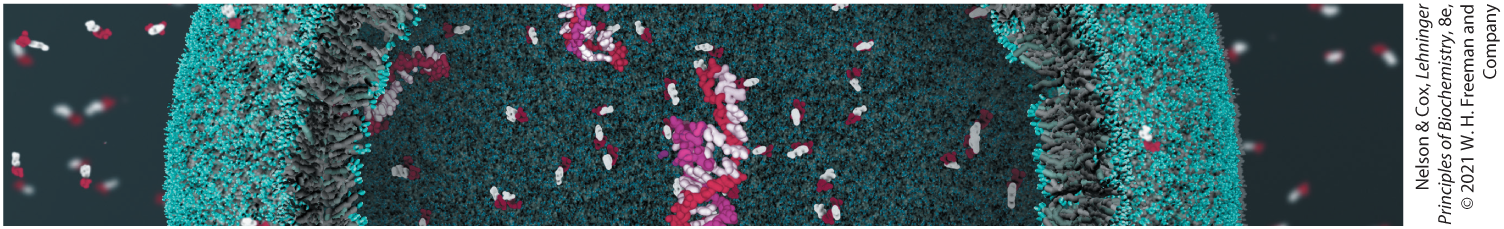
Cells use a relatively small set of carbon-based metabolites to create polymeric machines, supramolecular structures, and information repositories. The chemical structure of these components defines their cellular function. The collection of molecules carries out a program, the end result of which is reproduction of the program and self-perpetuation of that collection of molecules — in short, life.
Living organisms exist in a dynamic steady state, never at equilibrium with their surroundings. Following the laws of thermodynamics, living organisms extract energy from their surroundings and employ it to maintain homeostasis and do useful work. Essentially all of the energy obtained by a cell comes from the flow of electrons, driven by sunlight or by metabolic redox reactions.
Cells have the capacity for precise self-replication and self-assembly using chemical information stored in the genome. A single bacterial cell placed in a sterile nutrient medium can give rise to a billion identical “daughter” cells in 24 hours. Each cell is a faithful copy of the original, its construction directed entirely by information contained in the genetic material of the original cell. On a larger scale, the progeny of vertebrate animals share a striking resemblance to their parents, also the result of their inheritance of parental genes.
Living organisms change over time by gradual evolution. The result of eons of evolution is an enormous diversity of life forms, fundamentally related through their shared ancestry, which can be seen at the molecular level in the similarity of gene sequences and protein structures.
Despite these common properties and the fundamental unity of life they reveal, it is difficult to make generalizations about living organisms. Earth has an enormous diversity of organisms living in a wide range of habitats, from hot springs to Arctic tundra, from animal intestines to college dormitories. These habitats are matched by a correspondingly wide range of specific biochemical adaptations, achieved within a common chemical framework. For the sake of clarity, in this book we sometimes risk certain generalizations, which, though not perfect, remain useful; we also frequently point out the exceptions to these generalizations, which can prove illuminating.
Biochemistry describes in molecular terms the structures, mechanisms, and chemical processes shared by all organisms and provides organizing principles that underlie life in all its diverse forms. Although biochemistry provides important insights and practical applications in medicine, agriculture, nutrition, and industry, its ultimate concern is with the wonder of life itself.
In this introductory chapter we give an overview of the cellular, chemical, physical, and genetic backgrounds of biochemistry and the overarching principle of evolution — how life emerged and evolved into the diversity of organisms we see today. As you read through the book, you may find it helpful to refer back to this chapter at intervals to refresh your memory of this background material.
 Cells are the fundamental unit of life. Although they vary in complexity and can be highly specialized for their environment or function within a multicellular organism, they share remarkable similarities.
Cells are the fundamental unit of life. Although they vary in complexity and can be highly specialized for their environment or function within a multicellular organism, they share remarkable similarities. Cells use a relatively small set of carbon-based metabolites to create polymeric machines, supramolecular structures, and information repositories. The chemical structure of these components defines their cellular function. The collection of molecules carries out a program, the end result of which is reproduction of the program and self-perpetuation of that collection of molecules — in short, life.
Cells use a relatively small set of carbon-based metabolites to create polymeric machines, supramolecular structures, and information repositories. The chemical structure of these components defines their cellular function. The collection of molecules carries out a program, the end result of which is reproduction of the program and self-perpetuation of that collection of molecules — in short, life. Living organisms exist in a dynamic steady state, never at equilibrium with their surroundings. Following the laws of thermodynamics, living organisms extract energy from their surroundings and employ it to maintain homeostasis and do useful work. Essentially all of the energy obtained by a cell comes from the flow of electrons, driven by sunlight or by metabolic redox reactions.
Living organisms exist in a dynamic steady state, never at equilibrium with their surroundings. Following the laws of thermodynamics, living organisms extract energy from their surroundings and employ it to maintain homeostasis and do useful work. Essentially all of the energy obtained by a cell comes from the flow of electrons, driven by sunlight or by metabolic redox reactions. Cells have the capacity for precise self-replication and self-assembly using chemical information stored in the genome. A single bacterial cell placed in a sterile nutrient medium can give rise to a billion identical “daughter” cells in 24 hours. Each cell is a faithful copy of the original, its construction directed entirely by information contained in the genetic material of the original cell. On a larger scale, the progeny of vertebrate animals share a striking resemblance to their parents, also the result of their inheritance of parental genes.
Cells have the capacity for precise self-replication and self-assembly using chemical information stored in the genome. A single bacterial cell placed in a sterile nutrient medium can give rise to a billion identical “daughter” cells in 24 hours. Each cell is a faithful copy of the original, its construction directed entirely by information contained in the genetic material of the original cell. On a larger scale, the progeny of vertebrate animals share a striking resemblance to their parents, also the result of their inheritance of parental genes. Living organisms change over time by gradual evolution. The result of eons of evolution is an enormous diversity of life forms, fundamentally related through their shared ancestry, which can be seen at the molecular level in the similarity of gene sequences and protein structures.
Living organisms change over time by gradual evolution. The result of eons of evolution is an enormous diversity of life forms, fundamentally related through their shared ancestry, which can be seen at the molecular level in the similarity of gene sequences and protein structures.