11.2 Membrane Dynamics
One remarkable feature of all biological membranes is their plasticity — their ability to change shape without losing their integrity and becoming leaky. The bases for this property are the noncovalent interactions among lipids in the bilayer and the mobility allowed to individual lipids because they are not covalently anchored to one another. We turn now to the dynamics of membranes: the motions that occur and the transient structures that are allowed by these motions.
Acyl Groups in the Bilayer Interior Are Ordered to Varying Degrees
Although the lipid bilayer structure is stable, its individual phospholipid molecules have much freedom of motion (Fig. 11-17), depending on the temperature and the lipid composition. Below normal physiological temperatures, the lipids in a bilayer form a gel-like liquid-ordered state, in which all types of motion of individual lipid molecules are strongly constrained (Fig. 11-17a). Above physiological temperatures, individual hydrocarbon chains of fatty acids are in constant motion produced by rotation about the carbon–carbon bonds of the long acyl side chains and by lateral diffusion of individual lipid molecules in the plane of the bilayer. This is the liquid-disordered state (Fig. 11-17b). In the transition from the state to the state, the general shape and dimensions of the bilayer are maintained; what changes is the degree of motion (lateral and rotational) allowed to individual lipid molecules.
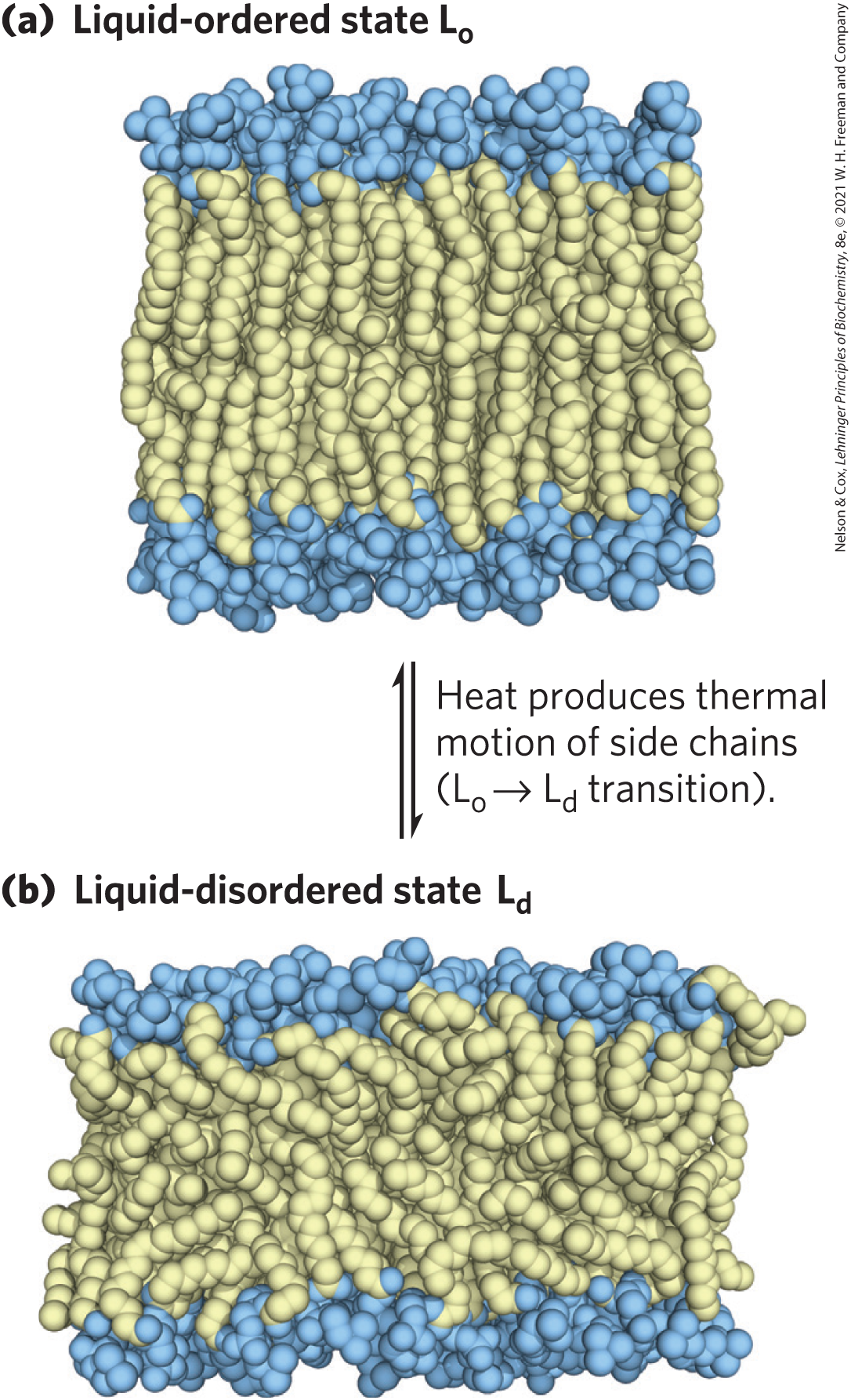
FIGURE 11-17 Two extreme states of bilayer lipids. (a) In the liquid-ordered () state, polar head groups are uniformly arrayed at the surface, and the acyl chains are nearly motionless and packed with regular geometry. (b) In the liquid-disordered state, or fluid state, acyl chains undergo much thermal motion and have no regular organization. The state of membrane lipids in biological membranes is maintained somewhere between these extremes. [Information from H. Heller et al., J. Phys. Chem. 97:8343, 1993.]
At temperatures in the physiological range for a mammal (about 20 to 40 °C), long-chain saturated fatty acids (such as 16:0 and 18:0) tend to pack into an gel phase, but the kinks in unsaturated fatty acids interfere with packing, favoring the state (see Fig. 10-1). Shorter-chain fatty acyl groups are more mobile than longer-chain fatty acyl groups and thus also favor the state. The sterol content of a membrane, which varies greatly with organism and organelle, is another important determinant of lipid state. Sterols (such as cholesterol) have paradoxical effects on bilayer fluidity: they interact with phospholipids containing unsaturated fatty acyl chains, compacting them and constraining their motion in bilayers. In contrast, their association with sphingolipids and phospholipids having long, saturated fatty acyl chains tends to make a bilayer fluid that would otherwise, without cholesterol, adopt the state. In biological membranes composed of a variety of phospholipids and sphingolipids, cholesterol tends to associate with sphingolipids and to form regions in the state surrounded by cholesterol-poor regions in the state (see the discussion of membrane rafts below).
Transbilayer Movement of Lipids Requires Catalysis
At physiological temperatures, lateral diffusion in the plane of the bilayer is very rapid, but the movement of a lipid molecule from one leaflet of the bilayer to the other occurs very slowly, if at all, in most membranes (Fig. 11-18). Transbilayer — or “flip-flop” — movement requires that a polar or charged head group leave its aqueous environment and move into the hydrophobic interior of the bilayer, a process with a large, positive free-energy change. There are, however, situations in which such movement is essential. For example, in the ER, membrane glycerophospholipids are synthesized on the cytosolic face, whereas sphingolipids are synthesized or modified on the lumenal surface. To get from their site of synthesis to their eventual point of deposition, these lipids must undergo flip-flop diffusion.
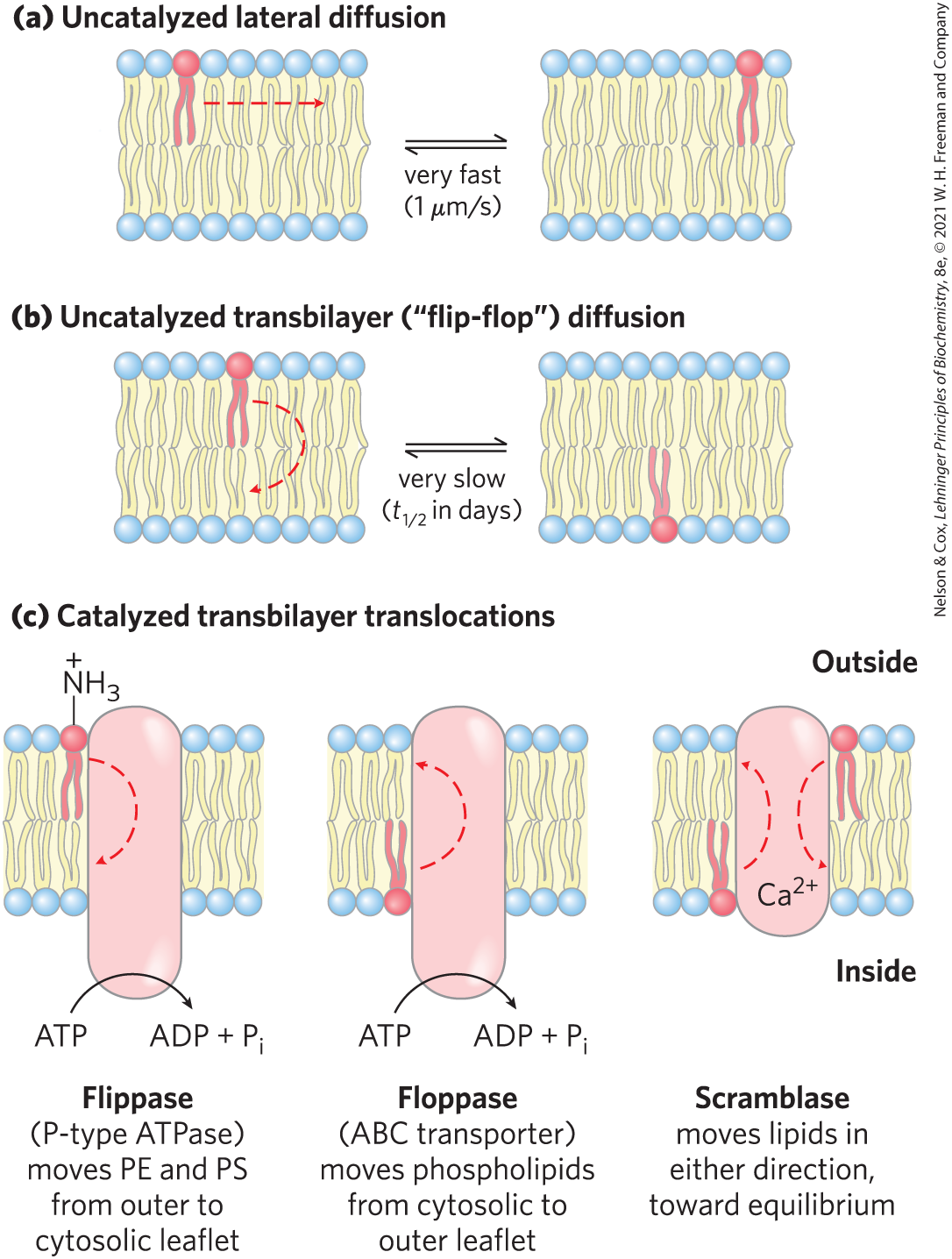
FIGURE 11-18 Motion of single phospholipids in a bilayer. (a) Lateral diffusion within a leaflet is very rapid, but (b) uncatalyzed movement from one leaflet to the other is very slow. (c) Three types of phospholipid translocators in the plasma membrane. PE is phosphatidylethanolamine; PS is phosphatidylserine.
Membrane proteins called flippases, floppases, and scramblases (Fig. 11-18c) facilitate the transbilayer movement (translocation) of individual lipid molecules. Like enzymes, these translocators act by providing a path that is energetically more favorable and therefore much faster than the uncatalyzed movement. The combination of asymmetric biosynthesis of membrane lipids, no uncatalyzed flip-flop diffusion, and the presence of selective, energy-dependent lipid translocators accounts for the transbilayer asymmetry in lipid composition discussed in Section 11.1.
Flippases catalyze translocation of the aminophospholipids phosphatidylethanolamine and phosphatidylserine from the extracellular to the cytoplasmic leaflet of the plasma membrane, contributing to the asymmetric distribution of phospholipids: phosphatidylethanolamine and phosphatidylserine primarily in the cytoplasmic leaflet, and the sphingolipids and phosphatidylcholine in the outer leaflet. Keeping phosphatidylserine out of the extracellular leaflet is important: its exposure on the outer surface triggers apoptosis (programmed cell death; see Chapter 12) and engulfment by macrophages that carry phosphatidylserine receptors. Flippases also act in the ER, where they move newly synthesized phospholipids from their site of synthesis in the cytosolic leaflet to the lumenal leaflet. Flippases consume about one ATP per molecule of phospholipid translocated, and they are structurally and functionally related to the P-type ATPases (active transporters) described in Section 11.3.
There are three other types of lipid-translocating activities: floppases, scramblases, and phosphatidylinositol transfer proteins. Floppases move plasma membrane phospholipids and sterols from the cytoplasmic leaflet to the extracellular leaflet and, like flippases, are ATP-dependent. Floppases are members of the ABC transporter family, described on page 395; all ABC transporters actively transport hydrophobic substrates outward across the plasma membrane. Each floppase specializes in movement of specific lipids: cholesterol, phosphatidylcholine, sphingomyelin, and phosphatidylserine. Scramblases are proteins that move any membrane phospholipid across the bilayer down its concentration gradient (from the leaflet where it has a higher concentration to the leaflet where it has a lower concentration); their activity is not dependent on ATP, although some require . Scramblase activity leads to controlled randomization of the head-group composition on the two faces of the bilayer. The activity of some scramblases rises sharply with an increase in cytosolic concentration, which may result from cell activation, cell injury, or apoptosis. Finally, a group of proteins that act primarily to move phosphatidylinositol lipids across lipid bilayers, the phosphatidylinositol transfer proteins, are believed to have important roles in lipid signaling and membrane trafficking.
Lipids and Proteins Diffuse Laterally in the Bilayer
Individual lipid molecules can move laterally in the plane of the membrane by changing places with neighboring lipid molecules; that is, they undergo Brownian movement within the bilayer (Fig. 11-18a). This rapid lateral diffusion in the plane of the bilayer tends to randomize the positions of individual molecules in a few seconds.
Lateral diffusion can be shown experimentally by attaching fluorescent probes to the head groups of lipids and using fluorescence microscopy to follow the probes over time (Fig. 11-19). In one technique, a small region of a cell surface with fluorescence-tagged lipids is bleached by intense laser radiation so that the irradiated patch no longer fluoresces when viewed with less-intense (nonbleaching) light in the fluorescence microscope. However, within milliseconds, the region recovers its fluorescence as unbleached lipid molecules diffuse into the bleached patch and bleached lipid molecules diffuse away from it. The rate of fluorescence recovery after photobleaching, or FRAP, is a measure of the rate of lateral diffusion of the lipids. Using the FRAP technique, researchers have shown that some membrane lipids diffuse laterally at rates of up to 1 μm/s. At this rate, a lipid molecule could move from one end of a eukaryotic cell to the other in a few seconds.

FIGURE 11-19 Measurement of lateral diffusion rates of lipids by fluorescence recovery after photobleaching (FRAP). Lipids in the outer leaflet of the plasma membrane are labeled by reaction with a membrane-impermeant fluorescent probe (red) so that the surface is uniformly labeled when viewed with a fluorescence microscope. A small area is bleached with a laser, then recovers its fluorescence. With time, unbleached lipid molecules diffuse into the bleached region, and it again becomes fluorescent. The FRAP method can also be used to measure lateral diffusion of membrane proteins labeled with a fluorescent tag.
Another technique, single particle tracking, has allowed researchers to follow the movement of a single lipid molecule in the plasma membrane on a much shorter time scale. Results from these studies confirm that lipid molecules diffuse laterally with rapidity within small, discrete regions of the cell surface but that movement from one such region to a nearby region (“hop diffusion”) is rarer; membrane lipids behave as though corralled by fences that they can occasionally cross by hop diffusion (Fig. 11-20).
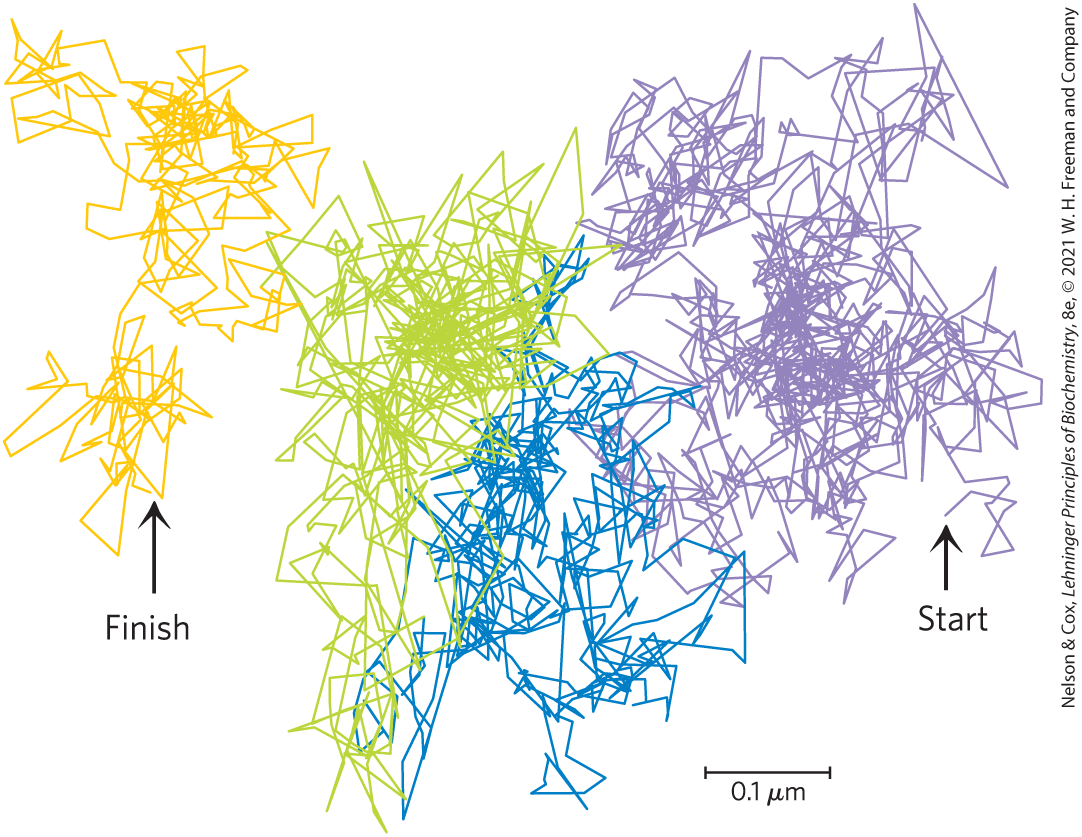
FIGURE 11-20 Hop diffusion of individual lipid molecules. The motion of a single fluorescently labeled lipid molecule in a cell surface is recorded on video by fluorescence microscopy, with a time resolution of 25 µs (equivalent to 40,000 frames/s). The track shown here represents a molecule followed for 56 ms (2,250 frames); the trace begins in the purple area and continues through blue, green, and orange. The pattern of movement indicates rapid diffusion within a confined region (about 250 nm in diameter, shown by a single color), with occasional hops into an adjoining region. This finding suggests that the lipids are corralled by molecular fences that they occasionally jump. [Data from Takahiro Fujiwara, Ken Ritchie, Hideji Murakoshi, Ken Jacobson, and Akihiro Kusumi.]
Like membrane lipids, many membrane proteins are free to diffuse laterally in the plane of the bilayer and are in constant motion, as shown by the FRAP technique with fluorescence-tagged plasma membrane proteins. Some membrane proteins associate to form large aggregates (“patches”) on the surface of a cell or an organelle in which individual protein molecules do not move relative to one another; for example, acetylcholine receptors form dense, near-crystalline patches on neuronal plasma membranes at synapses. Other membrane proteins are anchored to internal structures that prevent their free diffusion. In the erythrocyte membrane, both glycophorin and the chloride-bicarbonate exchanger (p. 389) are tethered to spectrin, a filamentous cytoskeletal protein (Fig. 11-21). One possible explanation for the pattern of lateral diffusion of lipid molecules shown in Figure 11-20 is that membrane proteins immobilized by their association with spectrin form the “fences” that define the regions within which relatively unrestricted lipid motion can occur.
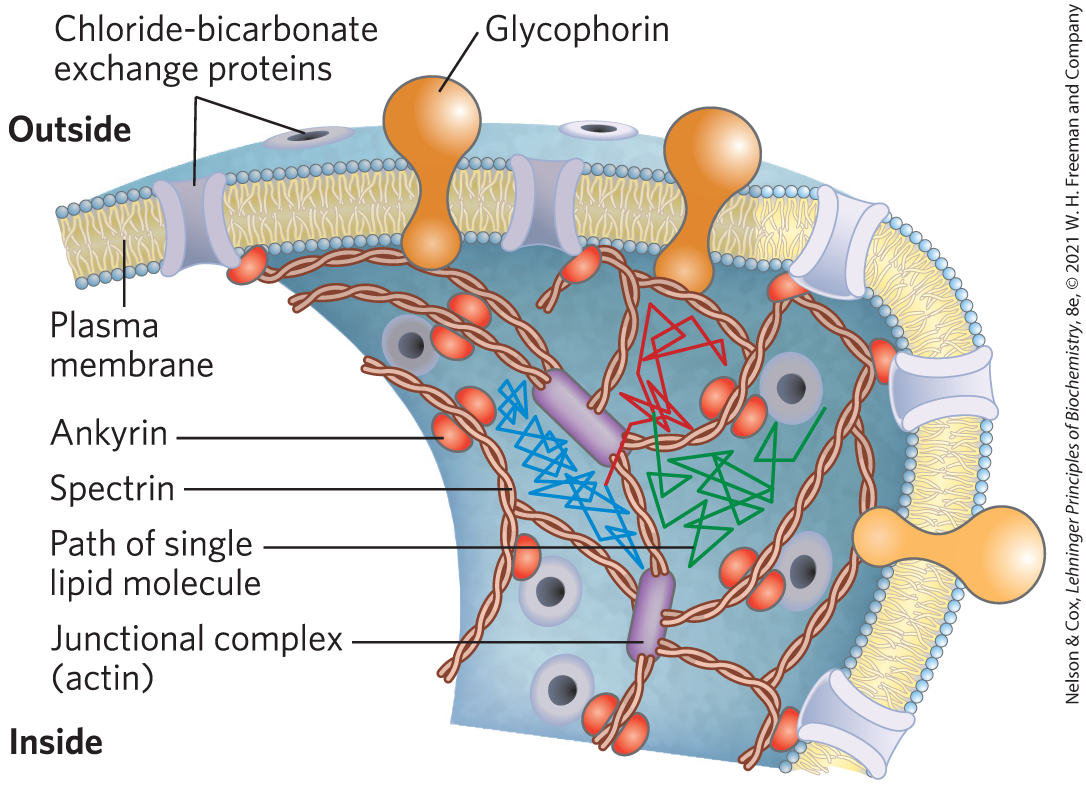
FIGURE 11-21 Restricted motion of the erythrocyte chloride-bicarbonate exchanger and glycophorin. The proteins span the membrane and are tethered to spectrin, a cytoskeletal protein, by another protein, ankyrin, limiting their lateral mobility. Ankyrin is anchored in the membrane by a covalently bound palmitoyl side chain (see Fig. 11-16). Spectrin, a long, filamentous protein, is cross-linked at junctional complexes containing actin. A network of cross-linked spectrin molecules attached to the cytoplasmic face of the plasma membrane stabilizes the membrane, making it resistant to deformation. This network of anchored membrane proteins may form the “corral” suggested by the experiment shown in Figure 11-20; the lipid tracks shown here are confined to different regions defined by the tethered membrane proteins. Occasionally a lipid molecule (green track) jumps from one corral to another (blue track), then another (red track).
Sphingolipids and Cholesterol Cluster Together in Membrane Rafts
We have seen that diffusion of membrane lipids from one bilayer leaflet to the other does not occur unless catalyzed and that the different lipid species of the plasma membrane are asymmetrically distributed in the two leaflets of the bilayer (Fig. 11-6). Even within a single leaflet, the lipid distribution is not uniform. Glycosphingolipids (cerebrosides and gangliosides), which typically contain long-chain saturated fatty acids, form transient clusters in the outer leaflet; these clusters largely exclude glycerophospholipids, which typically contain one unsaturated fatty acyl group and a shorter saturated acyl group. The long, saturated acyl groups of sphingolipids can form more compact, more stable associations with the long ring system of cholesterol than can the shorter, often unsaturated, chains of phospholipids. The cholesterol-sphingolipid microdomains of the plasma membrane make the bilayer slightly thicker and more ordered (less fluid) than neighboring regions, which are rich in phospholipids. These microdomains are more difficult to dissolve with nonionic detergents; they behave like liquid-ordered sphingolipid rafts adrift on an ocean of liquid-disordered phospholipids (Fig. 11-22). Proteins with relatively short hydrophobic helical sections (19 to 20 residues) cannot span the thicker bilayer in rafts, and thus they tend to be excluded. Proteins with longer hydrophobic helices (24 to 25 residues) segregate into the thicker bilayer regions of rafts, where the entire length of the helix is stabilized by the hydrophobic effect.
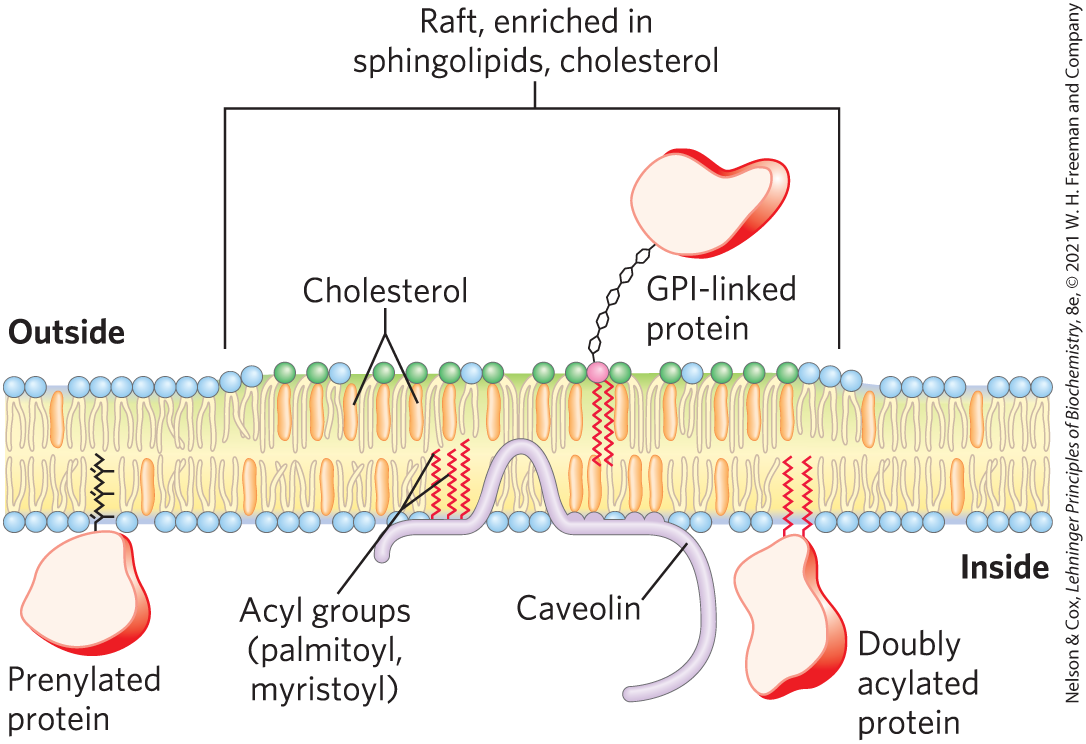
FIGURE 11-22 Membrane microdomains (rafts). Stable associations of sphingolipids and cholesterol in the outer leaflet produce a microdomain, slightly thicker than other membrane regions, that is enriched with specific types of membrane proteins. GPI-anchored proteins are prominent in the outer leaflet of these rafts, and proteins with one or several covalently attached long-chain acyl groups are common in the inner leaflet. Inwardly curved rafts called caveolae are especially enriched in proteins called caveolins (see Fig. 11-23).
Lipid rafts are remarkably enriched in two classes of integral proteins, with two specific types of covalently attached lipids. The integral proteins of one class have two long-chain saturated fatty acids (two palmitoyl groups, or a palmitoyl and a myristoyl group) covalently attached through Cys residues. Caveolin is one such protein; there are many others. Those of the second class, the GPI-anchored proteins, have a glycosyl phosphatidylinositol on their carboxyl-terminal residue (Fig. 11-16). Presumably, these lipid anchors, like the long, saturated acyl chains of sphingolipids, form more stable associations with the cholesterol and long acyl groups in rafts than they form with the surrounding phospholipids. (It is notable that other lipid-linked proteins, those with covalently attached isoprenyl groups such as farnesyl, are not preferentially associated with the outer leaflet of sphingolipid rafts; see Fig. 11-22.) The “raft” and “sea” domains of the plasma membrane are not rigidly separated; membrane proteins can move into and out of lipid rafts in a fraction of a second. But in the shorter time scale (microseconds) more relevant to many membrane-mediated biochemical processes, many of these proteins reside primarily in a raft.
The approximate fraction of the cell surface occupied by rafts can be as high as 50% in some cases; the rafts cover half of the ocean. Indirect measurements in cultured fibroblasts suggest a diameter of roughly 50 nm for an individual raft, which corresponds to a patch containing a few thousand sphingolipids and perhaps 10 to 50 membrane proteins. Because most cells express more than 50 different kinds of plasma membrane proteins, it is likely that a single raft contains only a subset of membrane proteins and that this segregation of membrane proteins is functionally significant. Their presence in a single raft would hugely increase the likelihood of their collision. Certain membrane receptors and signaling proteins, for example, seem to be segregated together in membrane rafts. Experiments show that signaling through these proteins can be disrupted by manipulations that deplete the plasma membrane of cholesterol and destroy lipid rafts.
Plasma membranes of many cells have specialized rafts called caveolae (“little caves”), which can represent about half of the total area of the plasma membrane (Fig. 11-23a). Closely associated with caveolae is caveolin, an integral protein with two globular domains connected by a hairpin-shaped hydrophobic domain that binds the protein to the cytoplasmic leaflet of the plasma membrane (Fig. 11-23b). Three palmitoyl groups attached to the carboxyl-terminal globular domain further anchor the protein to the membrane. Caveolins form dimers and associate with cholesterol-rich regions in the membrane. The presence of caveolin dimers forces the associated lipid bilayer to curve inward, forming caveolae. Caveolae involve both leaflets of the bilayer — the cytoplasmic leaflet, from which the caveolin globular domains project, and the extracellular leaflet, a cholesterol and sphingolipid raft with associated GPI-anchored proteins. Caveolae are implicated in a variety of cellular functions, including membrane trafficking within cells and the transduction of external signals into cellular responses.
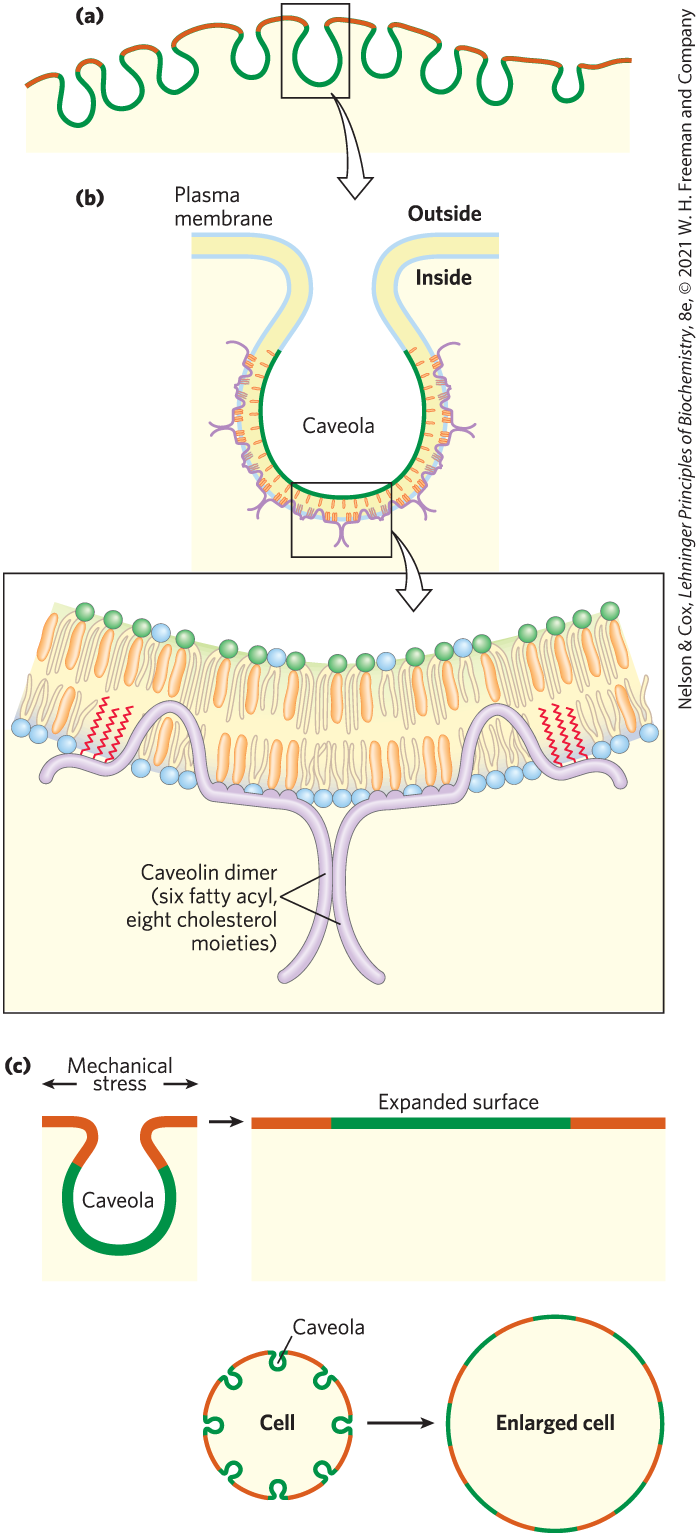
FIGURE 11-23 Caveolin forces inward curvature of a membrane. (a) Caveolae are small invaginations in the plasma membrane. (b) Cartoon showing the location and role of a caveolin dimer in causing inward membrane curvature. Each caveolin monomer has a central hydrophobic domain and three long-chain acyl groups (red), which hold the molecule to the inside of the plasma membrane. When several caveolin dimers are concentrated in a small region (a raft), they force a curvature in the lipid bilayer, forming a caveola. Cholesterol molecules in the bilayer are shown in orange. (c) Flattening of caveolae allows the plasma membrane to expand in response to various stresses. [Information from R. G. Parton, Nat. Rev. Mol. Cell Biol. 8:185–194, 2007.]
Caveolae may also provide a means of expanding the cell surface. The lipid bilayer itself is not elastic, but if existing caveolae lose their associated caveolin as the result of a regulatory signal, the caveolae flatten into the plasma membrane (Fig. 11-23c). The effect is to add surface area, allowing the cell to expand without bursting in response to osmotic or other stress.
Membrane Curvature and Fusion Are Central to Many Biological Processes
Caveolins are not unique in their ability to induce curvature in membranes. Changes of curvature are central to one of the most remarkable features of biological membranes: their ability to undergo fusion with other membranes without losing their continuity. Within the eukaryotic endomembrane system, the membranous compartments constantly reorganize. Vesicles bud from the ER to carry newly synthesized lipids and proteins to other organelles and to the plasma membrane. Exocytosis, endocytosis, cell division, fusion of egg and sperm cells, and entry of a membrane-enveloped virus into a host cell all involve a membrane reorganization that requires the fusion of two membrane segments without loss of continuity (Fig. 11-24). Most of these processes begin with a local increase in membrane curvature.
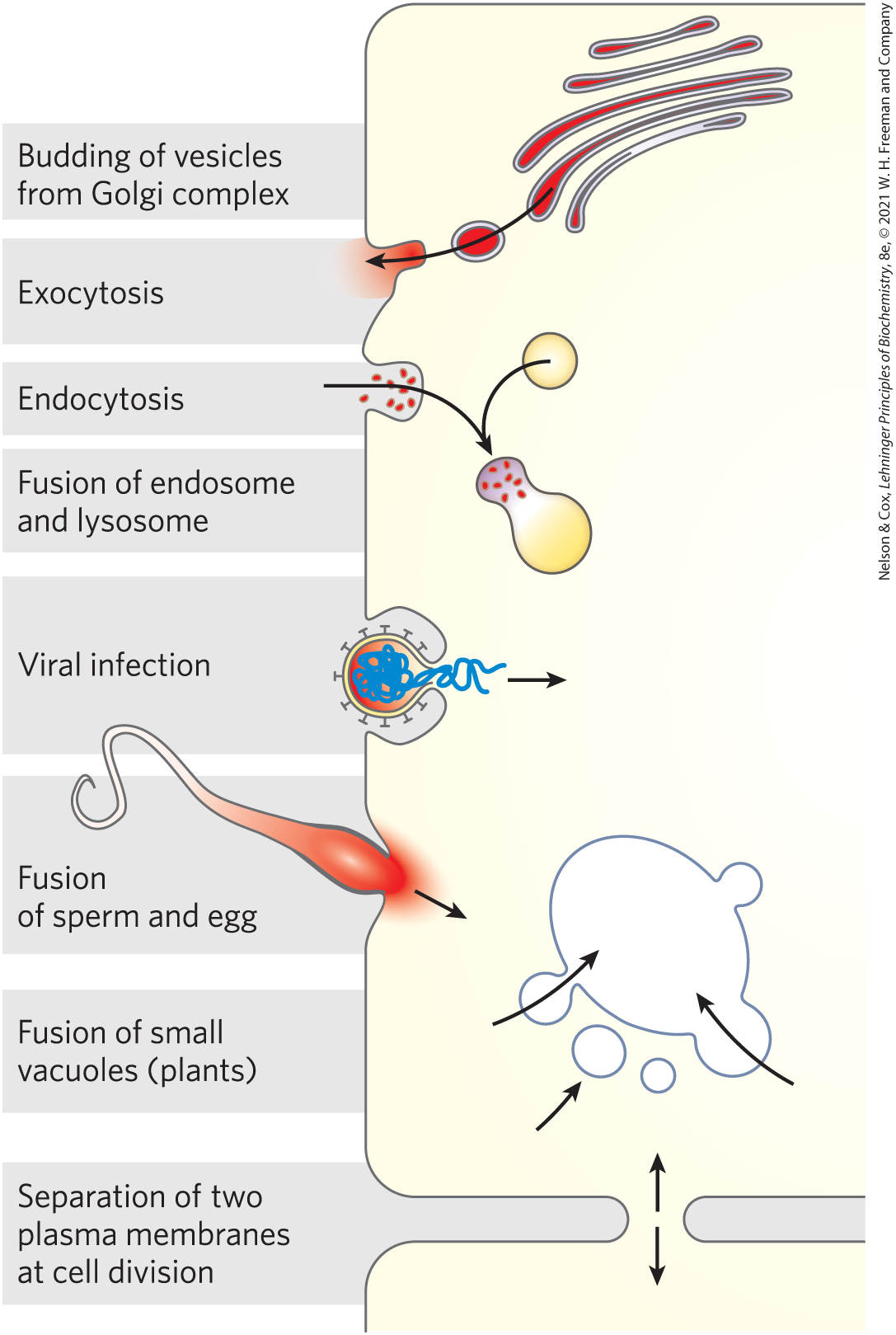
FIGURE 11-24 Membrane fusion. The fusion of two membranes is central to a variety of cellular processes involving organelles and the plasma membrane.
Cardiolipin, present primarily in mitochondrial membranes of eukaryotes, but also a major component of the membrane lipids of bacteria, can create or recognize membrane curvature. It is cone-shaped — its head group is small relative to its four fatty acyl chains — so it can act as a wedge in the monolayer, contracting that monolayer relative to the other.
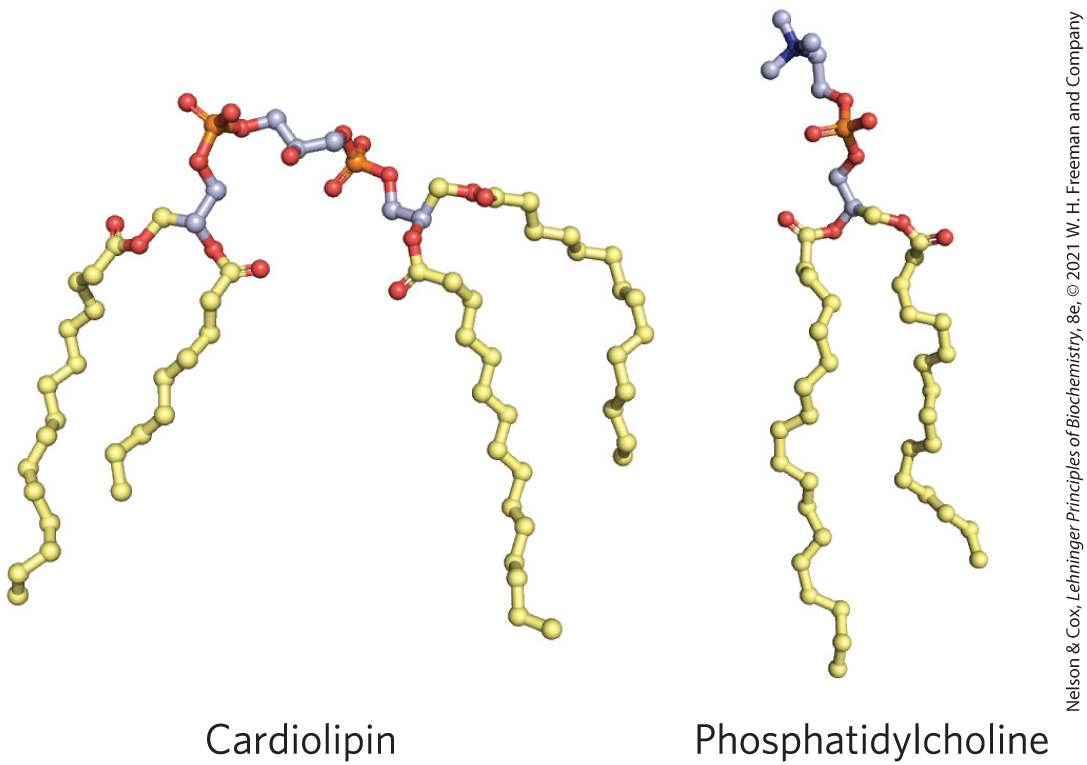
Membrane curvature is the result. In E. coli, cardiolipin is highly localized at the two poles of the rod-shaped cell, where its curvature is sharpest. It seems very likely that other membrane lipids will be found to influence local curvature of the bilayer.
A protein that is intrinsically curved may force a bilayer to curve by binding to it (Fig. 11-25); the binding energy provides the driving force for the increase in bilayer curvature. Alternatively, multiple subunits of a scaffold protein may assemble into curved supramolecular complexes and stabilize curves that spontaneously form in the bilayer. For example, a superfamily of proteins containing BAR domains (named for the first three members of the family that were identified: BIN1, amphiphysin, and RVS167) can assemble into a crescent-shaped scaffold that binds to the membrane surface, forcing or favoring membrane curvature. BAR domains consist of coiled coils that form long, thin, curved dimers with a positively charged concave surface that tends to form ionic interactions with the negatively charged head groups of membrane lipids and . The enzymatic formation of these inositol lipids can tag a plasma membrane area for creation of inward curvature by a BAR protein (Fig. 11-25). Some of these BAR proteins also have an amphipathic helix (having one polar face and one hydrophobic face; see Fig. 11-32) that inserts like a wedge into one leaflet of the bilayer, expanding its area relative to the other leaflet and thereby forcing curvature. Such a protein might also serve as a detector of preexisting membrane curvature due to differences in lipid composition of two leaflets of a bilayer.
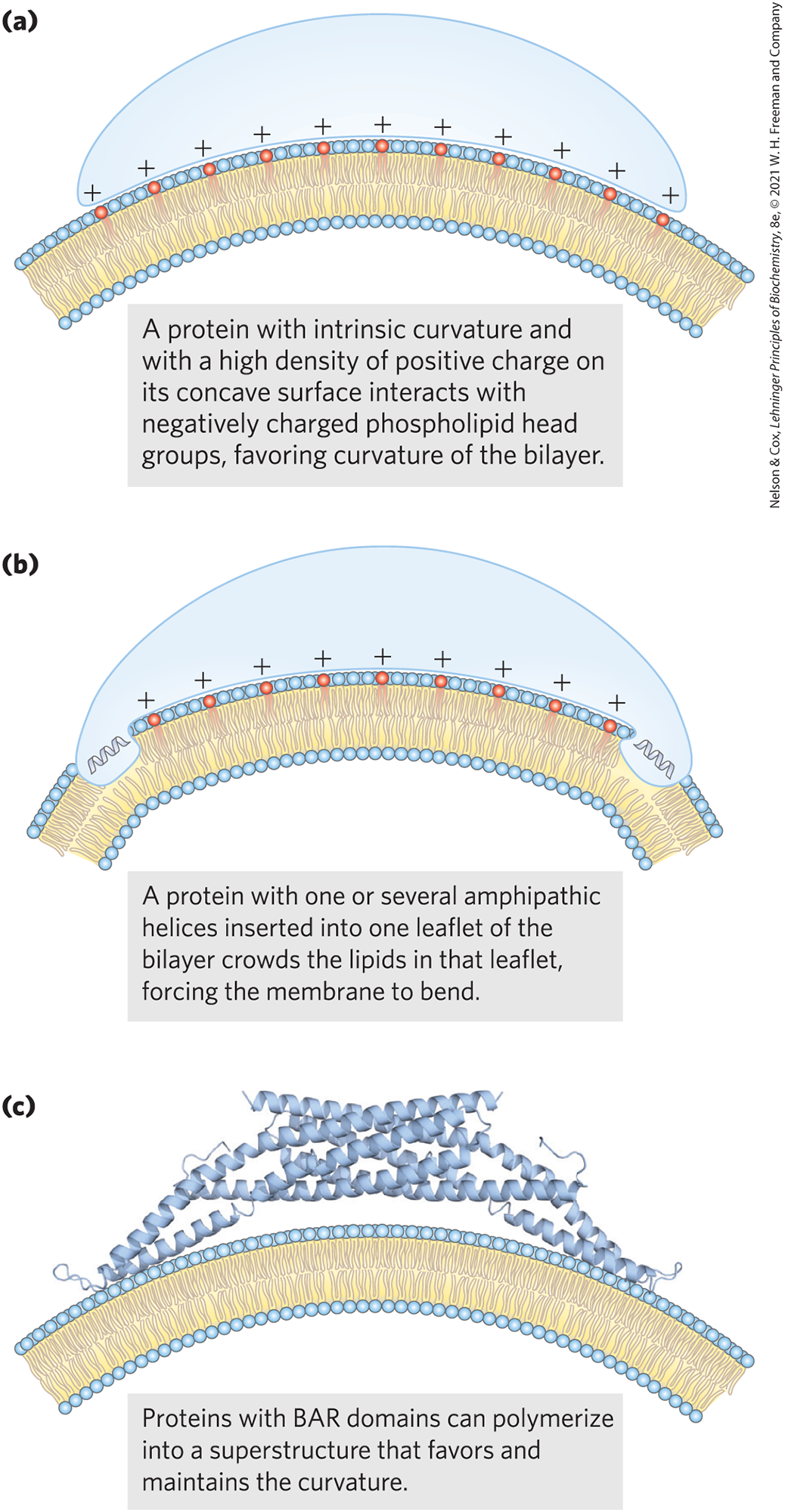
FIGURE 11-25 Three models for protein-induced curvature of membranes. [Information from (a, b) B. Qualmann et al., EMBO J. 30:3501, 2011, Fig. 1; (c) B. J. Peter et al., Science 303:495, 2004, Fig. 1A.]
Septins make up a family of GTP-binding proteins (14 genes in humans) that polymerize at curved regions of the plasma membrane and participate in cellular processes such as cell division, exocytosis, phagocytosis, and apoptosis. All septins have an amphipathic helix that can submerge its hydrophobic side in one leaflet of the bilayer, forcing one leaflet of the bilayer to expand laterally, either causing or sensing local curvature in the membranes. Studies of cells with mutations in this helix show its biological importance in vesicle trafficking and neurotransmitter release.
Specific fusion of two membranes requires mediation by fusion proteins. Fusion proteins ensure (1) that the two membranes recognize each other; (2) that their surfaces become closely apposed, which requires removal of the water molecules normally associated with the polar head groups of lipids; (3) that their bilayer structures become locally disrupted, resulting in fusion of the outer leaflets of the two membranes (hemifusion); and (4) that their bilayers fuse to form a single continuous bilayer. The fusion occurring in receptor-mediated endocytosis, or regulated secretion, also requires (5) that the process is triggered at the appropriate time or in response to a specific signal. Fusion proteins mediate these events, bringing about specific recognition and a transient local distortion of the bilayer structure that favors membrane fusion. (Note that these fusion proteins are unrelated to the products encoded by two fused genes, also called fusion proteins, discussed in Chapter 9.)
A well-studied example of membrane fusion occurs at synapses, when intracellular (neuronal) vesicles loaded with neurotransmitter fuse with the plasma membrane. Yeast cells provide another experimentally accessible system in which vesicles fuse with the plasma membrane, releasing their secretion products. Both processes involve a family of proteins called SNAREs (snap receptors; Fig. 11-26). SNAREs in the cytoplasmic face of the intracellular vesicle are called v-SNAREs (v for vesicle); those in the target membrane with which the vesicle fuses (the plasma membrane during exocytosis) are t-SNAREs (t for target). The protein NSF (N-ethylmaleimide sensitive factor) regulates the interactions among SNAREs. During fusion, a v-SNARE and a t-SNARE bind to each other and undergo a structural change that produces a bundle of long, thin rods made up of helices from both SNAREs and two helices from the protein SNAP25 (Fig. 11-26). The two SNAREs initially interact at their ends, then zip up into the bundle of helices. This structural change pulls the two membranes into contact and initiates the fusion of their lipid bilayers. An alternative designation of SNARE types is based on structural features of the proteins: R-SNAREs have an Arg residue critical to their function, and Q-SNAREs have a critical Gln residue. Typically, R-SNAREs act as v-SNAREs, and Q-SNAREs act as t-SNAREs.
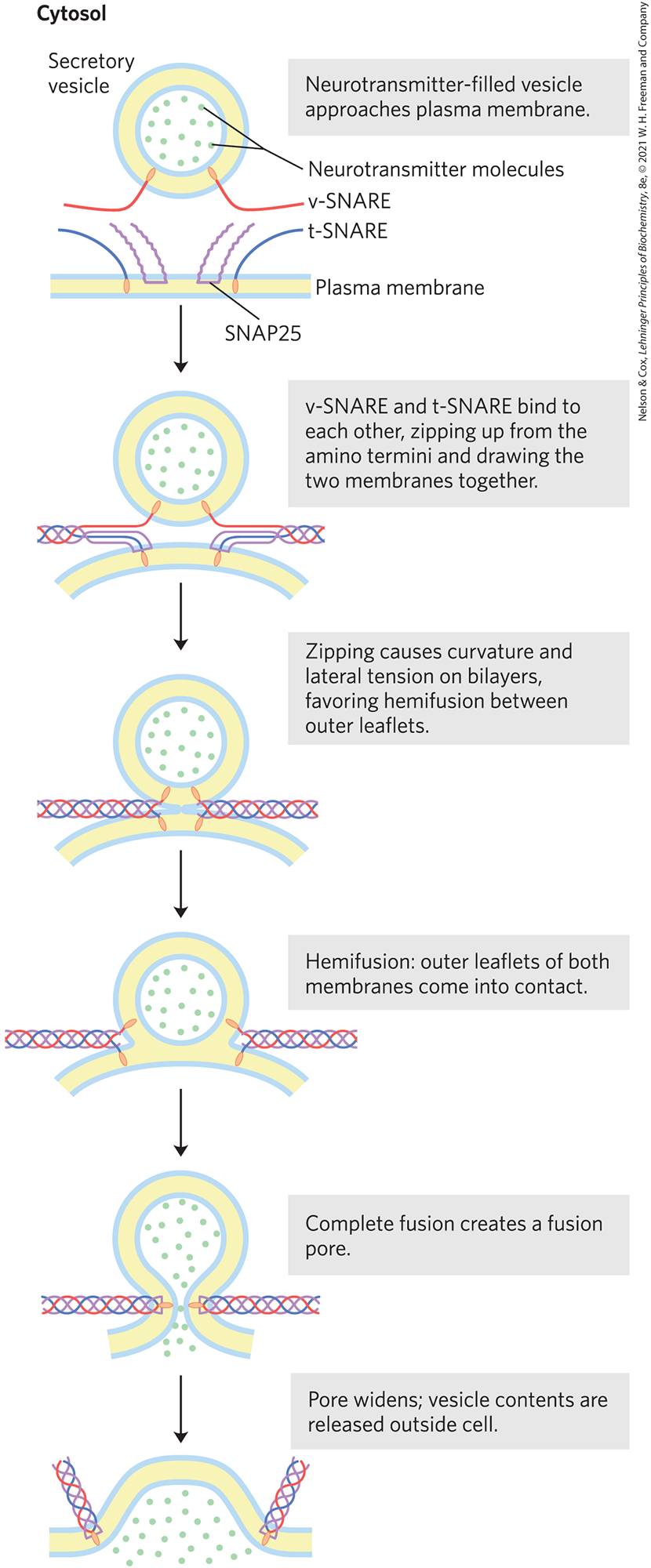
FIGURE 11-26 Membrane fusion during neurotransmitter release at a synapse. The secretory vesicle membrane contains the v-SNARE synaptobrevin (red). The target (plasma) membrane contains the t-SNAREs syntaxin (blue) and SNAP25 (violet). When a local increase in [] signals release of neurotransmitter, the v-SNARE, SNAP25, and t-SNARE interact, forming a coiled bundle of four α helices, pulling the two membranes together and disrupting the bilayer locally. This leads first to hemifusion, joining the outer leaflets of the two membranes, then to complete membrane fusion and neurotransmitter release. When fusion is complete, the SNARE complex is disassembled. [Information from Y. A. Chen and R. H. Scheller, Nat. Rev. Mol. Cell Biol. 2:98, 2001.]
The complex of SNAREs and SNAP25 is the target of several powerful neurotoxins. Clostridium botulinum toxin is a bacterial protease that cleaves specific bonds in SNARE proteins, preventing neurotransmission and causing paralysis and death. Because of its very high specificity for these proteins, purified botulinum toxin has served as a powerful tool for dissecting the mechanism of neurotransmitter release in vivo and in vitro. Used in small amounts, botulinum toxin (Botox) is used in medicine to treat disorders of eye and neck muscles, as well as cosmetically for the removal of skin wrinkles. Tetanus toxin, produced by the bacterium Clostridium tetani, also is a protease with high specificity for SNARE proteins. It causes painful muscle spasms and rigidity of voluntary muscles — hence the characteristic symptom “lockjaw.”
Integral Proteins of the Plasma Membrane Are Involved in Surface Adhesion, Signaling, and Other Cellular Processes
Several families of integral proteins in the plasma membrane provide specific points of attachment between cells or between a cell and proteins of the extracellular matrix. Integrins are surface adhesion proteins that mediate a cell’s interaction with the extracellular matrix and with other cells, including some pathogens. Integrins also carry signals in both directions across the plasma membrane, integrating information about the extracellular and intracellular environments. All integrins are heterodimeric proteins composed of two unlike subunits, α and β, each anchored to the plasma membrane by a single transmembrane helix. The large extracellular domains of the α and β subunits combine to form a specific binding site for extracellular proteins such as collagen and fibronectin, which contain a common determinant of integrin binding, the sequence Arg–Gly–Asp (RGD).
Other plasma membrane proteins involved in surface adhesion are the cadherins, which undergo homophilic (“with the same kind”) interactions with identical cadherins in an adjacent cell. Selectins have extracellular domains that, in the presence of , bind specific polysaccharides on the surface of an adjacent cell. Selectins are present primarily in the various types of blood cells and in the endothelial cells that line blood vessels (see Fig. 7-29). They are an essential part of the blood-clotting process.
SUMMARY 11.2 Membrane Dynamics
- Lipids in a biological membrane can exist in liquid-ordered or liquid-disordered states. Membrane fluidity is affected by temperature, fatty acid composition, and sterol content.
- Flip-flop diffusion of lipids between the inner and outer leaflets of a membrane occurs only when the diffusion is specifically catalyzed by flippases, floppases, scramblases, or PI transporters.
- Proteins and lipids can diffuse laterally within the plane of the membrane, but this mobility is limited by interactions of membrane proteins with internal cytoskeletal structures and interactions of lipids with lipid rafts.
- One class of lipid rafts is enriched for sphingolipids and cholesterol with a subset of membrane proteins that are GPI-linked or attached to several long-chain fatty acyl moieties. Caveolins are integral proteins that associate with the inner leaflet of the plasma membrane, forcing it to curve inward to form caveolae, which are involved in membrane transport, signaling, and the expansion of plasma membranes.
- Proteins containing BAR domains cause local membrane curvature and mediate the fusion of two membranes, which accompanies processes such as endocytosis, exocytosis, and viral invasion. Septins are proteins that sense or cause membrane curvature. Membrane fusion depends on SNARE proteins, which draw two membranes close together and favor fusion.
- Integrins, cadherins, and selectins are transmembrane proteins of the plasma membrane that act both to attach cells to each other and to carry messages between the extracellular matrix and the cytoplasm.
 the lipids in a bilayer form a gel-like liquid-ordered state, in which all types of motion of individual lipid molecules are strongly constrained (
the lipids in a bilayer form a gel-like liquid-ordered state, in which all types of motion of individual lipid molecules are strongly constrained ( Membrane proteins called flippases, floppases, and scramblases (
Membrane proteins called flippases, floppases, and scramblases ( The complex of SNAREs and SNAP25 is the target of several powerful neurotoxins. Clostridium botulinum toxin is a bacterial protease that cleaves specific bonds in SNARE proteins, preventing neurotransmission and causing paralysis and death. Because of its very high specificity for these proteins, purified botulinum toxin has served as a powerful tool for dissecting the mechanism of neurotransmitter release in vivo and in vitro. Used in small amounts, botulinum toxin (Botox) is used in medicine to treat disorders of eye and neck muscles, as well as cosmetically for the removal of skin wrinkles. Tetanus toxin, produced by the bacterium Clostridium tetani, also is a protease with high specificity for SNARE proteins. It causes painful muscle spasms and rigidity of voluntary muscles — hence the characteristic symptom “lockjaw.”
The complex of SNAREs and SNAP25 is the target of several powerful neurotoxins. Clostridium botulinum toxin is a bacterial protease that cleaves specific bonds in SNARE proteins, preventing neurotransmission and causing paralysis and death. Because of its very high specificity for these proteins, purified botulinum toxin has served as a powerful tool for dissecting the mechanism of neurotransmitter release in vivo and in vitro. Used in small amounts, botulinum toxin (Botox) is used in medicine to treat disorders of eye and neck muscles, as well as cosmetically for the removal of skin wrinkles. Tetanus toxin, produced by the bacterium Clostridium tetani, also is a protease with high specificity for SNARE proteins. It causes painful muscle spasms and rigidity of voluntary muscles — hence the characteristic symptom “lockjaw.” 
 Lipids in a biological membrane can exist in liquid-ordered or liquid-disordered states. Membrane fluidity is affected by temperature, fatty acid composition, and sterol content.
Lipids in a biological membrane can exist in liquid-ordered or liquid-disordered states. Membrane fluidity is affected by temperature, fatty acid composition, and sterol content.