10.2 Structural Lipids in Membranes
The central architectural feature of biological membranes is a double layer of lipids, which acts as a barrier to the passage of polar molecules and ions. Membrane lipids are amphipathic: one end of the molecule is hydrophobic, the other hydrophilic. The association of their hydrophobic regions with each other and their hydrophilic interactions with water direct their packing into sheets called membrane bilayers. In this section, we describe four general types of membrane lipids: phospholipids, which have hydrophobic regions composed of two fatty acids joined to glycerol or sphingosine; glycolipids, which contain a simple sugar or a complex oligosaccharide at the polar ends; archaeal tetraether lipids, which have two very long alkyl chains ether-linked to glycerol at both ends; and sterols, compounds characterized by a rigid system of four fused hydrocarbon rings (Fig. 10-6). Within these groups of membrane lipids, enormous diversity results from various combinations of fatty acid “tails” and polar “heads.” The arrangement of these lipids in membranes, and their structural and functional roles therein, are considered in the next chapter.
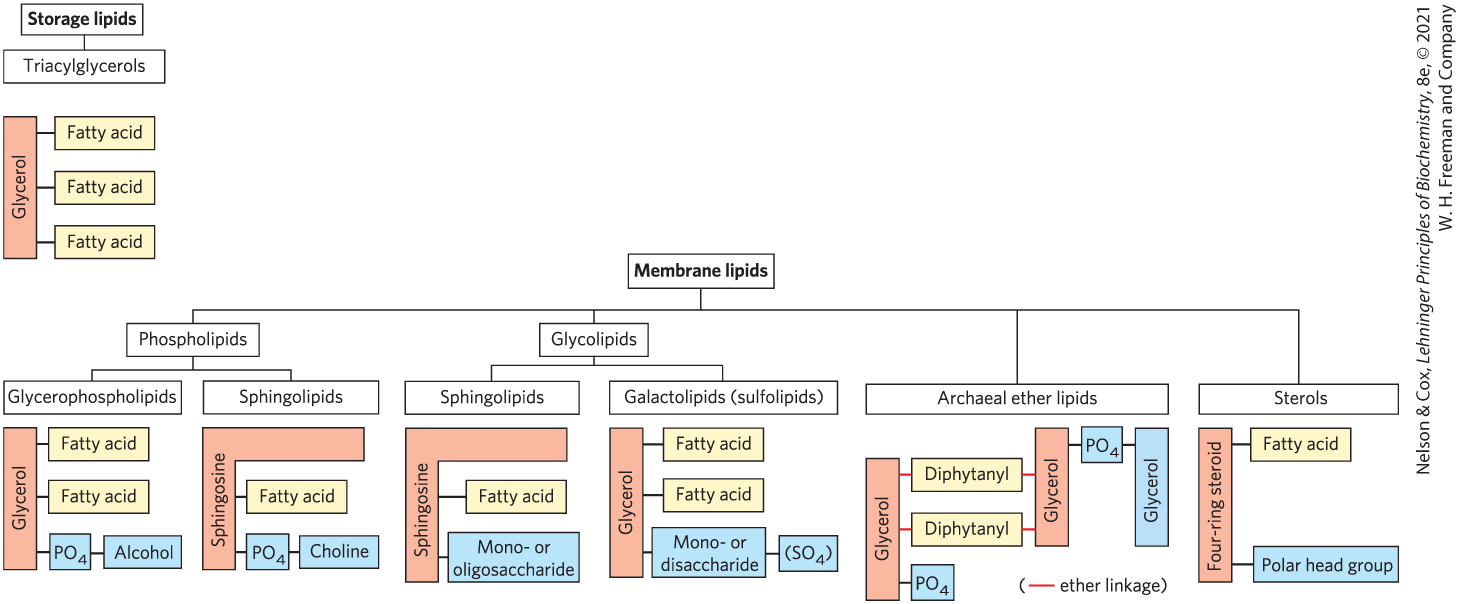
FIGURE 10-6 Some common types of storage and membrane lipids. The triacylglycerol, phospholipid, glycolipid, and archaeal ether lipid types shown here have either glycerol or sphingosine as the backbone (light red screen), to which are attached one or more long-chain alkyl groups (yellow) and a polar head group (blue). Sterols have a core composed of four fused carbocylic rings called the steroid nucleus. In triacylglycerols, glycerophospholipids, galactolipids, and sulfolipids, the alkyl groups are fatty acids in ester linkage. Sphingolipids contain a single fatty acid, in amide linkage to the sphingosine backbone. The membrane lipids of archaea are variable; that shown here has two very long, branched alkyl chains, each end in ether linkage with a glycerol moiety. Sterols have many different types of alkyl chains that can be attached to the steroid D ring at C-17. In phospholipids, the polar head group is joined through a phosphodiester, whereas glycolipids have a direct glycosidic linkage between the head-group sugar and the backbone glycerol. Many sterols contain a polar head group such as a carbonyl or hydroxyl group attached at C-3 of the steroid A ring.
Glycerophospholipids Are Derivatives of Phosphatidic Acid
Glycerophospholipids, also called phosphoglycerides, are membrane lipids in which two fatty acids are attached in ester linkage to the first and second carbons of glycerol, and a highly polar or charged group is attached through a phosphodiester linkage to the third carbon. Glycerol is prochiral; it has no asymmetric carbons, but attachment of phosphate at one end converts it into a chiral compound, which can be correctly named either l-glycerol 3-phosphate, d-glycerol 1-phosphate, or sn-glycerol 3-phosphate (Fig. 10-7). Glycerophospholipids are named as derivatives of the parent compound, phosphatidic acid (Fig. 10-8), according to the polar alcohol in the head group. Phosphatidylcholine and phosphatidylethanolamine have choline and ethanolamine as their polar head groups, for example. Cardiolipin is a two-tailed glycerophospholipid in which two phosphatidic acid moieties share the same glycerol as their head group (Fig. 10-8). Cardiolipin is found in most bacterial membranes; in eukaryotic cells, cardiolipin is located almost exclusively in the inner mitochondrial membrane (where it is synthesized), a location consistent with the endosymbiosis hypothesis for the origin of these organelles (see Fig. 1-37).
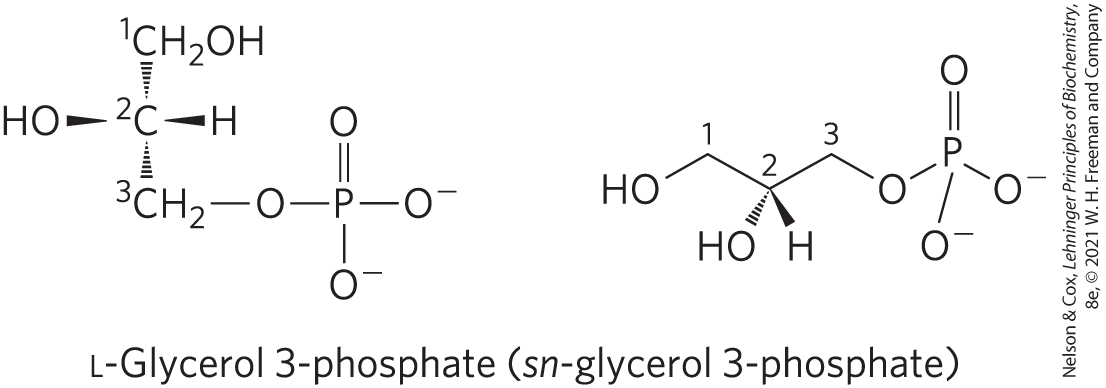
FIGURE 10-7 l-Glycerol 3-phosphate, the backbone of phospholipids. Glycerol itself is not chiral, as it has a plane of symmetry through C-2. However, glycerol is prochiral — it can be converted to a chiral compound by adding a substituent such as phosphate to either of the groups. One unambiguous nomenclature for glycerol phosphate is the d, l system (described on p. 73), in which the isomers are named according to their stereochemical relationships to glyceraldehyde isomers. By this system, the stereoisomer of glycerol phosphate found in most lipids is correctly named either l-glycerol 3-phosphate or d-glycerol 1-phosphate. Another way to specify stereoisomers is the sn (stereospecific numbering) system, in which C-1 is, by definition, the group of the prochiral compound that occupies the pro-S position. The common form of glycerol phosphate in phospholipids is, by this system, sn-glycerol 3-phosphate (in which C-2 has the R configuration). In archaea, the glycerol in lipids has the other configuration; it is d-glycerol 3-phosphate.
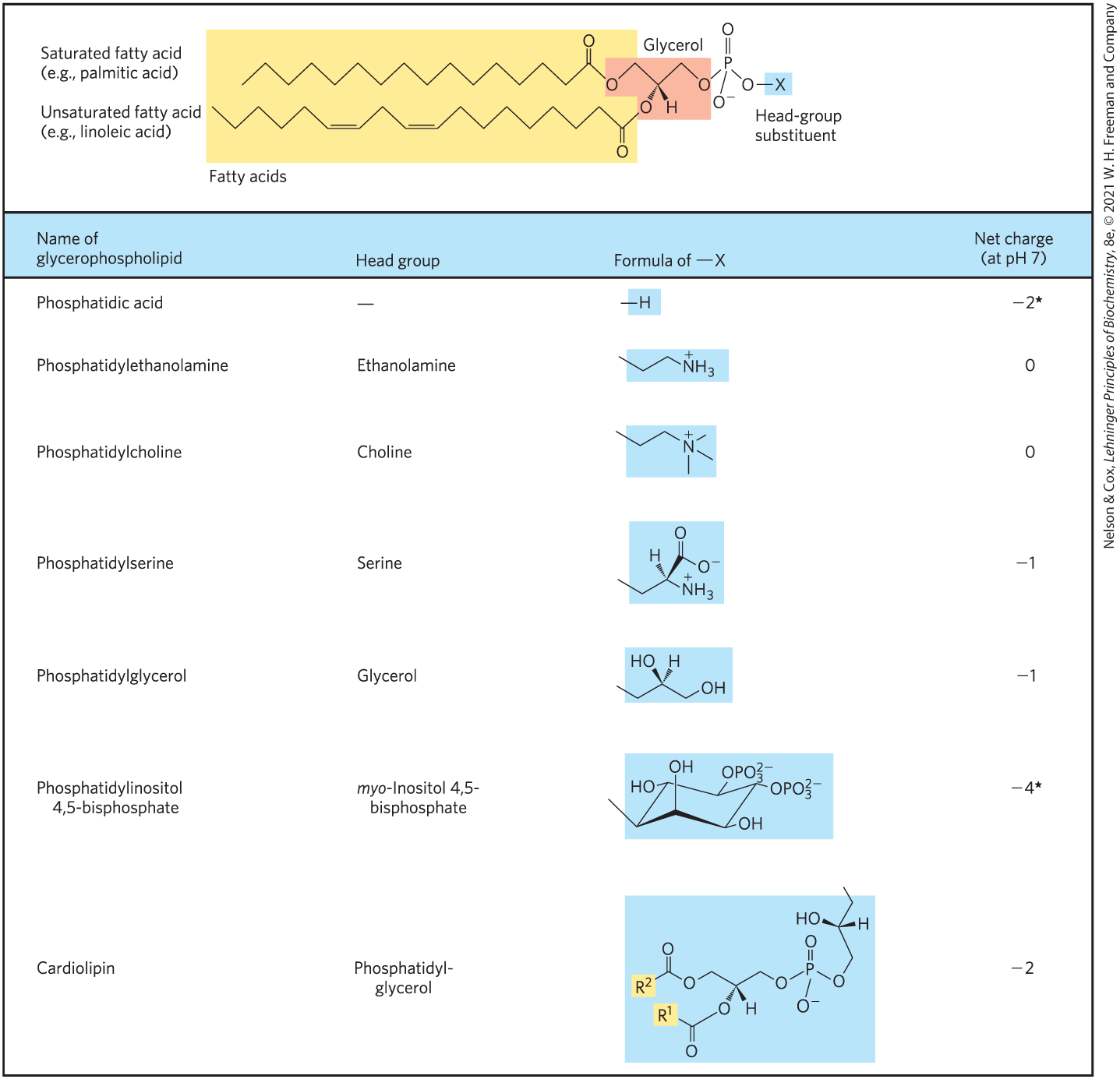
FIGURE 10-8 Glycerophospholipids. The common glycerophospholipids are diacylglycerols linked to head-group alcohols through a phosphodiester bond. Phosphatidic acid, a phosphomonoester, is the parent compound. Each derivative is named for the head-group alcohol, with the prefix “phosphatidyl-.” In cardiolipin, two phosphatidic acids share a single glycerol ( and are fatty acyl groups), resulting in a symmetric molecule. *Note that phosphate esters each have a charge of about –1.5; one of their groups is only partially ionized at pH 7.
In all glycerophospholipids, the head group is joined to glycerol through a phosphodiester bond, in which the phosphate group bears a negative charge at neutral pH. The polar alcohol may be negatively charged (as in phosphatidylinositol 4,5-bisphosphate), neutral (phosphatidylserine), or positively charged (phosphatidylcholine, phosphatidylethanolamine). As we shall see in Chapter 11, these charges contribute greatly to the surface properties of membranes.
The fatty acids in glycerophospholipids can be any of a wide variety, so a given phospholipid (phosphatidylcholine, for example) may consist of several molecular species, each with its unique complement of fatty acids. The distribution of molecular species is specific to the organism, to the particular tissue within the organism, and to the particular glycerophospholipids in the same cell or tissue. In general, glycerophospholipids contain a or saturated fatty acid at C-1 and a or unsaturated fatty acid at C-2. With few exceptions, the biological significance of the variation in fatty acids and head groups is not yet understood.
Some Glycerophospholipids Have Ether-Linked Fatty Acids
Some animal tissues and some unicellular organisms are rich in ether lipids, in which one of the two acyl chains is attached to glycerol in ether, rather than ester, linkage. The ether-linked chain may be saturated, as in the alkyl ether lipids, or it may contain a double bond between C-1 and C-2, as in plasmalogens (Fig. 10-9). Vertebrate heart tissue is uniquely enriched in ether lipids; about half of the heart phospholipids are plasmalogens. The membranes of halophilic bacteria, ciliated protists, and certain invertebrates also contain high proportions of ether lipids. The functional significance of ether lipids in these membranes is unknown; perhaps their resistance to the phospholipases that cleave ester-linked fatty acids from membrane lipids is important in some roles.
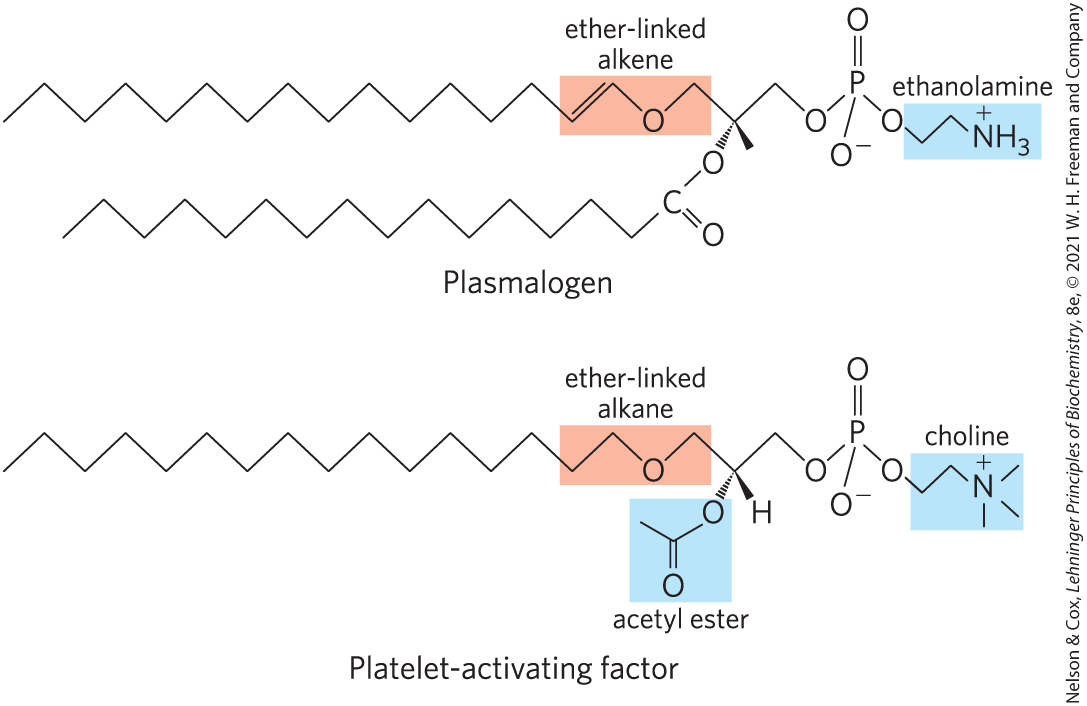
FIGURE 10-9 Ether lipids. Plasmalogens have an ether-linked alkenyl chain where most glycerophospholipids have an ester-linked fatty acid (compare to Fig. 10-8). Platelet-activating factor has a long ether-linked alkyl chain at C-1 of glycerol, but C-2 is ester-linked to acetic acid, which makes the compound much more water-soluble than most glycerophospholipids and plasmalogens. The head-group alcohol is ethanolamine in plasmalogens and choline in platelet-activating factor.
At least one ether lipid, platelet-activating factor, is a potent molecular signal. It is released from leukocytes called basophils and stimulates platelet aggregation and the release of serotonin (a vasoconstrictor) from platelets. It also exerts a variety of effects on liver, smooth muscle, heart, uterine, and lung tissues and plays an important role in inflammation and the allergic response.
Galactolipids of Plants and Ether-Linked Lipids of Archaea Are Environmental Adaptations
The second group of membrane lipids in Figure 10-6, the glycolipids, includes those that predominate in plant cells: the galactolipids, in which one or two galactose residues are connected by a glycosidic linkage to C-3 of a 1,2-diacylglycerol (Fig. 10-10). Galactolipids are localized in the thylakoid membranes (internal membranes) of chloroplasts; they make up 70% to 80% of the total membrane lipids of a vascular plant, and are therefore probably the most abundant membrane lipids in the biosphere. Phosphate is often the limiting plant nutrient in soil, and perhaps the evolutionary pressure to conserve phosphate for more critical roles favored plants that made phosphate-free lipids. Plant membranes also contain sulfolipids, in which a sulfonated glucose residue is joined to a diacylglycerol in glycosidic linkage. The sulfonate group bears a negative charge like that of the phosphate group in phospholipids.
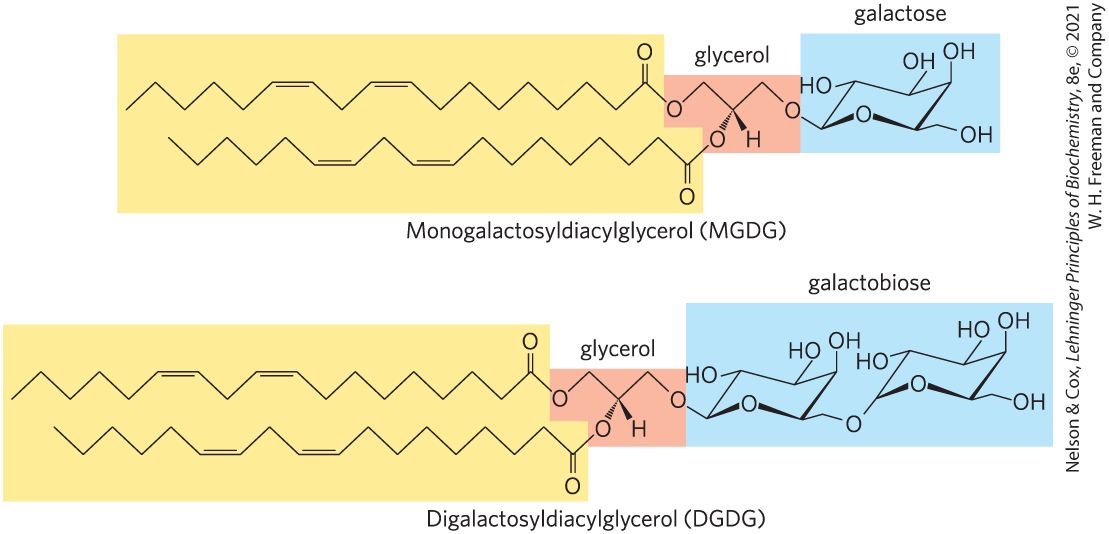
FIGURE 10-10 Two galactolipids of chloroplast thylakoid membranes. In monogalactosyldiacylglycerols (MGDGs) and digalactosyldiacylglycerols (DGDGs), both acyl groups are polyunsaturated and the head groups are uncharged.
Some archaea that live in ecological niches with extreme conditions — for example, high temperatures (boiling water), low pH, or high ionic strength — have membrane lipids containing long-chain (32 carbons) branched hydrocarbons linked at each end to glycerol (Fig.10-6). These linkages are through ether bonds, which are much more stable to hydrolysis at low pH and high temperature than are the ester bonds found in the lipids of bacteria and eukaryotes. In their fully extended form, these archaeal lipids are twice the length of phospholipids and sphingolipids, and can span the full width of the plasma membrane.
Sphingolipids Are Derivatives of Sphingosine
Sphingolipids, a large class of membrane phospholipids and glycolipids, have a polar head group and two nonpolar tails, but unlike glycerophospholipids and galactolipids they contain no glycerol (Fig. 10-6). Sphingolipids are composed of one molecule of the long-chain amino alcohol sphingosine (also called 4-sphingenine) or one of its derivatives, one molecule of a long-chain fatty acid, and a polar head group that is joined by a glycosidic linkage in some cases and a phosphodiester in others (Fig. 10-11).
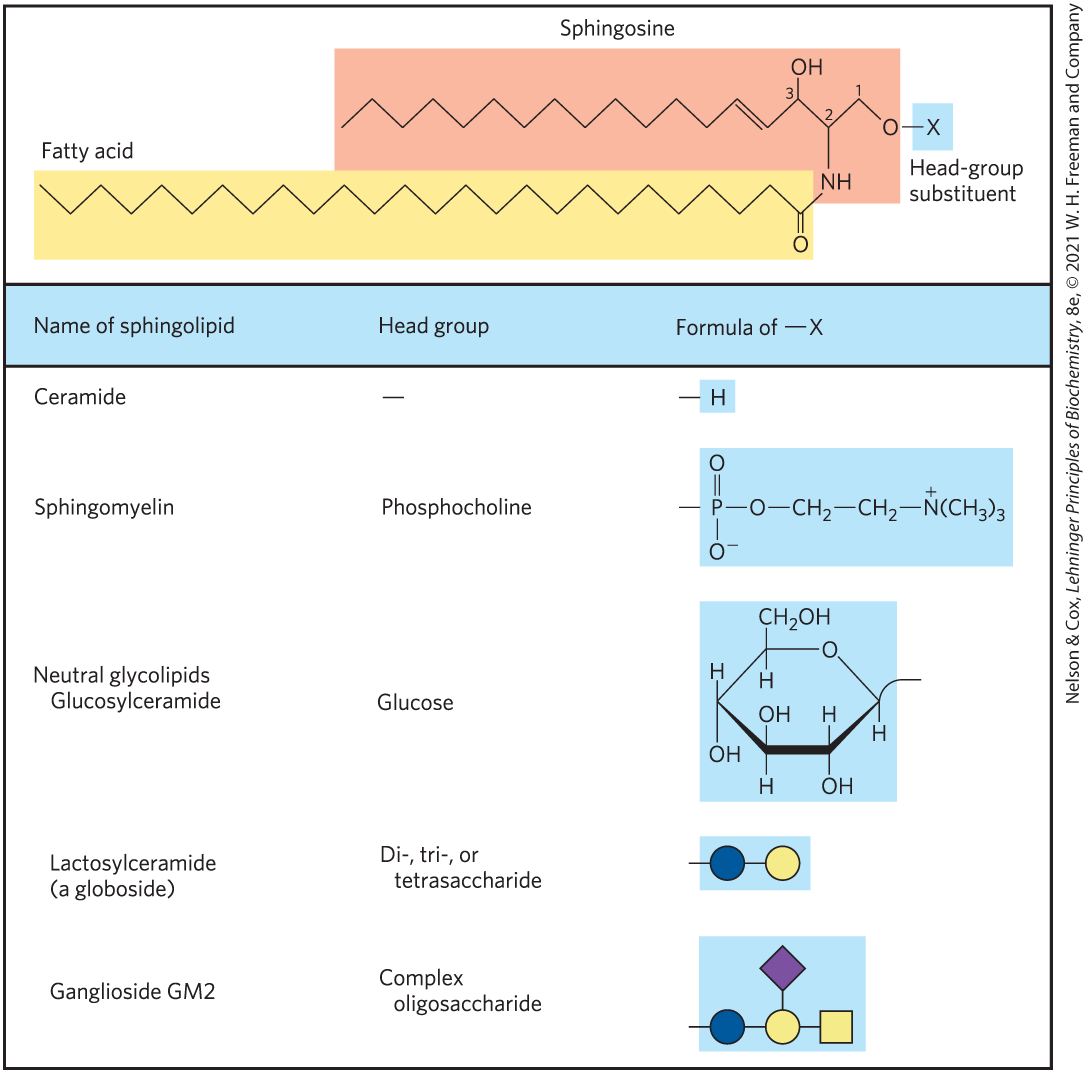
FIGURE 10-11 Sphingolipids. The first three carbons at the polar end of sphingosine are analogous to the three carbons of glycerol in glycerophospholipids. The amino group at C-2 bears a fatty acid in amide linkage. The fatty acid is usually saturated or monounsaturated, with 16, 18, 22, or 24 carbon atoms. Ceramide is the parent compound for this group. Other sphingolipids differ in the polar head group attached at C-1. Gangliosides have very complex oligosaccharide head groups. Standard symbols for sugars are used in this figure, as shown in Table 7-1.
Carbons C-1, C-2, and C-3 of the sphingosine molecule are structurally analogous to the three carbons of glycerol in glycerophospholipids. When a fatty acid is attached in amide linkage to the on C-2, the resulting compound is a ceramide, which is structurally similar to a diacylglycerol. Ceramides are the structural parents of all sphingolipids.
There are three subclasses of sphingolipids, all derivatives of ceramide but differing in their head groups: sphingomyelins, neutral (uncharged) glycolipids, and gangliosides. Sphingomyelins contain phosphocholine or phosphoethanolamine as their polar head group and are therefore classified along with glycerophospholipids as phospholipids (Fig. 10-6). Indeed, sphingomyelins resemble phosphatidylcholines in their general properties and three-dimensional structure, and in having no net charge on their head groups (Fig. 10-12). Sphingomyelins are present in the plasma membranes of animal cells and are especially prominent in myelin, a membranous sheath that surrounds and insulates the axons of some neurons — thus the name “sphingomyelins.”
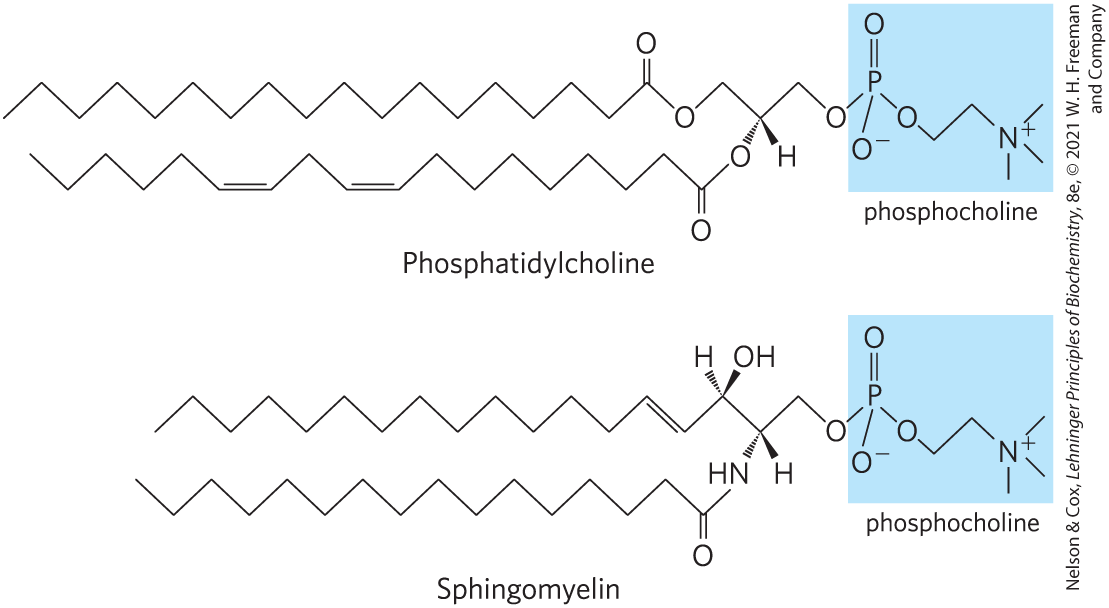
FIGURE 10-12 The similar molecular structures of two classes of membrane lipid. Phosphatidylcholine (a glycerophospholipid) and sphingomyelin (a sphingolipid) have similar dimensions and physical properties, but presumably play different roles in membranes.
Glycosphingolipids, which occur largely in the outer face of plasma membranes, have head groups with one or more sugars connected directly to the at C-1 of the ceramide moiety; they do not contain phosphate. Cerebrosides have a single sugar linked to ceramide; those with galactose are characteristically found in the plasma membranes of cells in neural tissue, and those with glucose are found in the plasma membranes of cells in nonneural tissues. Globosides are glycosphingolipids with two or more sugars, usually d-glucose, d-galactose, or N-acetyl-d-galactosamine. Cerebrosides and globosides are sometimes called neutral glycolipids, as they have no charge at pH 7.
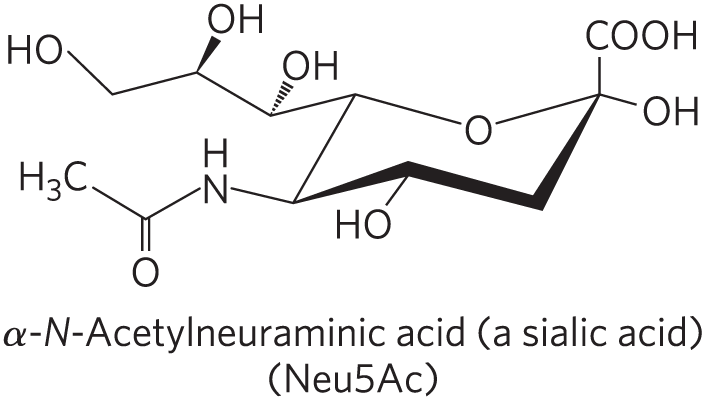
Gangliosides, the most complex sphingolipids, have oligosaccharides as their polar head groups and one or more residues of N-acetylneuraminic acid (Neu5Ac), a sialic acid (often simply called “sialic acid”), at the termini. Deprotonated sialic acid gives gangliosides the negative charge at pH 7 that distinguishes them from globosides. Gangliosides with one sialic acid residue are in the GM (M for mono-) series, those with two sialic acid residues are in the GD (D for di-) series, and so on (GT, three sialic acid residues; GQ, four).
Sphingolipids at Cell Surfaces Are Sites of Biological Recognition
When sphingolipids were discovered more than a century ago by the physician-chemist Johann Thudichum, their biological role seemed as enigmatic as the Sphinx, for which he therefore named them. In humans, at least 60 different sphingolipids have been identified in cellular membranes. Many of these are especially prominent in the plasma membranes of neurons, and some are clearly recognition sites on the cell surface, but a specific function for only a few sphingolipids has been discovered thus far. The carbohydrate moieties of certain sphingolipids define the human blood groups and therefore determine the type of blood that individuals can safely receive in blood transfusions (Fig. 10-13).
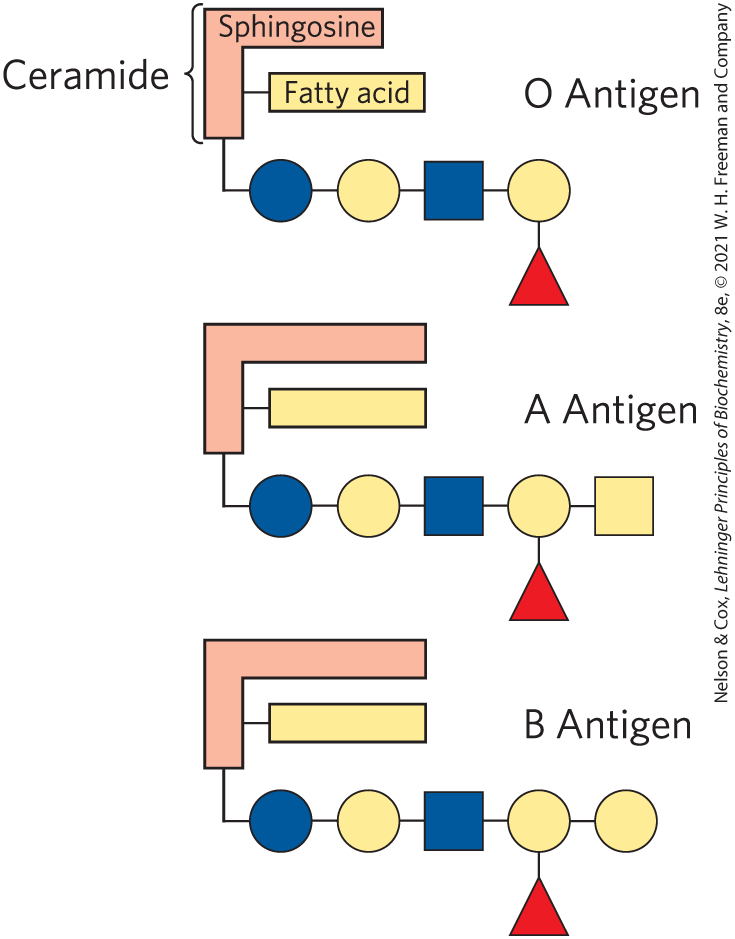
FIGURE 10-13 Glycosphingolipids as determinants of blood groups. The human blood groups (O, A, B) are determined in part by the oligosaccharide head groups of these glycosphingolipids. The same three oligosaccharides are also found attached to certain blood proteins of individuals of blood types O, A, and B, respectively. Standard symbols for sugars are used here (see Table 7-1).
Gangliosides are concentrated on the outer face of cell surface plasma membranes where they present points of recognition for extracellular molecules or the surfaces of neighboring cells. The kinds and amounts of gangliosides in the plasma membrane change dramatically during embryonic development. Tumor formation induces the synthesis of a new complement of gangliosides, and very low concentrations of a specific ganglioside have been found to induce differentiation of cultured neuronal tumor cells. Guillain-Barré syndrome is a serious autoimmune disorder in which the body makes antibodies against its own gangliosides, including those in neurons. The resulting inflammation damages the peripheral nervous system, leading to temporary (or sometimes permanent) paralysis. In cholera, cholera toxin produced by the intestinal bacterium Vibrio cholerae enters sensitive cells after attaching to specific gangliosides on the intestinal epithelial cell surface. Investigation of the biological roles of diverse gangliosides remains fertile ground for future research.
Phospholipids and Sphingolipids Are Degraded in Lysosomes
Most cells continually degrade and replace their membrane lipids. For each hydrolyzable bond in a glycerophospholipid, there is a specific hydrolytic enzyme in the lysosome (Fig. 10-14). Phospholipases of the A type remove one of the two fatty acids, producing a lysophospholipid. (These esterases do not attack the ether link of plasmalogens.) Lysophospholipases remove the remaining fatty acid.
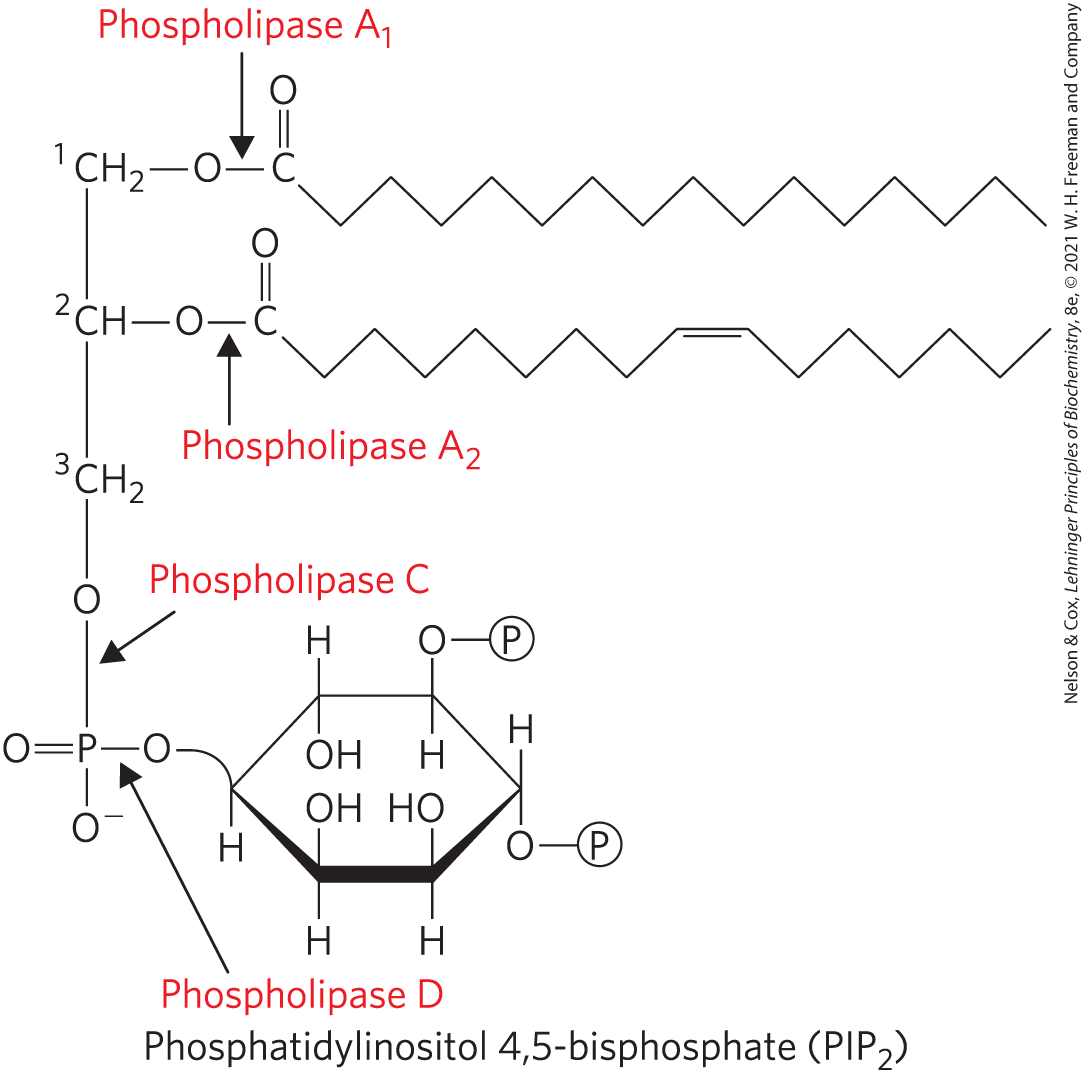
FIGURE 10-14 The specificities of phospholipases. Phospholipases and hydrolyze the ester bonds of intact glycerophospholipids at C-1 and C-2 of glycerol, respectively. When one of the fatty acids has been removed by a type A phospholipase, the second fatty acid is removed by a lysophospholipase (not shown). Phospholipases C and D each split one of the phosphodiester bonds in the head group. Some phospholipases act on only one type of glycerophospholipid, such as phosphatidylinositol 4,5-bisphosphate (, shown here) or phosphatidylcholine; others are less specific.
Gangliosides are degraded by a set of lysosomal enzymes that catalyze the stepwise removal of sugar units, finally yielding a ceramide. A genetic defect in any of these hydrolytic enzymes leads to the accumulation of gangliosides in the cell, with severe medical consequences (Box 10-1).
Sterols Have Four Fused Carbon Rings
Sterols are structural lipids present in the membranes of most eukaryotic cells. The characteristic structure of this group of membrane lipids is the steroid nucleus, consisting of four fused rings, three with six carbons and one with five (Fig. 10-15). The steroid nucleus is almost planar and is relatively rigid; the fused rings do not allow rotation about bonds. Cholesterol, the major sterol in animal tissues, is amphipathic, with a polar head group (the hydroxyl group at C-3) and a nonpolar hydrocarbon body (the steroid nucleus and the hydrocarbon side chain at C-17) about as long as a 16-carbon fatty acid in its extended form. Similar sterols are found in other eukaryotes: stigmasterol in plants and ergosterol in fungi, for example. Bacteria cannot synthesize sterols; a few bacterial species, however, can incorporate exogenous sterols into their membranes. The sterols of all eukaryotes are synthesized from simple five-carbon isoprene subunits, as are the fat-soluble vitamins, quinones, and dolichols described in Section 10.3.
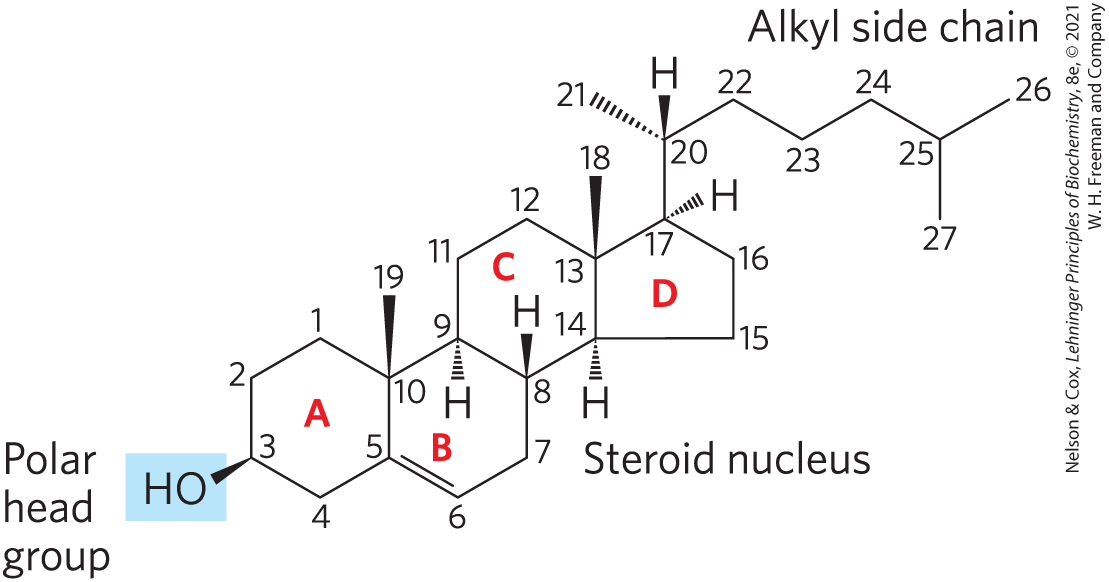
FIGURE 10-15 Cholesterol. In this chemical structure of cholesterol, the rings are labeled A through D to simplify reference to derivatives of the steroid nucleus. The C-3 hydroxyl group (shaded blue) is the polar head group. For storage and transport of the sterol, this hydroxyl group condenses with a fatty acid to form a sterol ester.
The molecule has a six-carbon ring labeled A on the left that has C 1 at the top vertex, C 2 at the upper left vertex, C 3 at the lower left vertex that is further solid wedge bonded to O H in a blue box and labeled polar head group, C 4 at the bottom vertex, C 5 at the lower right vertex, and C 10 at the upper right vertex and solid wedge bonded to C 19. The bond between C 5 and C 10 on its right side is shared with a six-membered ring to the right labeled B that has a double bond at its lower left side, C 6 at its bottom vertex, C 7 at its lower right vertex, C 8 at its upper right vertex and solid wedge bonded to H, and C 9 at its top vertex and hashed wedge bonded to H below. The bond between C 8 and C 9 is shared with a ring, labeled C, above and to the right. The shared bond forms the lower left side of ring C. Ring C has C at the lower right vertex hashed wedge bonded to H, C 13 at the upper right vertex solid wedge bonded to 18, C 12 at the top vertex, and C 11 at the upper left vertex. The bond between C 14 and C 13 is shared with a five-membered ring to the right labeled D. Ring D has C 15 at the lower right vertex, C 16 at the upper right vertex, and C 17 at the top vertex hashed wedge bonded to H and bonded to C 20 above that is hashed wedge bonded to C 21 to its upper left, solid wedge bonded to H above, and bonded to a five-carbon zigzag chain to the right that runs from C 22 to C 26 with a substituent, C 27, bonded to C 25. The rings are labeled steroid nucleus.
In addition to their roles as membrane constituents, the sterols serve as precursors for a variety of products with specific biological activities. Steroid hormones, for example, are potent biological signals that regulate gene expression. Bile acids are polar derivatives of cholesterol that act as detergents in the intestine, emulsifying dietary fats to make them more readily accessible to digestive lipases.
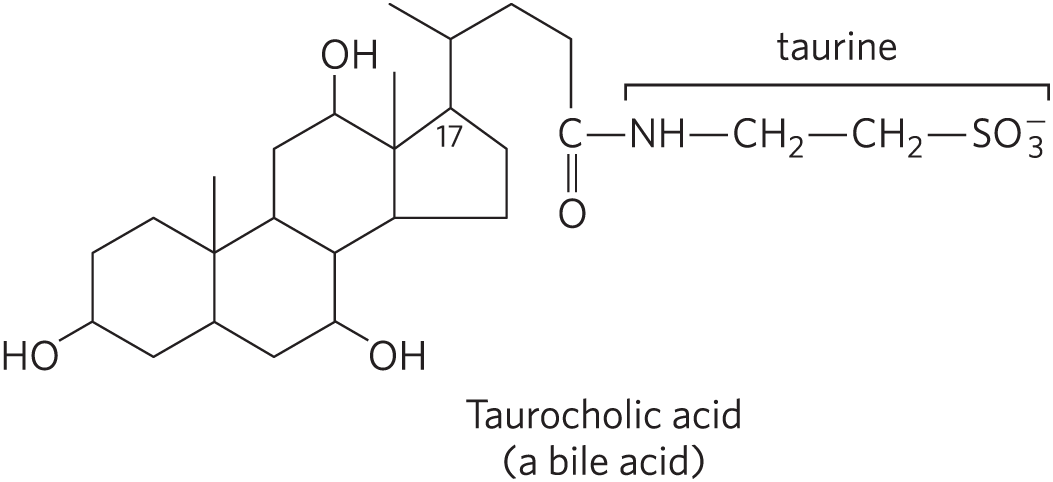
The molecule has the standard four-ring structure of cholesterol with no double bonds, C 3 bonded to O H, C 10 bonded to C H 3, C 7 bonded to O H, C 12 bonded to O H, C 13 bonded to C H 3, and C 17, which is numbered, bonded to C H bonded to C H 3 to the left and bonded to the right to C H 2 bonded to C H 2 bonded to C double bonded to O below and bonded to N H of taurine to the right. Taurine has N H bonded to C H 2 C H 2 S O 3 minus.
We return to cholesterol and other sterols in later chapters, to consider the structural role of cholesterol in biological membranes (Chapter 11), signaling by steroid hormones (Chapter 12), and the remarkable biosynthetic pathway to cholesterol and transport of cholesterol by lipoprotein carriers (Chapter 21).
SUMMARY 10.2 Structural Lipids in Membranes
- Lipids with polar heads and nonpolar tails are major components of membranes. The most abundant are the glycerophospholipids, which contain fatty acids esterified to two of the hydroxyl groups of glycerol. The third hydroxyl of glycerol is esterified with the polar head group via a phosphodiester bond. Common glycerophospholipids such as phosphatidylethanolamine and phosphatidylcholine differ in the structure of their head group.
- Heart tissue plasmalogens and platelet-activating factor are examples of glycerophospholipids containing ether-linked acyl chains.
- Chloroplast membranes are rich in galactolipids, composed of a diacylglycerol with one or two linked galactose residues, and sulfolipids, diacylglycerols with a linked sulfonated sugar residue. Some archaea have unique membrane lipids, with long-chain alkyl groups ether-linked to glycerol at each end. These lipids are stable under the harsh conditions in which these archaea live.
- The sphingolipids contain sphingosine instead of glycerol. The three subclasses of sphingolipids are all ceramide derivatives: sphingomyelins, neutral glycolipids, and gangliosides. Sphingomyelins have choline head groups; the other classes have sugar components.
- Sphingolipids are abundant in the plasma membranes of neurons, and they define human blood groups.
- Phospholipases degrade glycerophospholipids and catalyze hydrolysis at specific positions within the structure.
- Sterols have four fused rings and a hydroxyl group. Cholesterol, the major sterol in animals, is both a structural component of membranes and precursor to a wide variety of steroids.
 Membrane lipids are amphipathic: one end of the molecule is hydrophobic, the other hydrophilic. The association of their hydrophobic regions with each other and their hydrophilic interactions with water direct their packing into sheets called membrane bilayers. In this section, we describe four general types of membrane lipids:
Membrane lipids are amphipathic: one end of the molecule is hydrophobic, the other hydrophilic. The association of their hydrophobic regions with each other and their hydrophilic interactions with water direct their packing into sheets called membrane bilayers. In this section, we describe four general types of membrane lipids:  At least one ether lipid, platelet-activating factor, is a potent molecular signal. It is released from leukocytes called basophils and stimulates platelet aggregation and the release of serotonin (a vasoconstrictor) from platelets. It also exerts a variety of effects on liver, smooth muscle, heart, uterine, and lung tissues and plays an important role in inflammation and the allergic response.
At least one ether lipid, platelet-activating factor, is a potent molecular signal. It is released from leukocytes called basophils and stimulates platelet aggregation and the release of serotonin (a vasoconstrictor) from platelets. It also exerts a variety of effects on liver, smooth muscle, heart, uterine, and lung tissues and plays an important role in inflammation and the allergic response. 
 In addition to their roles as membrane constituents, the sterols serve as precursors for a variety of products with specific biological activities. Steroid hormones, for example, are potent biological signals that regulate gene expression.
In addition to their roles as membrane constituents, the sterols serve as precursors for a variety of products with specific biological activities. Steroid hormones, for example, are potent biological signals that regulate gene expression.  Lipids with polar heads and nonpolar tails are major components of membranes. The most abundant are the glycerophospholipids, which contain fatty acids esterified to two of the hydroxyl groups of glycerol. The third hydroxyl of glycerol is esterified with the polar head group via a phosphodiester bond. Common glycerophospholipids such as phosphatidylethanolamine and phosphatidylcholine differ in the structure of their head group.
Lipids with polar heads and nonpolar tails are major components of membranes. The most abundant are the glycerophospholipids, which contain fatty acids esterified to two of the hydroxyl groups of glycerol. The third hydroxyl of glycerol is esterified with the polar head group via a phosphodiester bond. Common glycerophospholipids such as phosphatidylethanolamine and phosphatidylcholine differ in the structure of their head group.