11.1 The Composition and Architecture of Membranes
We begin our discussion of biological membranes by looking at the stable bilayer structures formed spontaneously by phospholipids in water. In biological membranes we see this basic lipid bilayer combined with a wide array of membrane proteins specialized for one of the many roles that membranes play in cells. Each intracellular compartment has membranes with specific protein and lipid content, uniquely disposed across the two leaflets of the bilayer. The intracellular membranes together make up a dynamic endomembrane system that synthesizes and distributes membrane components to create functionally specialized cell compartments. Depending on its function, a protein may form one or several transmembrane segments, or it may adhere to the membrane bilayer through lipids attached to the protein, or it may alternate between membrane-associated and free forms.
The Lipid Bilayer Is Stable in Water
Glycerophospholipids, sphingolipids, and sterols are virtually insoluble in water. Recall that glycerophospholipids contain two long-chain fatty acids (which are hydrophobic) and a head group consisting of glycerol and one of several polar or charged substituents such as phosphocholine or phosphoethanolamine (see Fig. 10-8). Sphingolipids are constructed from a long-chain alkyl amine (sphingosine), a saturated long-chain fatty acid, and a polar head group that may be as simple as phosphocholine or may be a complex oligosaccharide (see Fig. 10-11). Sterols (cholesterol in animal membranes) have a very nonpolar steroid nucleus of four fused rings, and a polar hydroxyl group at one end of the ring system (see Fig. 10-15). Each type of membrane lipid has its particular effect on membrane structure and function. When mixed with water, these lipids spontaneously form microscopic lipid aggregates, clustering together, with their hydrophobic moieties in contact with each other and their hydrophilic groups in contact with the surrounding water. The clustering reduces the amount of hydrophobic surface exposed to water. This minimizes the number of molecules in the shell of ordered water at the lipid-water interface (see Fig. 2-7), resulting in an increase in entropy. This hydrophobic effect provides the thermodynamic driving force for the formation and maintenance of these clusters of lipid molecules. The term hydrophobic interactions is sometimes used to describe the clustering of hydrophobic molecular surfaces in an aqueous environment, but the molecules are not interacting chemically; they are simply finding the lowest-energy environment by reducing the hydrophobic, or nonpolar, surface area exposed to water.
Depending on the precise conditions and the nature of the lipids, several types of lipid aggregate can form when amphipathic lipids are mixed with water (Fig. 11-1). Micelles are spherical structures that contain anywhere from a few dozen to a few thousand amphipathic molecules. These molecules are arranged with their hydrophobic regions aggregated in the interior, where water is excluded, and their hydrophilic head groups at the outer surface, in contact with water. Micelle formation is favored when the cross-sectional area of the head group is greater than that of the acyl side chain(s), as in free fatty acids, lysophospholipids (phospholipids lacking one fatty acid), and many detergents, such as sodium dodecyl sulfate (SDS; p. 88).
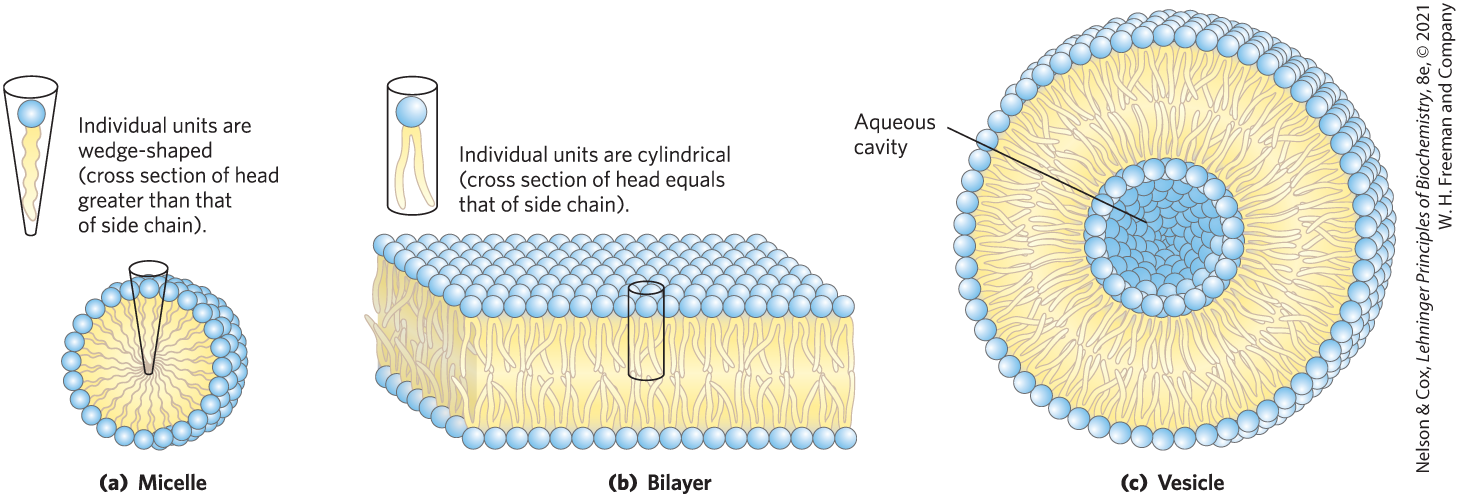
FIGURE 11-1 Amphipathic lipid aggregates that form in water. (a) In micelles, the hydrophobic chains of the fatty acids are sequestered at the core of the sphere. There is virtually no water in the hydrophobic interior. (b) In an open bilayer, all acyl side chains except those at the edges of the sheet are protected from interaction with water. (c) When a two-dimensional bilayer folds on itself, it forms a closed bilayer, a three-dimensional hollow vesicle (liposome) enclosing an aqueous cavity.
A second type of lipid aggregate in water is the bilayer, in which two lipid monolayers (leaflets) form a two-dimensional sheet. Bilayer formation is favored if the cross-sectional areas of the head group and acyl side chain(s) are similar, as in glycerophospholipids and sphingolipids. The hydrophobic portions in each monolayer, excluded from water, make contact with each other. The hydrophilic head groups interact with water at one or the other surface of the bilayer. Because the hydrophobic regions at its edges (Fig. 11-1b) are in contact with water, a bilayer sheet is relatively unstable and spontaneously folds back on itself to form a hollow sphere, called a vesicle or liposome (Fig. 11-1c). The continuous surface of vesicles eliminates exposed hydrophobic regions, allowing bilayers to achieve maximal stability in their aqueous environment. Vesicle formation also creates a separate internal aqueous compartment (the vesicle lumen). It is likely that the antecedents to the first living cells resembled lipid vesicles, their aqueous contents segregated from their surroundings by a lipid bilayer.
Studies of lipid bilayers in vitro (Fig. 11-2) show that the hydrocarbon core of the bilayer, made up of the and of the fatty acyl groups, is about as nonpolar as decane, and about 3 nm (30 Å) thick, roughly the width of two extended fatty acyl chains. Biological membranes, to which we turn next, are 50–80 Å thick, when proteins protruding on either side are included.
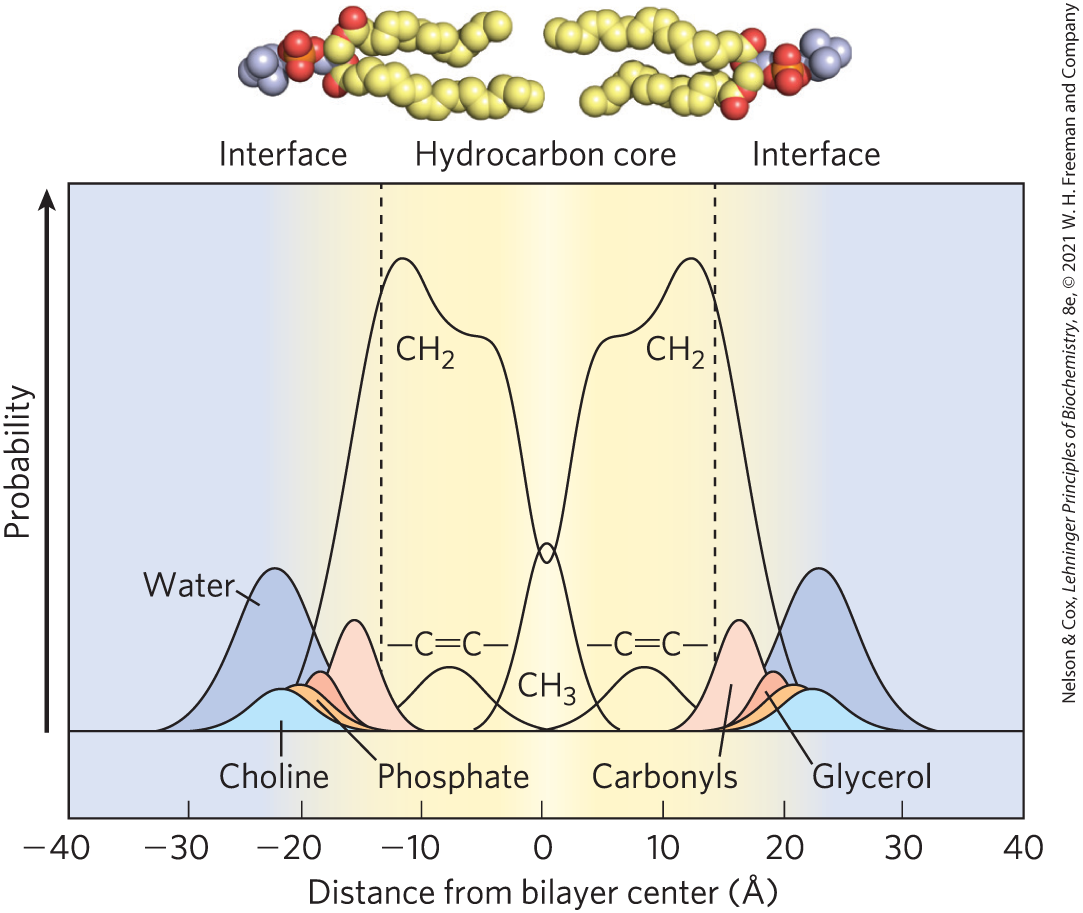
FIGURE 11-2 Distribution of membrane lipids across an artificial membrane. The membrane, composed of pure phosphatidylcholine in which both fatty acids are oleic acid , was studied with x-ray and neutron diffraction. The y axis shows the probability that one of the chemical moieties will be found at the position indicated on the x axis. The phosphatidylcholine molecules are shown at the same scale as the x axis. Fatty acyl chains in the hydrocarbon core are fluid and are about as nonpolar as decane. At either side, a polar region about 15 Å from the center of the bilayer contains the fatty acyl carbonyls. The glycerol, choline, and phosphate that make up the polar head group are at 18–20 Å, outside the hydrocarbon core and in contact with water. [Information from A. Rath and C. M. Deber, Annu. Rev. Biophys. 41:135, 2012, Fig. 5a, based on M. C. Wiener and S. H. White, Biophys. J. 61:434, 1992.]
Bilayer Architecture Underlies the Structure and Function of Biological Membranes
In the generalized membrane in Figure 11-3, phospholipids form a bilayer in which proteins are embedded, their hydrophobic domains in contact with the fatty acyl chains of membrane lipids. Some proteins protrude from only one side of the membrane; others have domains exposed on both sides. The orientation of both lipids and proteins in the bilayer is asymmetric, giving the membrane structural and functional “sidedness.” The individual lipid and protein units in a membrane form a fluid mosaic with a pattern that, unlike a mosaic of ceramic tile and mortar, is able to change as lipids and proteins move in the plane of the membrane, while maintaining the permeability barrier to polar and charged solutes.
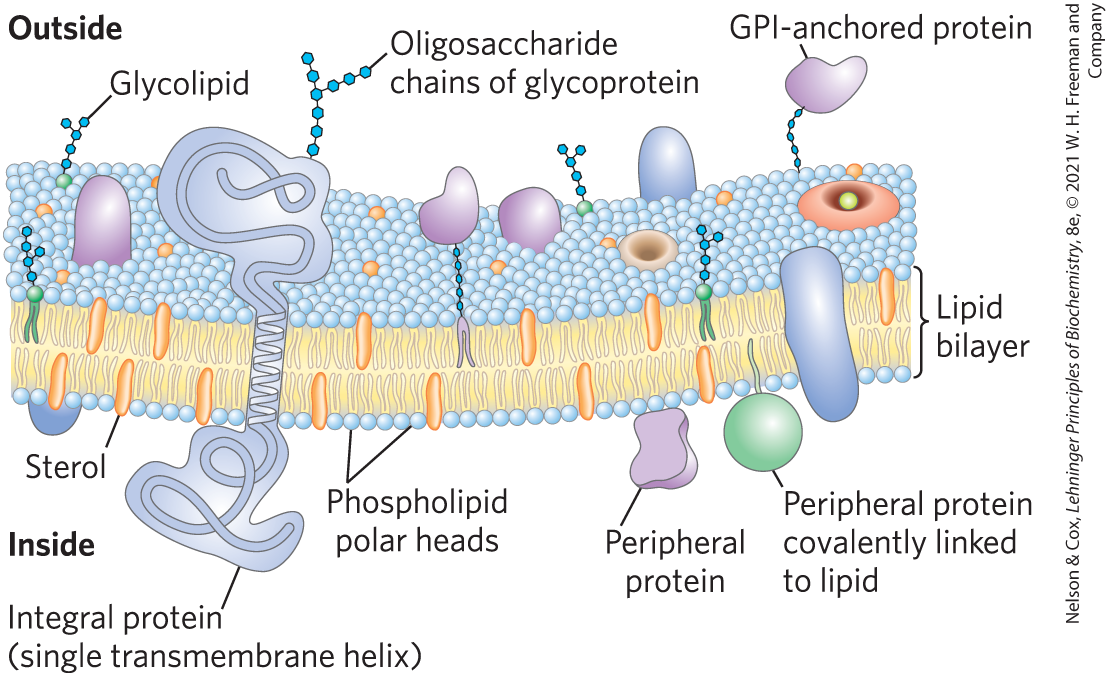
FIGURE 11-3 Fluid mosaic model for plasma membrane structure. In this simplified and general model, the fatty acyl chains in the interior of the membrane form a fluid, hydrophobic region. Integral proteins float in this sea of lipid, with their nonpolar amino acid side chains protected from interaction with water.
Membranes are not merely passive barriers. They are flexible, self-repairing, and selectively permeable. Their flexibility permits the shape changes that accompany cell growth and movement (such as amoeboid movement). With their ability to break and reseal, two membranes can fuse, as in exocytosis, or a single membrane-enclosed compartment can undergo fission to yield two sealed compartments, as in endocytosis or cell division, without creating gross leaks through cellular surfaces. Their selective permeability allows them to serve as molecular gatekeepers.
These membrane functions are accomplished primarily by a broad array of proteins and enzymes in and on membranes. At the cell surface, transporters move specific organic solutes and inorganic ions across the membrane; receptors sense extracellular signals and trigger molecular changes in the cell; ion channels mediate electrical signaling between cells; and adhesion molecules hold neighboring cells together. The relative proportions of protein and lipid vary with the type of membrane, reflecting the diversity of biological roles. For example, certain neurons have a myelin sheath — an extended plasma membrane that wraps around the cell many times and acts as a passive electrical insulator. The myelin sheath consists primarily of lipids (good insulators). In contrast, the plasma membranes of bacteria and the membranes of mitochondria and chloroplasts, the sites of many enzyme-catalyzed processes, contain more protein than lipid (in mass per total mass).
Within the cell, membranes organize cellular processes such as the synthesis of lipids and certain proteins and the energy transductions that produce ATP in mitochondria and chloroplasts. Proteins associated with membranes are essentially confined to two-dimensional space, where intermolecular collisions of membrane proteins and lipids are far more probable than in the three-dimensional cytoplasm, so the efficiency of enzyme-catalyzed processes organized within membranes can be vastly increased.
The Endomembrane System Is Dynamic and Functionally Differentiated
In eukaryotic cells, the endoplasmic reticulum (ER), Golgi apparatus, lysosomes, and various small vesicles that carry lipids and proteins throughout the cell are each surrounded and defined by a single membrane. This endomembrane system (Fig. 11-4) also includes three organelles with double membranes: the nucleus, mitochondrion, and (in plants) chloroplast. (Recall the likely origin of mitochondria and chloroplasts by an endosymbiont mechanism shown in Fig. 1-37.) The convoluted and tubular ER is dynamic and extends throughout the cell, touching the other compartments at specific membrane contact points. Although each compartment is closed, they actively exchange proteins and lipids in several ways.
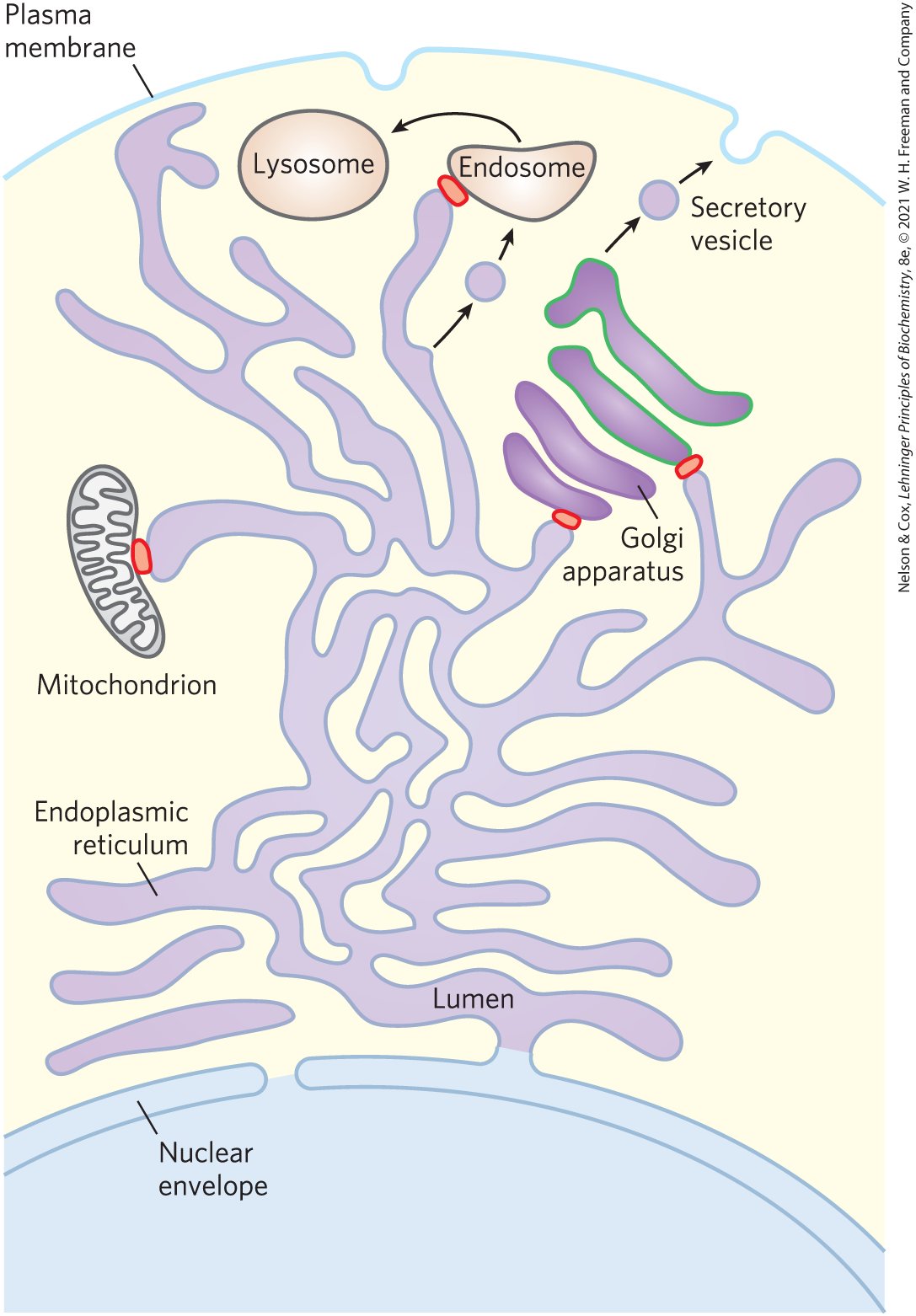
FIGURE 11-4 Trafficking in the endomembrane system of an animal cell. Lipids and proteins move from the site of their synthesis (ER) through the Golgi apparatus to the cell surface (or to organelles such as lysosomes). Mitochondria (and in plants, chloroplasts) are also part of the endomembrane system. Small vesicles bud off the ER, move to and fuse with the cis Golgi apparatus, exit the trans Golgi apparatus as secretory or transport vesicles, and fuse with the plasma membrane or with endosomes, giving rise to lysosomes. Individual lipid molecules can be carried throughout the cell by lipid transfer proteins (LTPs), which act at membrane contact points, shown here in red.
Cells have mechanisms to target specific lipids to particular organelles. Each species, each tissue or cell type, and the organelles of each cell type have a characteristic set of membrane lipids. Plasma membranes, for example, are enriched in cholesterol and sphingolipids, but they contain no detectable cardiolipin (Fig. 11-5); mitochondrial membranes are very low in cholesterol and sphingolipids, but they contain most of the cell’s phosphatidylglycerol and cardiolipin, which are synthesized within the mitochondria. Cardiolipin is specifically required for correct assembly of the respiratory complexes of mitochondria. In all but a few cases, the functional significance of these different combinations of lipids is not yet known.
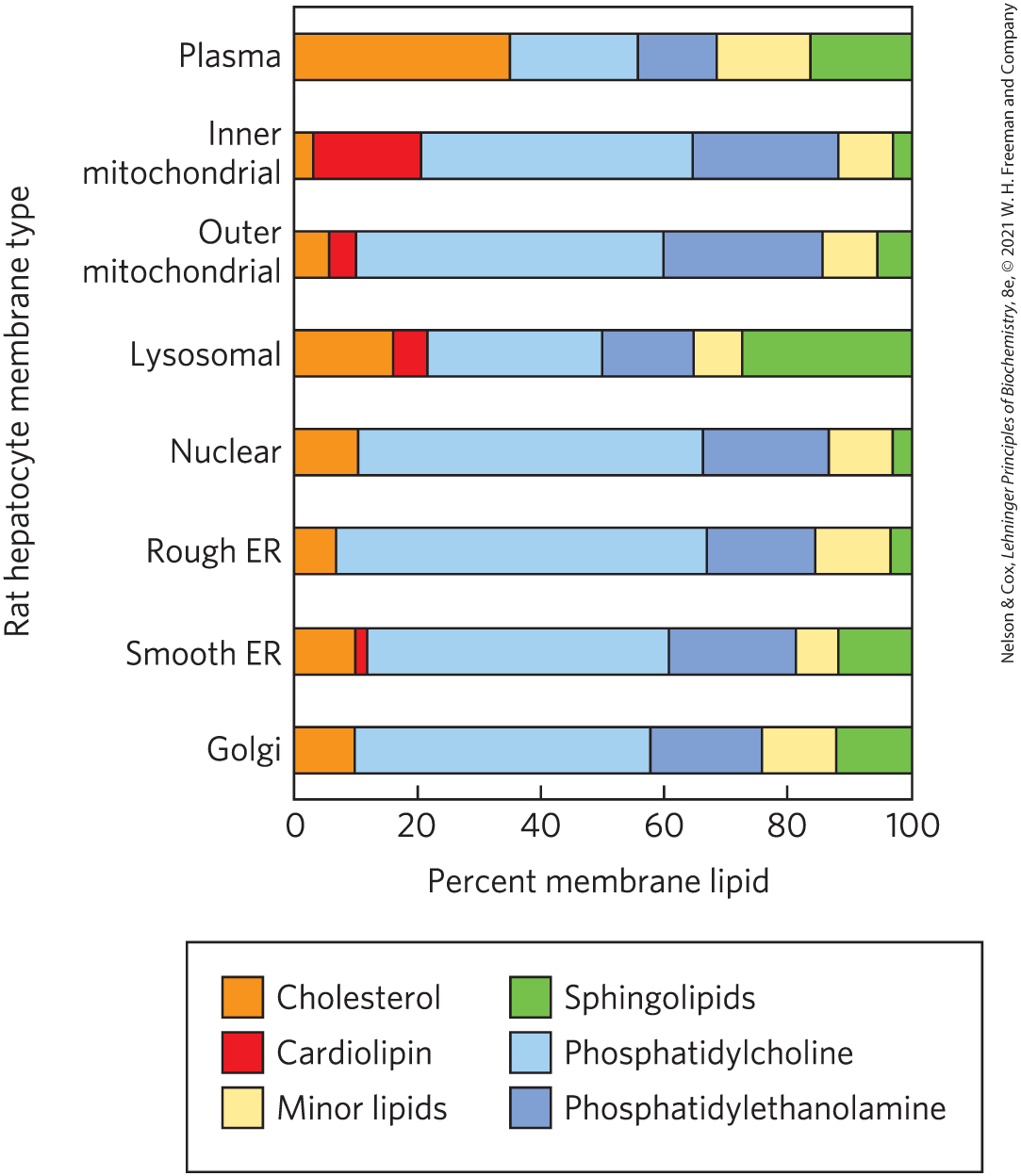
FIGURE 11-5 Lipid composition of the plasma and organelle membranes of a rat hepatocyte. The functional specialization of each membrane type is reflected in its unique lipid composition. Cholesterol is prominent in plasma membranes but barely detectable in mitochondrial membranes. Cardiolipin is a major component of the inner mitochondrial membrane but not of the plasma membrane. Phosphatidylserine, phosphatidylinositol, and phosphatidylglycerol are relatively minor components of most membranes but serve critical functions; phosphatidylinositol and its derivatives, for example, are important in signal transductions triggered by hormones. Sphingolipids, phosphatidylcholine, and phosphatidylethanolamine are present in most membranes but in varying proportions. Glycolipids, which are major components of the chloroplast membranes of plants, are virtually absent in animal cells.
Most membrane lipids and proteins are synthesized in the ER, and from there they move to their destination organelles or to the plasma membrane. In this process of membrane trafficking, small membrane vesicles bud from the ER, then move to and fuse with the cis Golgi apparatus. As lipids and proteins move across the Golgi apparatus to its trans side, they undergo covalent modifications required for their cellular function. In many cases, these covalent alterations act as molecular ZIP codes, dictating the eventual location of the mature protein.
Membrane trafficking achieves striking changes in lipid composition and disposition across the bilayer (Fig. 11-6). Phosphatidylcholine is the principal phospholipid in the lumenal leaflet of the Golgi membrane, but in transport vesicles leaving the trans Golgi apparatus, phosphatidylcholine has largely been replaced by sphingolipids and cholesterol, which, following fusion of the transport vesicles with the plasma membrane, make up the majority of the lipids in the outer leaflet of the cell’s plasma membrane. Plasma membrane lipids are asymmetrically distributed between the two leaflets of the bilayer. Choline-containing lipids (phosphatidylcholine and sphingomyelin) are typically found in the outer (extracellular, or exoplasmic) leaflet, whereas phosphatidylserine, phosphatidylethanolamine, and the phosphatidylinositols are almost exclusively in the inner (cytoplasmic) leaflet. Here the negatively charged serine and inositol phosphate head groups can interact electrostatically with positively charged regions of peripheral or amphitropic membrane proteins (described below).
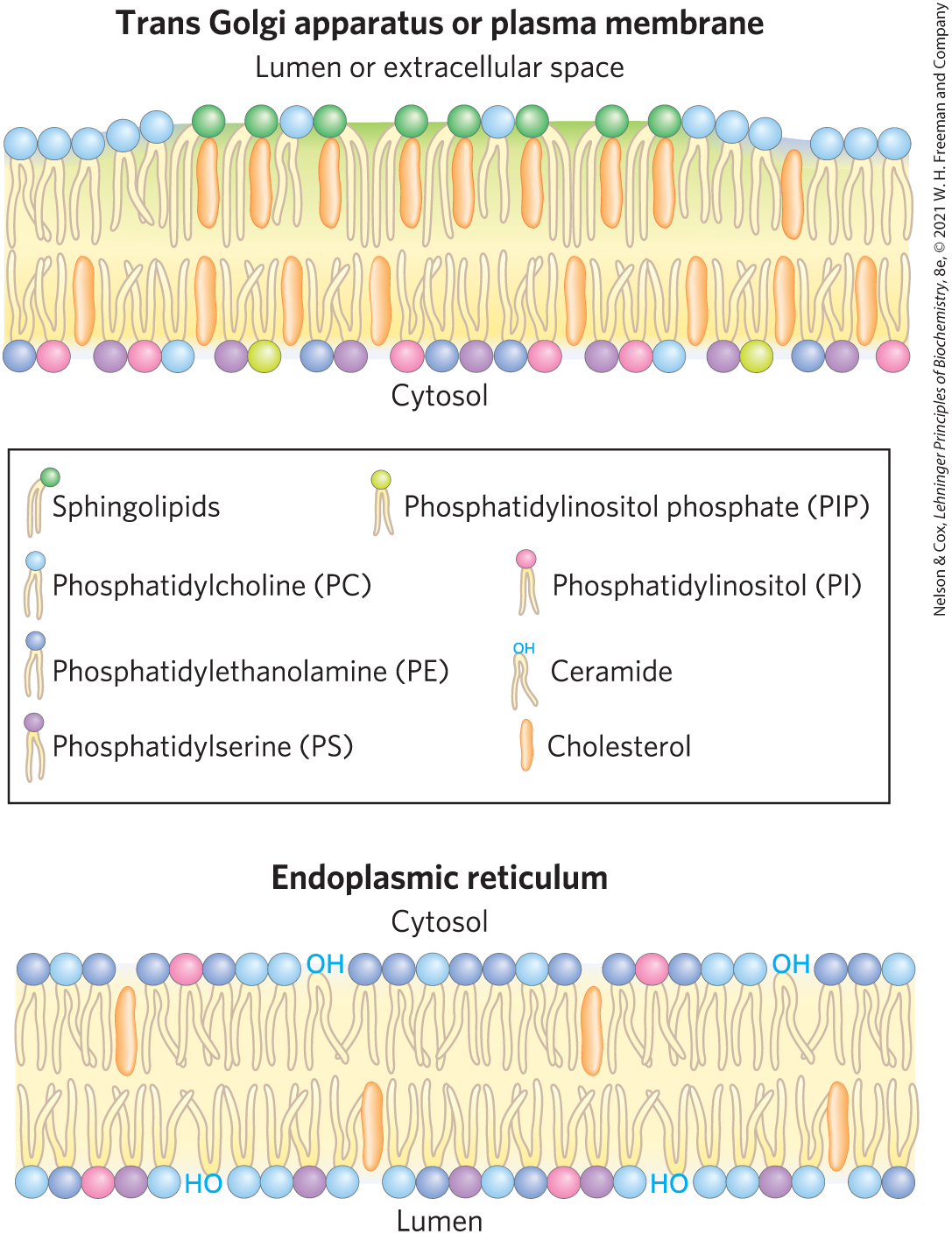
FIGURE 11-6 Change in lipid composition of secretory vesicles with their passage through the Golgi apparatus. Both the lipid composition of the bilayer and the disposition of specific lipids between inner and outer leaflets change remarkably as a result of vesicle trafficking. In the trans Golgi apparatus and plasma membrane, sphingolipids are greatly enriched in the leaflet facing the lumen or extracellular space, and phosphatidylserine, phosphatidylethanolamine, and the inositol phospholipids are almost exclusively in the cytosolic leaflet. The plasma membrane is also enriched with cholesterol. [Information from G. Drin, Annu. Rev. Biochem. 83:51, 2014, Fig. 1.]
A second route for redistributing lipids from their site of synthesis to their destination membrane is via lipid transfer proteins (LTPs; Fig. 11-7), which occur universally in various forms. LTPs are soluble in water, but they have a hydrophobic lipid-binding pocket in which they carry a lipid from one membrane to another through the cytosol. Some LTPs are bispecific: they carry, for example, a molecule of cholesterol to the plasma membrane, and they return with a phosphatidylinositol molecule. One class of lipid transfer protein forms a hydrophobic tunnel between two membranes at their contact points. In some cases, ATP is needed to drive the process, supplied by an ATP-binding cassette (ABC) transporter (described below). The rate of lipid movement from one membrane to another in vivo is significantly greater than the rate of vesicle budding and fusion.
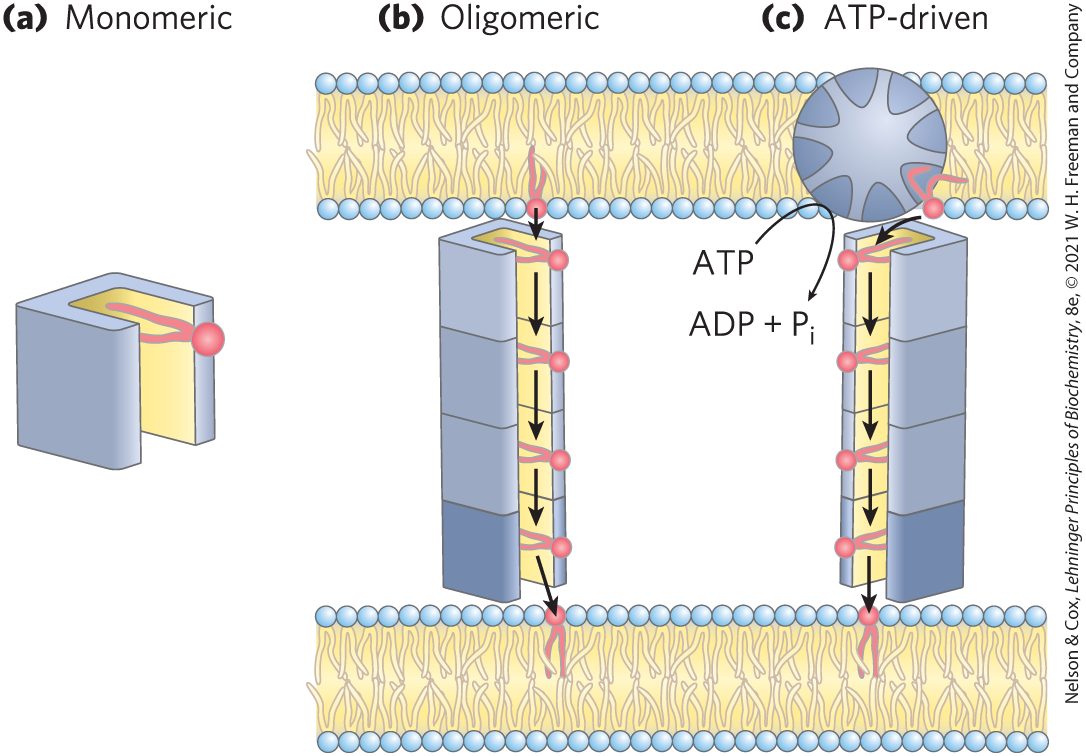
FIGURE 11-7 Lipid transfer protein (LTP) action. (a) Lipid transfer proteins move lipids and other nonpolar solutes through the very polar cytosol by providing a hydrophobic groove or pocket into which the hydrophobic solute can fit, with its nonpolar regions masked from the surrounding water. (b) Some LTPs form multisubunit structures to generate a hydrophobic groove long enough to connect two separate membranes at contact points (see Fig. 11-5). (c) To move lipid molecules against a concentration gradient (say, from a leaflet poor in phosphatidylserine to one relatively rich in it), the LTP is coupled to an ATP-dependent pump. [Information from L. H. Wong et al., Nat. Rev. Mol. Cell Biol. 20:85, 2019, Fig. 2.]
Finally, once phospholipids reach their destination membrane, they can undergo enzymatic remodeling of their fatty acid constituents, a subject discussed in Chapter 21.
Membrane Proteins Are Receptors, Transporters, and Enzymes
The protein composition of membranes reflects each membrane’s functional specialization. One large group of membrane proteins are the receptors for extracellular signals such as hormones and changes in membrane potential. Hundreds of membrane proteins are transporters that carry specific polar or charged compounds — sugars, amino acids, vitamins, a wide variety of metabolic intermediates, minerals , and trace metal ions — across the plasma membrane or between organelles. All transporters and many receptors must span the membrane at least once to play their roles. Some membrane-associated enzymes also span the membrane, but many carry out their catalytic functions on one side or the other of their membrane setting, partially penetrating just one leaflet of the bilayer.
The synthesis and delivery to the site of function are more complicated for membrane proteins than for typical cytosolic proteins. Many of the proteins destined for the plasma membrane undergo extensive posttranslational modification as they pass through the ER and the Golgi apparatus. One covalent modification is the glycosylation (attachment of oligosaccharides) of proteins bound for the plasma membrane. In glycophorin, a glycoprotein of the erythrocyte plasma membrane, 60% of the mass consists of complex oligosaccharides covalently attached to specific amino acid residues (Fig. 11-8). For such glycoproteins, Ser, Thr, and Asn residues are the most common points of carbohydrate attachment (see Fig. 7-27), and the carbohydrates are invariably on the outer face of the plasma membrane. Another posttranslational modification of some proteins is the covalent attachment of one or more lipids, which serve as hydrophobic anchors to hold a protein to the membrane or as tags to target the protein to a specific location.
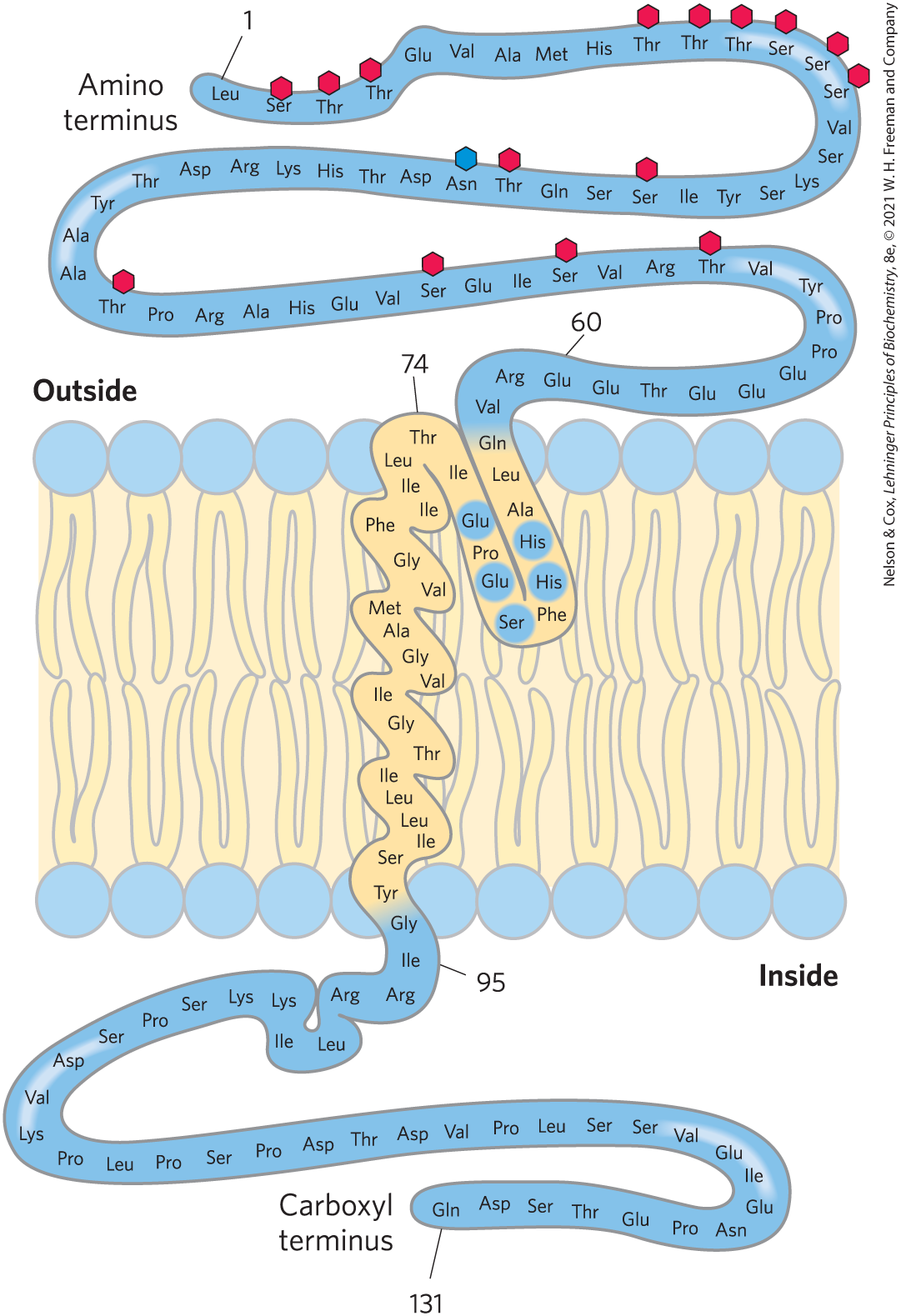
FIGURE 11-8 Transbilayer disposition of glycophorin in an erythrocyte. One hydrophilic domain, containing all the sugar residues, is on the outer surface, and another hydrophilic domain protrudes from the inner face of the membrane. Each red hexagon represents a tetrasaccharide (containing two Neu5Ac (sialic acid), Gal, and GalNAc) O-linked to a Ser or Thr residue; the blue hexagon represents an oligosaccharide N-linked to an Asn residue. The oligosaccharides are much larger than the hexagons used to represent them. A segment of 19 hydrophobic residues (residues 75 to 93) forms an α helix that traverses the membrane bilayer (see Fig. 11-13a). The segment from residues 64 to 74 has some hydrophobic residues and probably penetrates the outer face of the lipid bilayer, as shown. The functional unit of glycophorin is a homodimer; for clarity we show only one of the subunits here. [Information from V. T. Marchesi et al., Annu. Rev. Biochem. 45:667, 1976.]
Membrane Proteins Differ in the Nature of Their Association with the Membrane Bilayer
Integral membrane proteins are firmly embedded within the lipid bilayer and are removable only by agents such as detergents or organic solvents that overcome hydrophobic interactions (Fig. 11-9). Peripheral membrane proteins associate with the membrane through electrostatic interactions and hydrogen bonding with hydrophilic domains of integral proteins and with the charged head groups of membrane lipids. In the laboratory, they can be released from their membrane association by relatively mild treatments that interfere with electrostatic interactions or that break hydrogen bonds. Amphitropic proteins associate reversibly with membranes and are therefore found both in the membrane and in the cytosol. Their affinity for membranes results in some cases from the protein’s noncovalent interaction with another membrane protein or lipid, and in other cases from the presence of one or more covalently attached lipids (see Fig. 11-16).
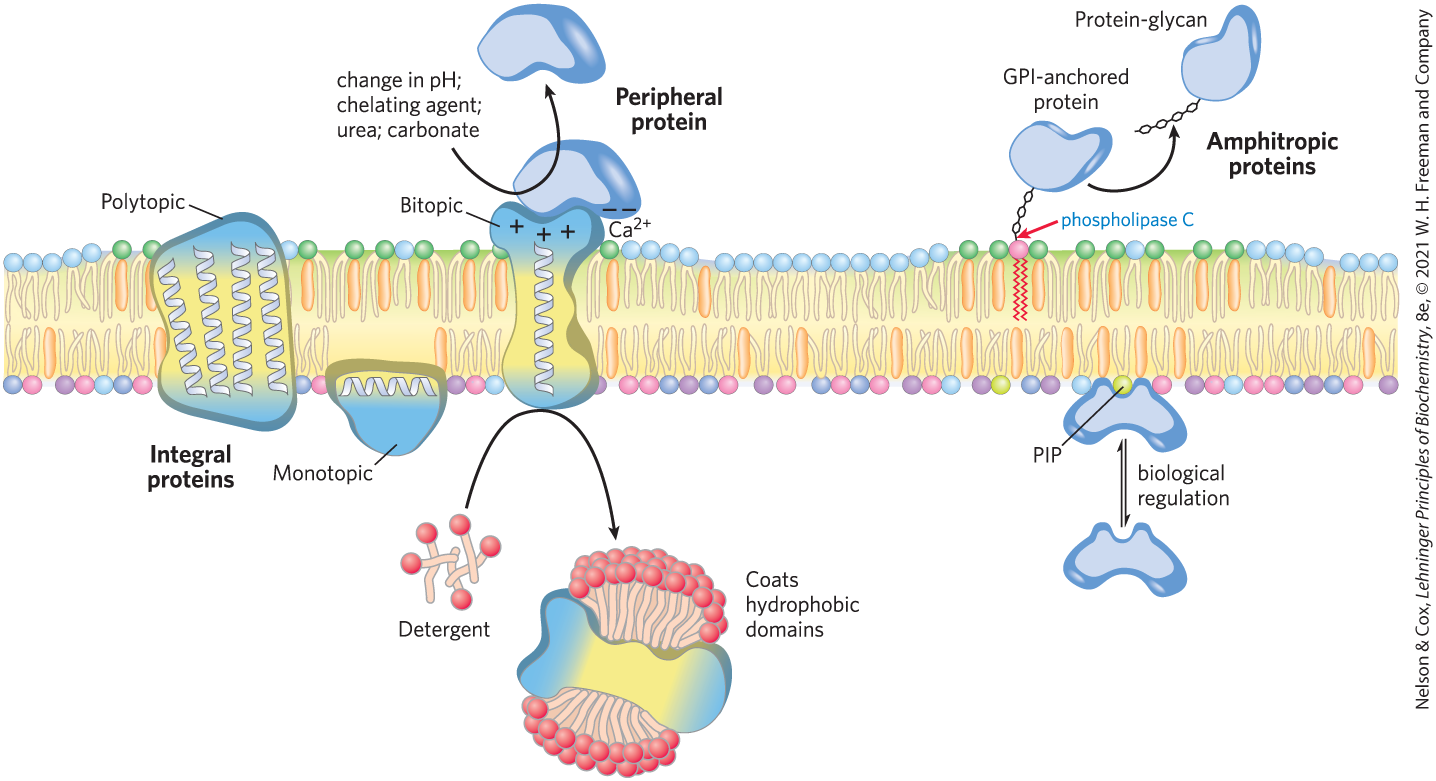
FIGURE 11-9 Integral, peripheral, and amphitropic proteins. Membrane proteins can be operationally distinguished by the conditions required to release them from the membrane. Integral proteins can only be removed from membranes with harsh treatment such as detergent. Peripheral proteins are more loosely associated. Amphitropic proteins have a reversible association with membranes that depends on a regulatory process, such as reversible palmitoylation or phosphorylation.
The firm attachment of integral proteins to membranes is the result of the hydrophobic interaction between the nonpolar core of the lipid bilayer and nonpolar side chains of the protein. Monotopic integral proteins have small hydrophobic domains that interact with only a single leaflet of the membrane (Fig. 11-10). These domains, which make up as little as a few percent of the protein’s total mass, provide enough hydrophobic surface to hold the proteins firmly to the membrane. Bitopic proteins span the bilayer once, extending on either surface. They have a single hydrophobic sequence somewhere in the molecule. Glycophorin (Fig. 11-8) is bitopic. Polytopic proteins cross the membrane several times. They have multiple hydrophobic sequences, each of which, when in the α-helical conformation, is long enough (about 20 residues) to span the lipid bilayer. (Recall from Worked Example 4-1 that each residue in an α helix adds 1.5 Å to its length.)
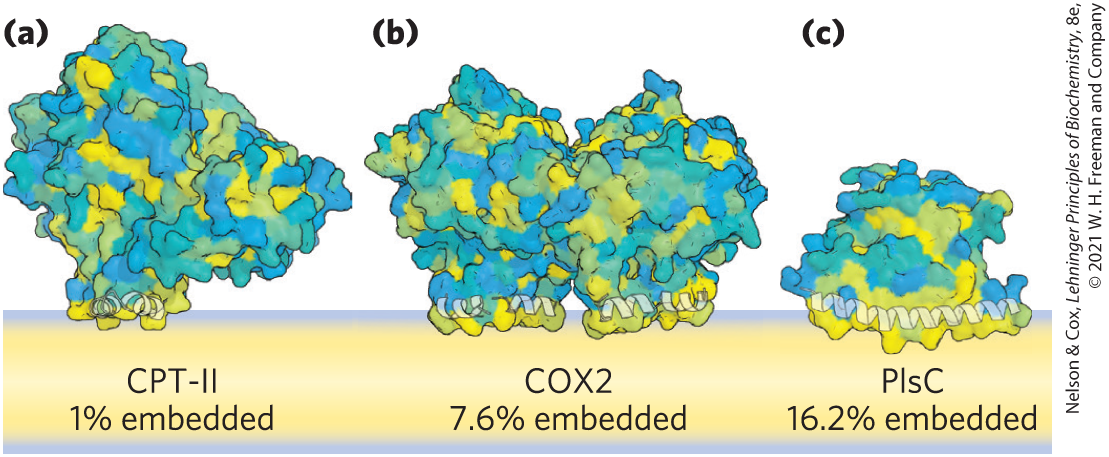
FIGURE 11-10 Monotopic proteins penetrate only one leaflet. Three typical monotopic membrane proteins have relatively little of their structure (1% to 16%) associated with lipids in one leaflet of the bilayer. They are colored to show surface hydrophobicity, with yellow hydrophobic, blue hydrophilic, and hues of green in between. Each of these proteins catalyzes a reaction that involves a hydrophobic substrate. (a) CPT-II is a mammalian carnitine palmitoyltransferase II; (b) COX2 is a mammalian prostaglandin H2 synthase-1; (c) PlsC is a bacterial acyltransferase (1-acyl-sn-glycerol-6-phosphate acyltransferase). [Data from (a) PDB ID 2H4T, Y. S. Hsiao et al., Biochem. Biophys. Res. Commun. 346:974, 2006; (b) PDB ID 1CQE, D. Picot et al., Nature 367:243, 1994; (c) PDB ID 5KYM, R. M. Robertson et al., Nat. Struct. Mol. Biol. 24:666, 2017.]
One of the best-studied polytopic proteins, bacteriorhodopsin, has seven very hydrophobic internal sequences and spans the lipid bilayer seven times. Bacteriorhodopsin is a light-driven proton pump densely packed in regular arrays in the purple membrane of the bacterium Halobacterium salinarum. X-ray crystallography and cryo-electron microscopy reveal a structure in which each of the seven α-helical segments crosses the lipid bilayer, and all are connected by nonhelical loops at the inner and outer faces of the membrane (Fig. 11-11). In the amino acid sequence of bacteriorhodopsin, seven segments of about 20 hydrophobic residues can be identified, each forming an α helix that spans the bilayer. The seven helices are clustered together and oriented not quite perpendicular to the bilayer plane, a motif that (as we shall see in Chapter 12) is very common in membrane proteins involved in signal reception. The hydrophobic interaction keeps the nonpolar amino acid residues firmly anchored among the fatty acyl groups of the membrane lipids in the membrane’s lipid core.
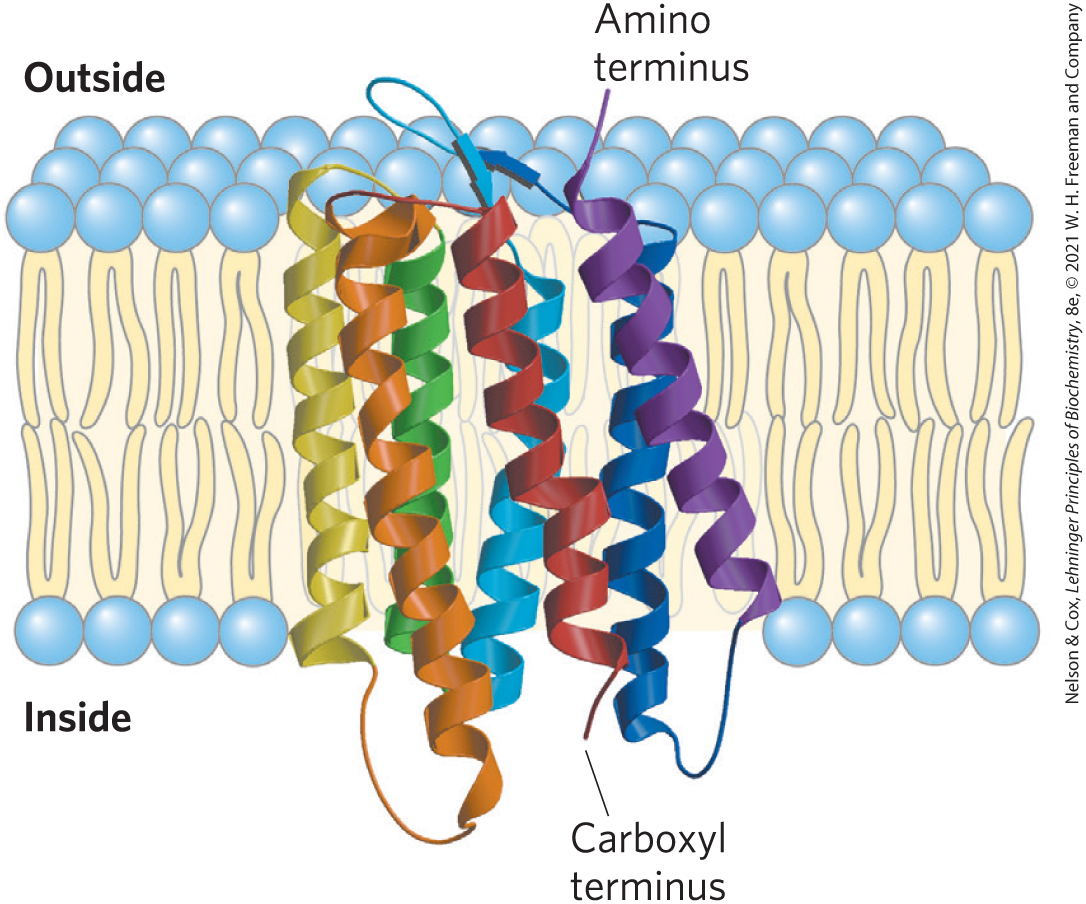
FIGURE 11-11 Bacteriorhodopsin, a membrane-spanning protein. The single polypeptide chain folds into seven hydrophobic α helices, each of which traverses the lipid bilayer roughly perpendicular to the plane of the membrane. The seven transmembrane helices are clustered, and the space around and between them is filled with the acyl chains of membrane lipids. The light-absorbing pigment retinal (see Fig. 10-20) is buried deep within the membrane in contact with several of the helical segments (not shown). The helices are colored to correspond with the hydropathy plot in Figure 11-13. [Data from PDB ID 2AT9, K. Mitsuoka et al., J. Mol. Biol. 286:861, 1999.]
Many membrane proteins co-crystalize with phospholipid molecules, which are presumed to be positioned in the native membranes as they are in the protein crystals. Many of these phospholipid molecules lie on the protein surface, their head groups interacting with polar amino acid residues at the inner and outer membrane–water interfaces and their side chains associated with nonpolar residues. Other phospholipids are found at the interfaces between monomers of multisubunit membrane proteins, where they form a “grease seal” (Fig. 11-12).
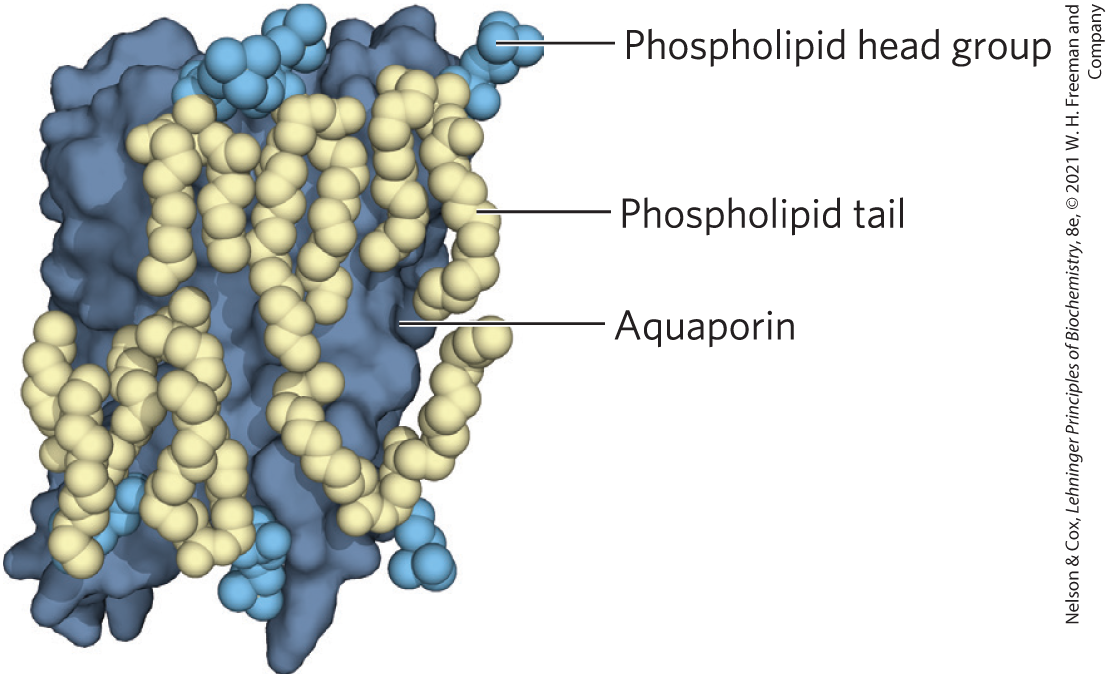
FIGURE 11-12 Lipid annuli associated with an integral protein. The crystal structure of sheep aquaporin, a transmembrane water channel, includes a shell of phospholipids positioned with their head groups (blue) at the expected positions on the inner and outer membrane surfaces and their hydrophobic acyl chains (gold) intimately associated with the surface of the protein exposed to the bilayer. The lipid forms a “grease seal” around the protein, which is depicted by a dark blue surface representation. [Data from PDB ID 2B6O, T. Gonen et al., Nature 438:633, 2005.]
The Topology of an Integral Membrane Protein Can Often Be Predicted from Its Sequence
Determination of the location of a membrane protein relative to the bilayer — that is, its topology — is generally much more difficult than determining its amino acid sequence, either directly or by gene sequencing. The amino acid sequences of many thousands of membrane proteins are known, but fewer three-dimensional structures have been established by x-ray crystallography or cryo-electron microscopy. The presence of unbroken sequences of more than 20 hydrophobic residues in a membrane protein is commonly taken as evidence that these sequences traverse the lipid bilayer, acting as hydrophobic anchors or forming transmembrane channels. All known integral proteins have at least one such sequence. Application of this logic to entire genomic sequences leads to the conclusion that in many organisms, 20% to 30% of all proteins are integral proteins.
What can we predict about the secondary structure of the membrane-spanning portions of integral proteins? At 1.5 Å (0.15 nm) per amino acid residue, an α-helical sequence of 20 to 25 residues is just long enough to span the thickness (30 Å) of the lipid bilayer. A polypeptide chain surrounded by lipids, having no water molecules with which to hydrogen-bond, will tend to form α helices or β sheets in which intrachain hydrogen bonding is maximized. If the side chains of all amino acids in a helix are nonpolar, the helix is further stabilized in the surrounding lipids by the hydrophobic effect.
Several simple methods of analyzing amino acid sequences yield reasonably accurate predictions of secondary structure for transmembrane proteins. The relative polarity of each amino acid has been determined experimentally by measuring the free-energy change accompanying the movement of that amino acid side chain from a hydrophobic environment into water. This free energy of transfer, which can be expressed as a hydropathy index (see Table 3-1), ranges from highly exergonic for charged or polar residues to highly endergonic for amino acids with aromatic or aliphatic hydrocarbon side chains. The overall hydropathy index (hydrophobicity) of a sequence of amino acids is estimated by summing the free energies of transfer for the residues in the sequence. To scan a polypeptide sequence for potential membrane-spanning segments, an investigator calculates the hydropathy index for successive segments (called windows) of a given size, from 7 to 20 residues. For a window of seven residues, for example, the average indices for residues 1 to 7, 2 to 8, 3 to 9, and so on, are plotted as in Figure 11-13 (plotted for the middle residue in each window — residue 4 for residues 1 to 7, for example). A region with more than 20 residues of high hydropathy index is presumed to be a transmembrane segment. When the sequences of membrane proteins of known three-dimensional structure are scanned using simple online bioinformatics tools, we find a reasonably good correspondence between predicted and known membrane-spanning segments. Hydropathy analysis predicts a single hydrophobic helix for glycophorin (Fig. 11-13a) and seven transmembrane segments for bacteriorhodopsin (Fig. 11-13b) — in agreement with structures known from x-ray crystallography.
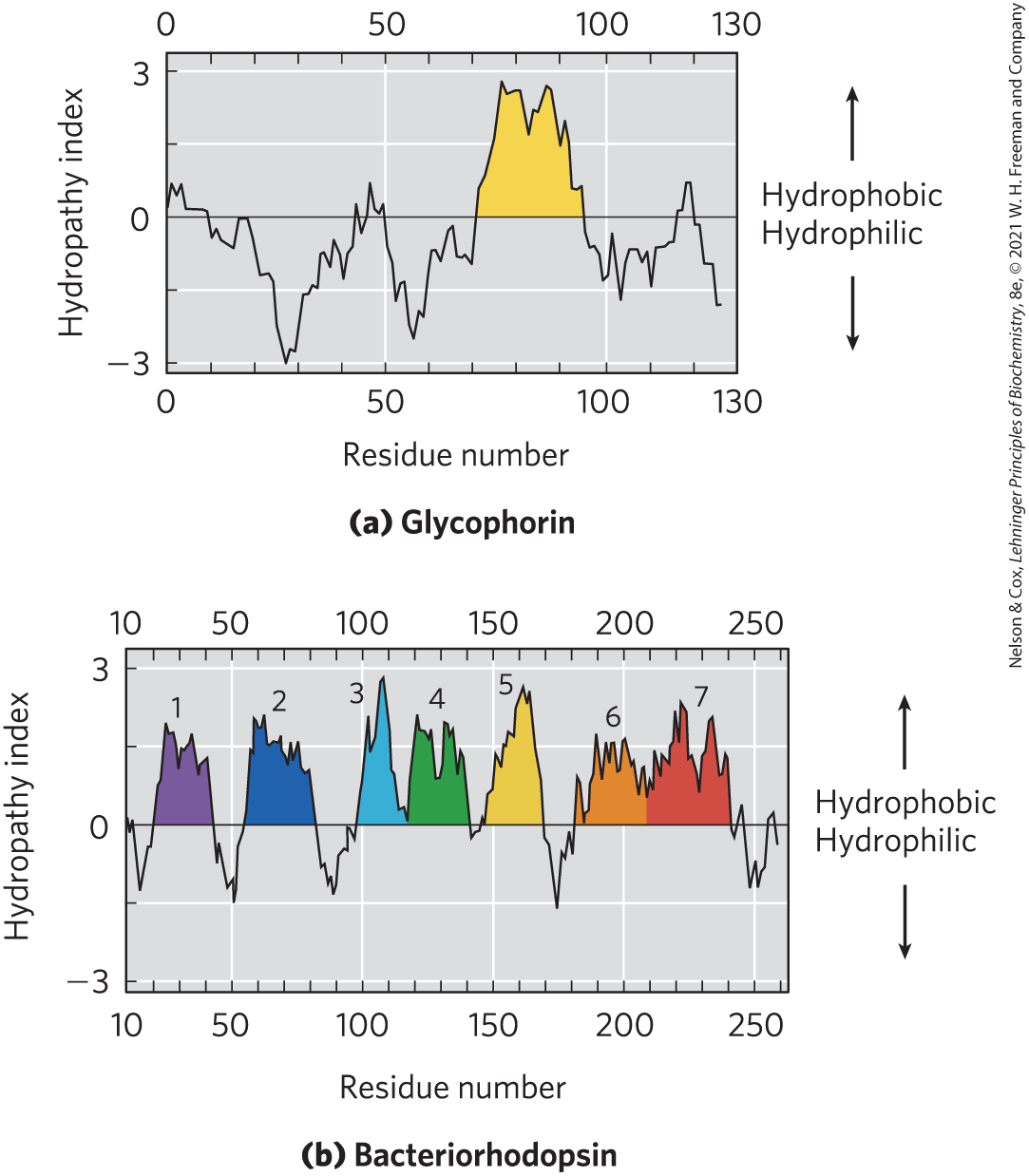
FIGURE 11-13 Hydropathy plots. Average hydropathy index (see Table 3-1) is plotted against residue number for two integral membrane proteins. The hydropathy index for each amino acid residue in a sequence of defined length, or “window,” is used to calculate the average hydropathy for that window. The horizontal axis shows the residue number in the middle of the window. (a) Glycophorin from human erythrocytes has a single hydrophobic sequence between residues 75 and 93 (yellow); compare this with Figure 11-8. (b) Bacteriorhodopsin, known from independent physical studies to have seven transmembrane helices (see Fig. 11-11), has seven hydrophobic regions. Note, however, that the hydropathy plot is ambiguous in the region of segments 6 and 7. X-ray crystallography has confirmed that this region has two transmembrane segments.
Not all integral proteins are composed of transmembrane α helices. Another structural motif common in bacterial and mitochondrial membrane proteins is the β barrel (see Fig. 4-16b), in which 20 or more transmembrane segments form β sheets that line a cylinder (Fig. 11-14). The same factors that favor α helix formation in the hydrophobic interior of a lipid bilayer also stabilize β barrels: when no water molecules are available to hydrogen-bond with the carbonyl oxygen and nitrogen of the peptide bond, maximal intrachain hydrogen bonding gives the most stable conformation. Planar β sheets do not maximize these interactions and are generally not found in the membrane interior; β barrels allow all possible hydrogen bonds and are common among membrane proteins. Porins, proteins that allow certain polar solutes to cross the outer membrane of gram-negative bacteria such as E. coli, have many-stranded β barrels lining the polar transmembrane passage. The outer membranes of mitochondria and chloroplasts also contain a variety of β barrels, as do the outer membranes of the modern bacteria descended from those believed to have evolved to mitochondria and chloroplasts.
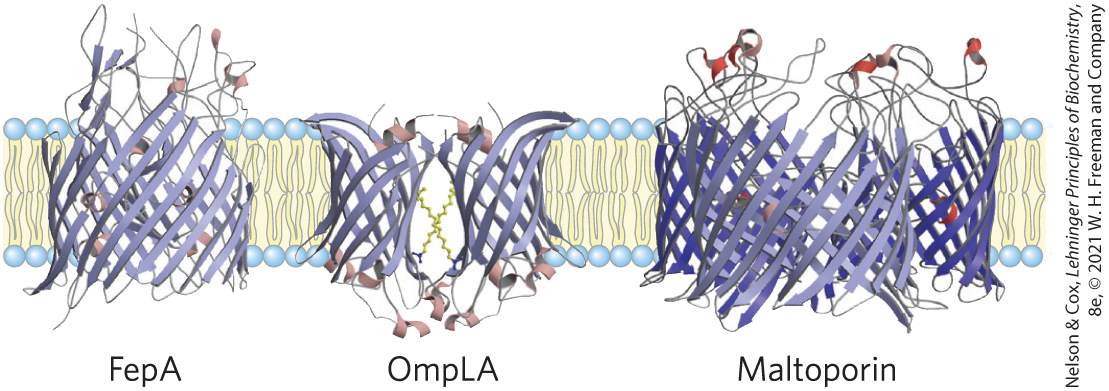
FIGURE 11-14 Polytopic integral proteins with β-barrel structure. Three proteins of the E. coli outer membrane are shown, viewed in the plane of the membrane. FepA, involved in iron uptake, has 22 membrane-spanning β strands. Outer membrane phospholipase A, or OmpLA, is a 12-stranded β barrel that exists as a dimer in the membrane. Maltoporin, a maltose transporter, is a trimer; each monomer consists of 16 β strands. [Data from FepA, PDB ID 1FEP, S. K. Buchanan et al., Nat. Struct. Biol. 6:56, 1999; OmpLA, PDB ID 1QD5, H. J. Snijder et al., Nature 401:717, 1999; maltoporin, PDB ID 1MAL, T. Schirmer et al., Science 267:512, 1995.]
A polypeptide is more extended in the β conformation than in an α helix; just seven to nine residues of β conformation are needed to span a membrane. Recall that in the β conformation, alternating side chains project above and below the sheet (see Fig. 4-5). In β strands of membrane proteins, every second residue in the membrane-spanning segment is hydrophobic and interacts with the lipid bilayer; aromatic side chains are commonly found at the lipid-protein interface. The other residues may or may not be hydrophilic.
A further remarkable feature of many transmembrane proteins of known structure is the presence of Tyr and Trp residues at the interface between lipid and water (Fig. 11-15). The side chains of these residues seem to serve as membrane interface anchors, able to interact simultaneously with the central lipid phase and the aqueous phases on either side of the membrane. Another generalization about amino acid location relative to the bilayer is described by the positive-inside rule: the positively charged Lys and Arg residues in the extramembrane loop of membrane proteins occur more commonly on the cytoplasmic face of plasma membranes.
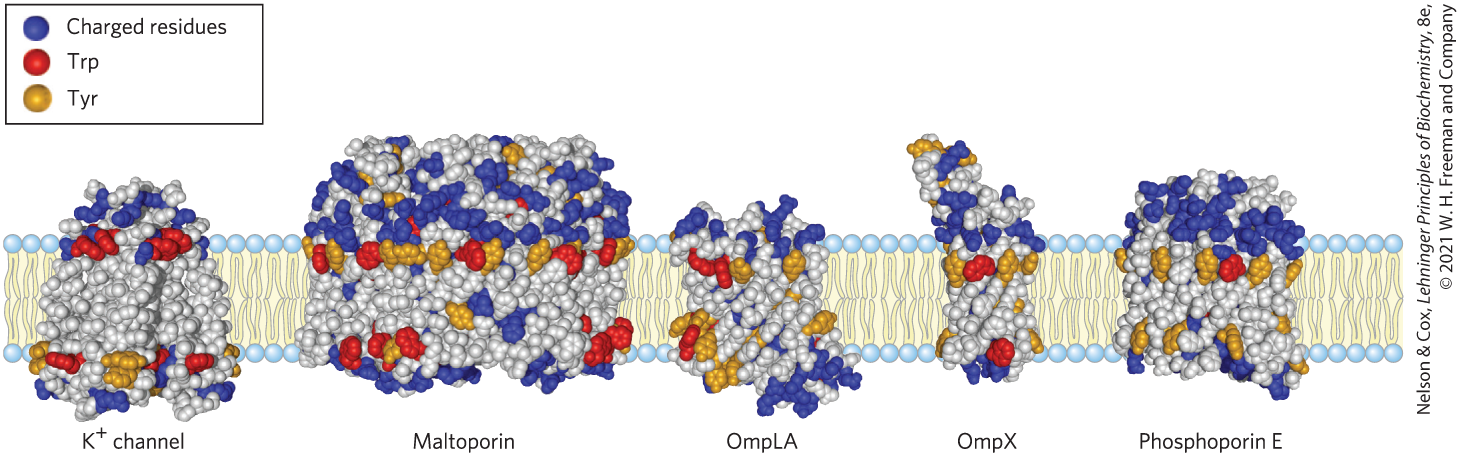
FIGURE 11-15 Tyr and Trp residues of integral proteins clustering at the water-lipid interface. The detailed structures of these five integral proteins are known from crystallographic studies. The channel is from the bacterium Streptomyces lividans (see Fig. 11-45); maltoporin, OmpLA, OmpX, and phosphoporin E are proteins of the outer membrane of E. coli. Residues of Tyr and Trp are found predominantly where the nonpolar region of acyl chains meets the polar head-group region. Charged residues (Lys, Arg, Glu, Asp) are found almost exclusively in the aqueous phases. [Data from channel, PDB ID 1BL8, D. A. Doyle et al., Science 280:69, 1998; maltoporin, PDB ID 1AF6, Y. F. Wang et al., J. Mol. Biol. 272:56, 1997; OmpLA, PDB ID 1QD5, H. J. Snijder et al., Nature 401:717, 1999; OmpX, PDB ID 1QJ9, J. Vogt and G. E. Schulz, Structure 7:1301, 1999; phosphoporin E, PDB ID 1PHO, S. W. Cowan et al., Nature 358:727, 1992.]
Covalently Attached Lipids Anchor or Direct Some Membrane Proteins
Some membrane proteins are covalently linked to one or more lipids, which may be of several types: long-chain fatty acids, isoprenoids, sterols, or glycosylated derivatives of phosphatidylinositol (GPIs; Fig. 11-16). The attached lipid provides a hydrophobic anchor that inserts into the lipid bilayer and holds the protein at the membrane surface. The strength of the hydrophobic interaction between a bilayer and a single hydrocarbon chain linked to a protein is barely enough to anchor the protein securely, but many proteins have more than one attached lipid moiety. Furthermore, other interactions, such as ionic attractions between positively charged Lys residues in the protein and negatively charged lipid head groups, can add to the anchoring effect of a covalently bound lipid. For example, the plasma membrane protein MARCKS (myristoylated alanine-rich C-kinase substrate), which interacts with actin filaments in the process of cell motility, has a covalently attached myristoyl moiety, but it also contains the sequence
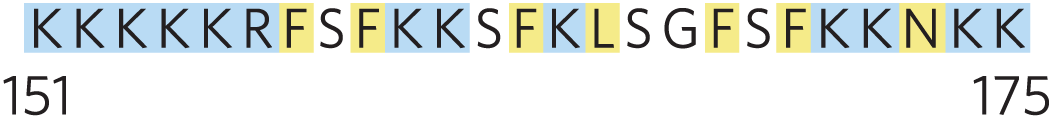
which adds to the protein’s affinity for the membrane. Three clusters of positively charged Lys and Arg residues (screened blue) interact with the negatively charged head group of phosphatidylinositol 4,5-bisphosphate on the cytoplasmic face of the plasma membrane; five aromatic residues (screened yellow) insert into the lipid bilayer. When the head-group phosphates of are enzymatically removed, MARCKS loses its hold on the plasma membrane and dissociates. The reversible nature of the association of MARCKS with the membrane makes it an amphitropic protein.
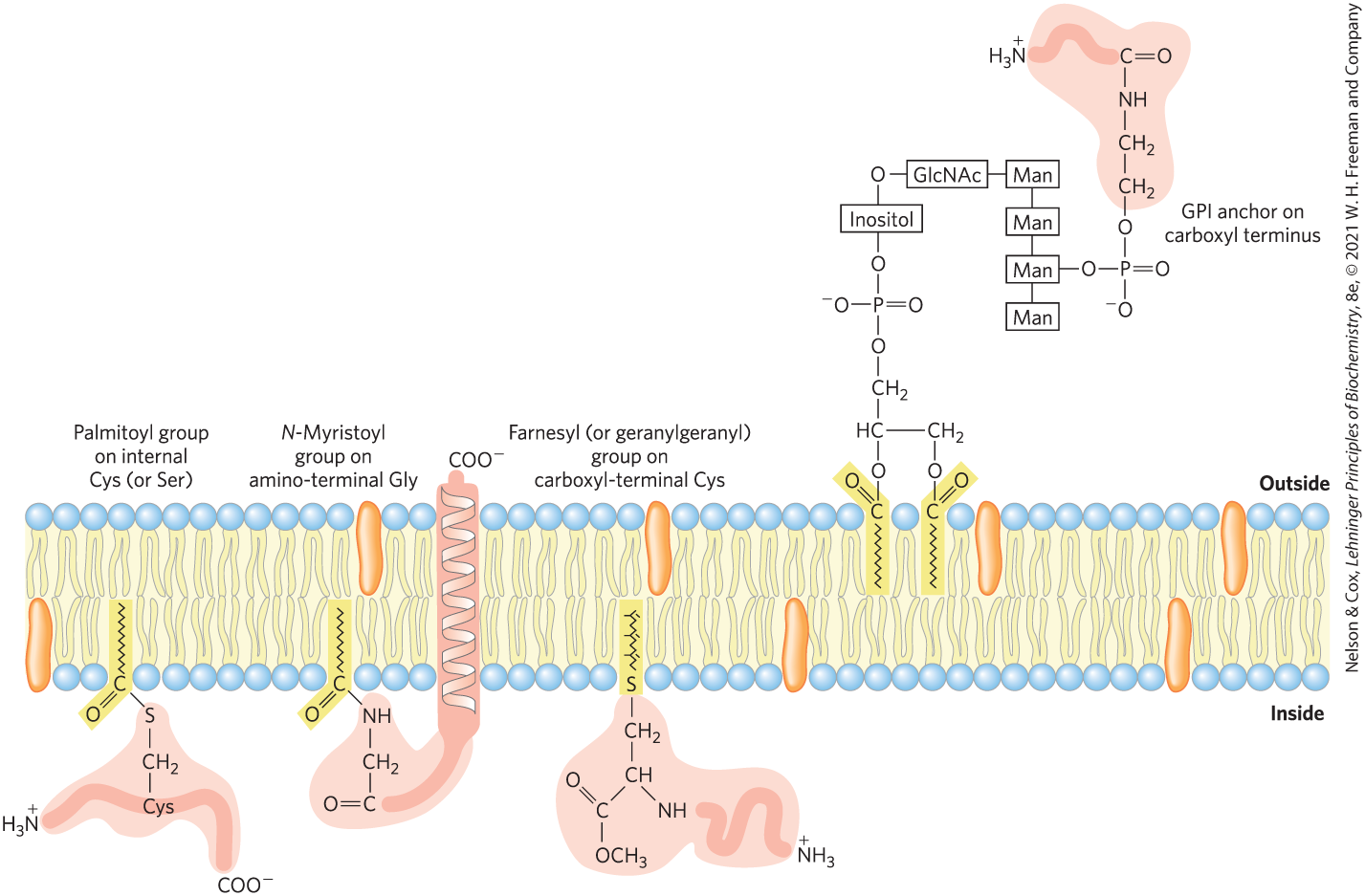
FIGURE 11-16 Lipid-linked membrane proteins. Covalently attached lipids anchor membrane proteins to the lipid bilayer. A palmitoyl group is shown attached by thioester linkage to a Cys residue; an N-myristoyl group is generally attached to an amino-terminal Gly residue, typically of a protein that also has a hydrophobic transmembrane segment; the farnesyl and geranylgeranyl groups attached to carboxyl-terminal Cys residues are isoprenoids of 15 and 20 carbons, respectively. The carboxyl-terminal Cys residue is invariably methylated. Glycosyl phosphatidylinositol (GPI) anchors are derivatives of phosphatidylinositol in which the inositol bears a short oligosaccharide covalently joined to the carboxyl-terminal residue of a protein through phosphoethanolamine. GPI-anchored proteins are always on the extracellular face of the plasma membrane. Farnesylated and palmitoylated membrane proteins are found on the inner surface of the plasma membrane, and myristoylated proteins have domains both inside and outside the plasma membrane. For some lipid-linked proteins, the lipid attachment process is reversible, so the protein is amphitropic: membrane-bound when lipid-linked, soluble when not.
Beyond merely anchoring a protein to the membrane, the attached lipid may have a more specific role. In the plasma membrane, GPI-anchored proteins are exclusively on the outer face and are clustered in certain regions, as discussed later in the chapter (p. 380), whereas other types of lipid-linked proteins (with farnesyl or geranylgeranyl groups attached; Fig. 11-16) are exclusively on the inner face. In polarized epithelial cells (such as intestinal epithelial cells), in which apical and basal surfaces have different roles, GPI-anchored proteins are directed specifically to the apical surface. Attachment of a specific lipid to a newly synthesized membrane protein therefore has a targeting function, directing the protein to its correct cellular location.
SUMMARY 11.1 The Composition and Architecture of Membranes
- Biological phospholipids and sterols spontaneously form a lipid bilayer to protect their hydrophobic hydrocarbon chains from energetically unfavorable interaction with water.
- In the fluid mosaic model, a lipid bilayer is the basic structural unit, and proteins associate with or span the bilayer. Biological membranes are flexible, self-repairing, and selectively permeable. They regulate the traffic of small molecules into and out of the cell and among the organelles, and they provide a scaffold on which proteins assemble into catalytic and structural aggregates that function in two-dimensional space.
- Proteins and lipids are trafficked through the dynamic endomembrane system of a eukaryote from their point of synthesis to their appropriate cellular locations. Small vesicles and soluble transporter proteins ensure that each membrane leaflet has its unique complement of lipids and proteins to achieve its specialized function.
- Cells have hundreds of membrane transporters that carry polar solutes and ions in and out of cells and throughout the endomembrane system. The plasma membrane contains protein receptors that sense signals from outside the cell and carry their message into the cell. Many enzymes are associated with membranes, where they interact with each other in essentially two-dimensional space.
- Integral proteins are embedded within membranes, their nonpolar amino acid side chains stabilized by contact with the lipid bilayer. Peripheral proteins associate with membranes through electrostatic interactions and hydrogen bonding with membrane phospholipids and integral proteins. Amphitropic proteins associate reversibly with the membrane.
- Many integral membrane proteins span the lipid bilayer several times, with hydrophobic sequences of about 20 amino acid residues forming transmembrane α helices. Multistranded β barrels are also common in integral proteins of bacterial and mitochondrial membranes. A hydropathy plot of the amino acid sequence identifies segments that are likely to cross the lipid bilayer as helices or barrels.
- Covalent attachment of a hydrophobic molecule such as a fatty acid serves to anchor some membrane proteins to the bilayer.
 The individual lipid and protein units in a membrane form a
The individual lipid and protein units in a membrane form a  In eukaryotic cells, the endoplasmic reticulum (ER), Golgi apparatus, lysosomes, and various small vesicles that carry lipids and proteins throughout the cell are each surrounded and defined by a single membrane. This endomembrane system (
In eukaryotic cells, the endoplasmic reticulum (ER), Golgi apparatus, lysosomes, and various small vesicles that carry lipids and proteins throughout the cell are each surrounded and defined by a single membrane. This endomembrane system ( Biological phospholipids and sterols spontaneously form a lipid bilayer to protect their hydrophobic hydrocarbon chains from energetically unfavorable interaction with water.
Biological phospholipids and sterols spontaneously form a lipid bilayer to protect their hydrophobic hydrocarbon chains from energetically unfavorable interaction with water.