10.3 Lipids as Signals, Cofactors, and Pigments
The two functional classes of lipids considered thus far (storage lipids and structural lipids) are major cellular components; membrane lipids make up 5% to 10% of the dry mass of most cells, and storage lipids make up more than 80% of the mass of an adipocyte. With some important exceptions, these lipids play a passive role in the cell; lipid fuels are stored until oxidized by enzymes, and membrane lipids form impermeable barriers around cells and cellular compartments. Another group of lipids, present in much smaller amounts, includes those with active roles in metabolic traffic as metabolites and messengers. Some serve as potent signals — as hormones, carried in the blood from one tissue to another, or as intracellular messengers generated in response to an extracellular signal (hormone or growth factor). Others function as enzyme cofactors in electron-transfer reactions in chloroplasts and mitochondria, or in the transfer of sugar moieties in a variety of glycosylation reactions. A third group consists of lipids with a system of conjugated double bonds: pigment molecules that absorb visible light. Some of these act as light-capturing pigments in vision and photosynthesis; others produce natural colorations, such as the orange of pumpkins and carrots and the yellow of canary feathers. Finally, a very large group of volatile lipids produced in plants consists of signaling molecules that pass through the air, allowing plants to communicate with each other and to invite animal friends and deter foes. In this section, we describe a few representatives of these biologically active lipids. In later chapters, we consider their synthesis and biological roles in more detail.
Phosphatidylinositols and Sphingosine Derivatives Act as Intracellular Signals
Phosphatidylinositol (PI) and its phosphorylated derivatives (Fig. 10-16) act at several levels to regulate cell structure and metabolism. Phosphatidylinositol 4,5-bisphosphate (; Fig. 10-14) in the cytoplasmic (inner) face of plasma membranes serves as a reservoir of messenger molecules that are released inside the cell in response to extracellular signals interacting with specific surface receptors. Extracellular signals such as the hormone vasopressin activate a specific phospholipase C in the membrane, which hydrolyzes to release two products that act as intracellular messengers: inositol 1,4,5-trisphosphate (), which is water soluble, and diacylglycerol, which remains associated with the plasma membrane. triggers release of from the endoplasmic reticulum, and the combination of diacylglycerol and elevated cytosolic activates the enzyme protein kinase C. By phosphorylating specific proteins, this enzyme brings about the cell’s response to the extracellular signal. This signaling mechanism is described more fully in Chapter 12 (see Fig. 12-15).
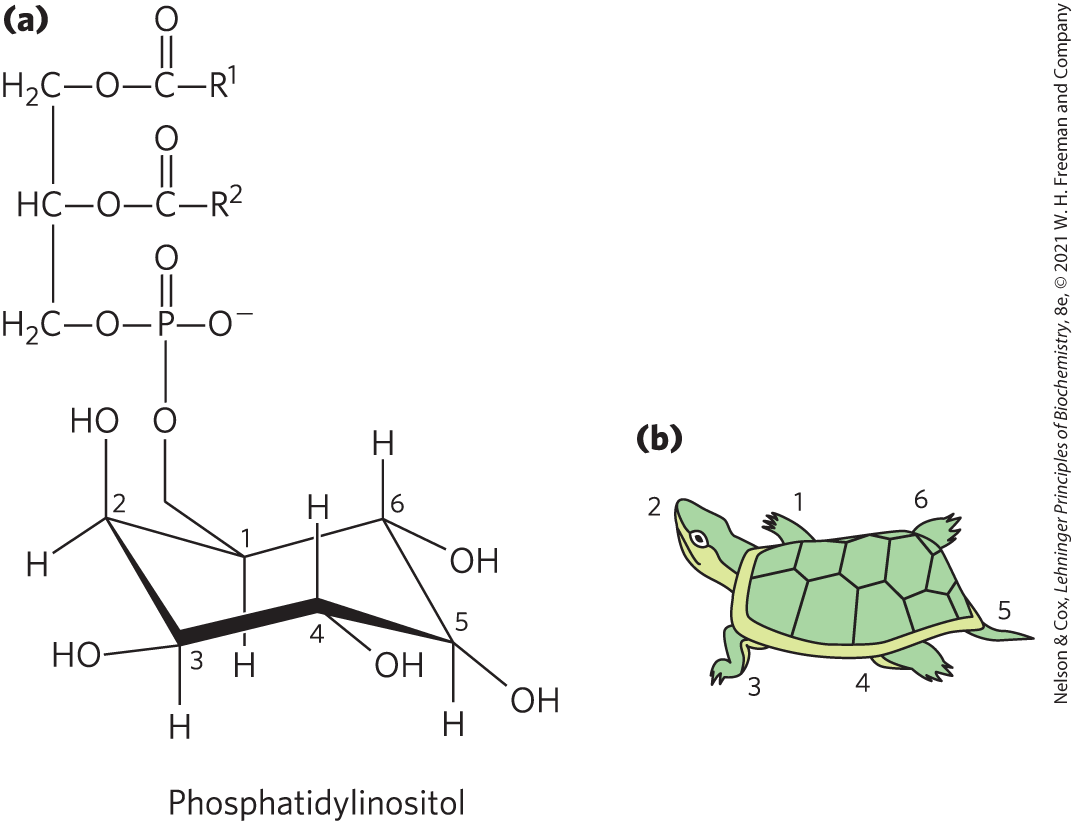
FIGURE 10-16 Phosphatidylinositol and its derivatives. (a) In phosphatidylinositol, the glycerol phospholipid is attached at C-1 of inositol. Phosphorylation of the remaining inositol hydroxyl groups forms signaling molecules such as phosphatidylinositol 4,5-bisphosphate (; see Fig. 10-14), inositol 1,4,5-bisphosphate (), and phosphatidylinositol 3,4,5-trisphosphate (). (b) A useful trick for remembering the inositol carbon-numbering scheme is to compare the chair conformation of inositol to a turtle. Begin numbering with C-1 as the right front flipper and move counterclockwise around the structure, with C-2 the head and C-5 the tail.
Inositol phospholipids also serve as points of nucleation for supramolecular complexes involved in signaling or in exocytosis. Certain signaling proteins bind specifically to phosphatidylinositol 3,4,5-trisphosphate () in the plasma membrane, initiating the formation of multienzyme complexes at the membrane’s cytosolic surface. Thus, formation of in response to extracellular signals brings the proteins together in signaling complexes at the surface of the plasma membrane (see Figs. 12-11 and 12-23).
Membrane sphingolipids also can serve as sources of intracellular messengers. Both ceramide and sphingomyelin (Fig. 10-11) are potent regulators of protein kinases, and ceramide or its derivatives are involved in the regulation of cell division, differentiation, migration, and programmed cell death (also called apoptosis; see Chapter 12).
Eicosanoids Carry Messages to Nearby Cells
Eicosanoids are paracrine hormones, substances that act only on cells near the point of hormone synthesis instead of being transported in the blood to act on cells in other tissues or organs. These fatty acid derivatives have a variety of dramatic effects on vertebrate tissues. They are involved in reproductive function; in the inflammation, fever, and pain associated with injury or disease; in the formation of blood clots and the regulation of blood pressure; in gastric acid secretion; and in various other processes important in human health or disease.
Eicosanoids are derived from arachidonate (arachidonic acid; 20:4()) and eicosapentaenoic acid (EPA; 20:5()), from which they take their general name (Greek eikosi, “twenty”). There are four major classes of eicosanoids: prostaglandins, thromboxanes, leukotrienes, and lipoxins (Fig. 10-17). Eicosanoid names include letter designations for the functional groups on the ring and numbers indicating the number of double bonds in the hydrocarbon chain.
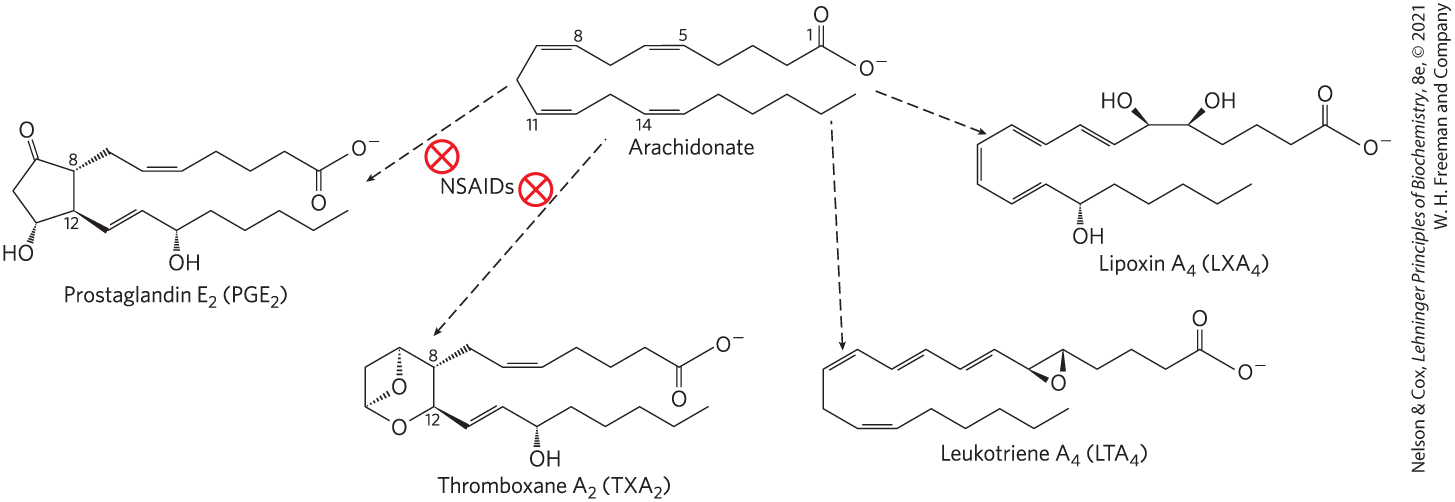
FIGURE 10-17 Arachidonic acid and some eicosanoid derivatives. Arachidonic acid (arachidonate at pH 7) is the precursor of eicosanoids, including the prostaglandins, thromboxanes, leukotrienes, and lipoxins. In prostaglandin , C-8 and C-12 of arachidonate are joined to form the characteristic five-membered ring. In thromboxane , the C-8 and C-12 are joined and an oxygen atom is added to form the six-membered ring. Nonsteroidal anti-inflammatory drugs (NSAIDs) such as aspirin and ibuprofen block the formation of prostaglandins and thromboxanes from arachidonate by inhibiting the enzyme cyclooxygenase (prostaglandin synthase). Leukotriene has a series of three conjugated double bonds, and no cyclic moiety. Lipoxins are also noncyclic derivatives of arachidonate, with several hydroxyl groups.
Prostaglandins (PG) contain a five-carbon ring, and their name derives from the prostate gland from which they were first isolated. and other series 2 prostaglandins are synthesized from arachidonate; series 3 prostaglandins are derived from EPA (see Fig. 21-12). Prostaglandins have an array of functions. Some stimulate contraction of the smooth muscle of the uterus during menstruation and labor. Others affect blood flow to specific organs, the wake-sleep cycle, and the responsiveness of certain tissues to hormones such as epinephrine and glucagon. Prostaglandins in a third group elevate body temperature (producing fever) and cause inflammation and pain.
The thromboxanes (TX) have a six-membered ring containing an ether. They are produced by platelets (also called thrombocytes) and act in the formation of blood clots and reduction of blood flow to the site of a clot. Nonsteroidal anti-inflammatory drugs (NSAIDs) — aspirin, ibuprofen, and meclofenamate, for example — inhibit the enzyme cyclooxygenase or COX (also called prostaglandin synthase), which catalyzes an early step in the pathway from arachidonate to series 2 prostaglandins and thromboxanes (Fig. 10-17) and from EPA to series 3 prostaglandins and thromboxanes (see Fig. 21-12).
Leukotrienes (LT), first found in leukocytes, contain three conjugated double bonds. They are powerful biological signals. For example, leukotriene , derived from leukotriene , induces contraction of the smooth muscle lining the airways to the lung. Overproduction of leukotrienes causes asthmatic attacks, and leukotriene synthesis is one target of antiasthmatic drugs such as prednisone. The strong contraction of the smooth muscle of the lungs that occurs during anaphylactic shock is part of the potentially fatal allergic reaction in individuals hypersensitive to bee stings, penicillin, or other agents.
Lipoxins (LX), like leukotrienes, are linear eicosanoids. Their distinguishing feature is the presence of several hydroxyl groups along the chain (Fig. 10-17). These compounds are potent anti-inflammatory agents. Because their synthesis is stimulated by low doses (81 mg) of aspirin taken daily, this low dose is commonly prescribed for individuals with cardiovascular disease.
Steroid Hormones Carry Messages between Tissues
Steroids are oxidized derivatives of sterols; they have the sterol nucleus but lack the alkyl chain attached to ring D of cholesterol, and they are more polar than cholesterol. Steroid hormones move through the bloodstream (on protein carriers) from their site of production to target tissues, where they enter cells, bind to highly specific receptor proteins in the nucleus, and trigger changes in gene expression and thus metabolism. Because hormones have very high affinity for their receptors, very low concentrations of hormones (nanomolar or less) are sufficient to produce responses in target tissues. The major groups of steroid hormones are the male and female sex hormones and the hormones produced by the adrenal cortex, cortisol and aldosterone (Fig. 10-18). Prednisone is a steroid drug with strong anti-inflammatory activity, mediated in part by the inhibition of arachidonate release by phospholipase and consequent inhibition of the synthesis of prostaglandins, thromboxanes, leukotrienes, and lipoxins. Prednisone and similar drugs have a variety of medical applications, including the treatment of asthma and rheumatoid arthritis.
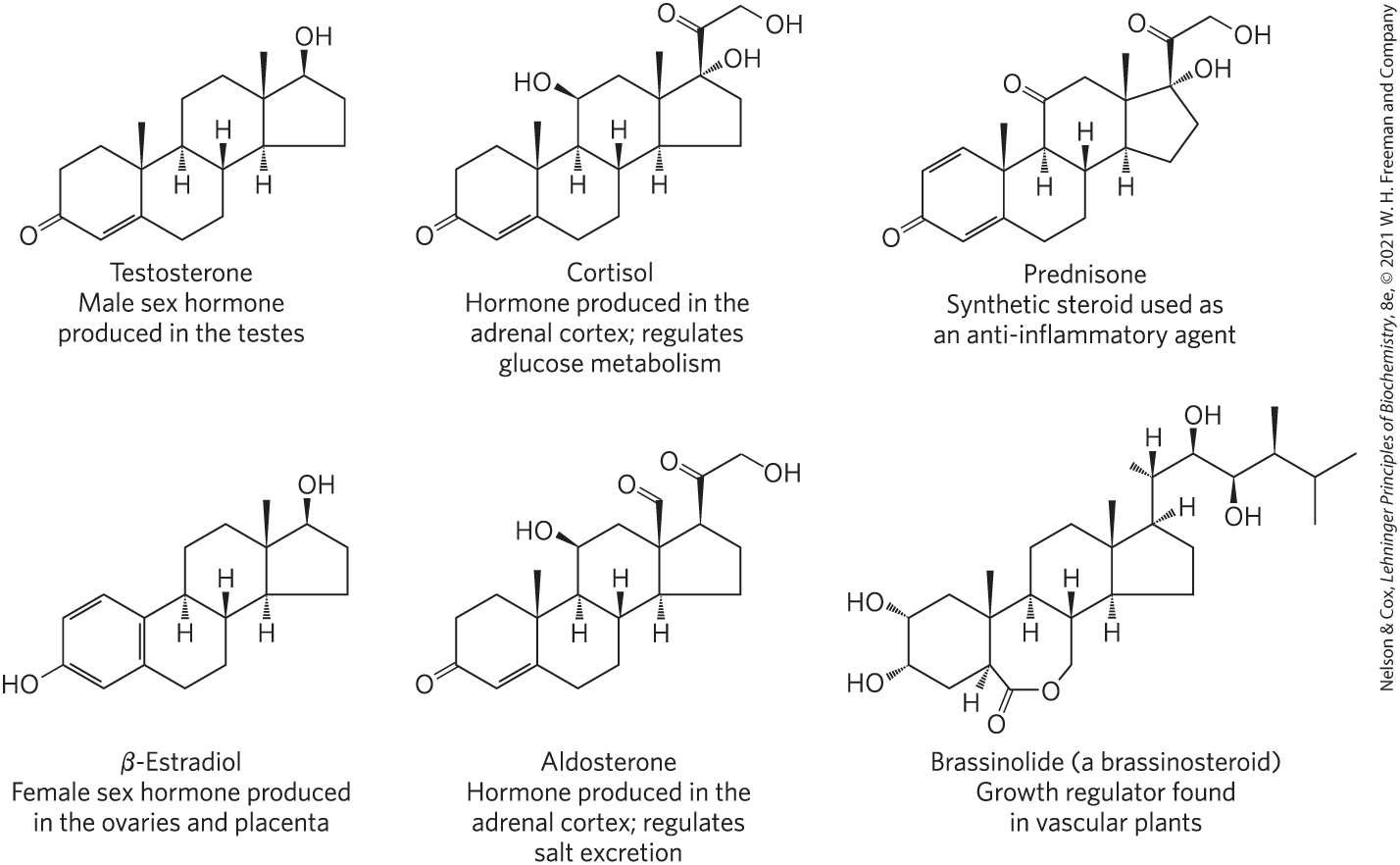
FIGURE 10-18 Steroids derived from cholesterol.
Vascular plants contain the steroidlike brassinolide (Fig. 10-18), a potent growth regulator that increases the rate of stem elongation and affects the orientation of cellulose microfibrils in the cell wall during growth.
Vascular Plants Produce Thousands of Volatile Signals
Plants produce thousands of different lipophilic compounds, volatile substances that are used to attract pollinators, repel herbivores, attract organisms that defend the plant against herbivores, and communicate with other plants. Jasmonate, for example, derived from the fatty acid 18:3() in membrane lipids, triggers the plant’s defenses in response to insect-inflicted damage. The methyl ester of jasmonate gives the characteristic fragrance of jasmine oil, which is widely used in the perfume industry. Many plant volatiles, including geraniol (the characteristic scent of geraniums), β-pinene (pine trees), limonene (limes), and menthol, are derived from fatty acids or from compounds made by the condensation of five-carbon isoprene units.
Vitamins A and D Are Hormone Precursors
During early decades of the twentieth century, a major focus of research in physiological chemistry was the identification of vitamins, compounds that are essential to the health of humans and other vertebrates but cannot be synthesized by these animals and must therefore be obtained in the diet. Early nutritional studies identified two general classes of such compounds: those soluble in nonpolar organic solvents (fat-soluble vitamins) and those that could be extracted from foods with aqueous solvents (water-soluble vitamins). Eventually, the fat-soluble group was resolved into the four vitamin groups A, D, E, and K, all of which are isoprenoid compounds synthesized by the condensation of multiple isoprene units. Two of these (D and A) serve as hormone precursors.
Vitamin , also called cholecalciferol, is normally formed in the skin from 7-dehydrocholesterol in a photochemical reaction driven by the UV component of sunlight (Fig. 10-19a). Vitamin is not itself biologically active, but it is converted by enzymes in the liver and kidney to 1α,25-dihydroxyvitamin (calcitriol), a hormone that regulates calcium uptake in the intestine and calcium levels in kidney and bone. Deficiency of vitamin D leads to defective bone formation and the disease rickets (Fig. 10-19b), for which administration of vitamin D produces a dramatic cure. Vitamin (ergocalciferol) is a commercial product formed by UV irradiation of the ergosterol of yeast. Vitamin is structurally similar to , with slight modification to the side chain attached to the sterol D ring. Both have the same biological effects, and is commonly added to milk and butter as a dietary supplement. The product of vitamin D metabolism, 1α,25-dihydroxyvitamin , regulates gene expression by interacting with specific nuclear receptor proteins. We discuss such regulation of gene expression in more detail in Chapter 28.
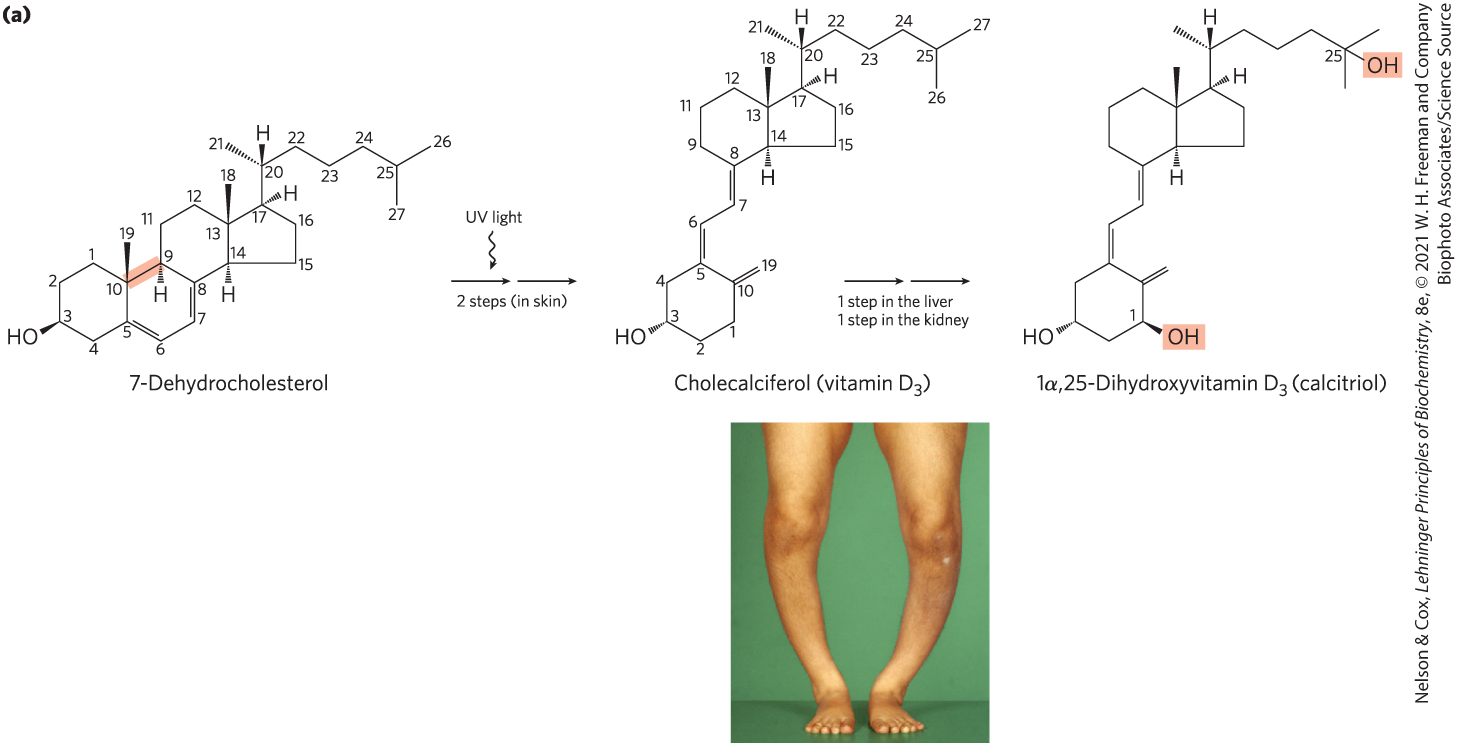
FIGURE 10-19 Vitamin production and metabolism. (a) Cholecalciferol (vitamin ) is produced in the skin by UV irradiation of 7-dehydrocholesterol, which breaks the bond shaded light red. In the liver, a hydroxyl group is added at C-25; in the kidney, a second hydroxylation at C-1 produces the active hormone, 1α,25-dihydroxyvitamin . This hormone regulates the metabolism of in kidney, intestine, and bone. (b) Dietary vitamin D prevents rickets, a disease once common in cold climates where heavy clothing blocks the UV component of sunlight necessary for the production of vitamin in skin. Rickets results in weak or soft bones in children; it can often be identified by bowed legs and other bone deformities.
Vitamin (all-trans-retinol) and its oxidized metabolites retinoic acid and retinal act in the processes of development, cell growth and differentiation, and vision (Fig. 10-20). Vitamin or β-carotene in the diet can be converted enzymatically to all-trans-retinoic acid, a retinoid hormone that acts through a family of nuclear receptor proteins (RAR, RXR, PPAR) to regulate gene expression central to embryonic development, stem cell differentiation, and cell proliferation. All-trans-retinoic acid is used to treat certain types of leukemia, and it is the active ingredient in the drug tretinoin (Retin-A), used to treat severe acne and wrinkled skin. In the vertebrate eye, retinal bound to the protein opsin forms the photoreceptor pigment rhodopsin. The photochemical conversion of 11-cis-retinal to all-trans-retinal is the fundamental event in vision (see Fig. 12-19).
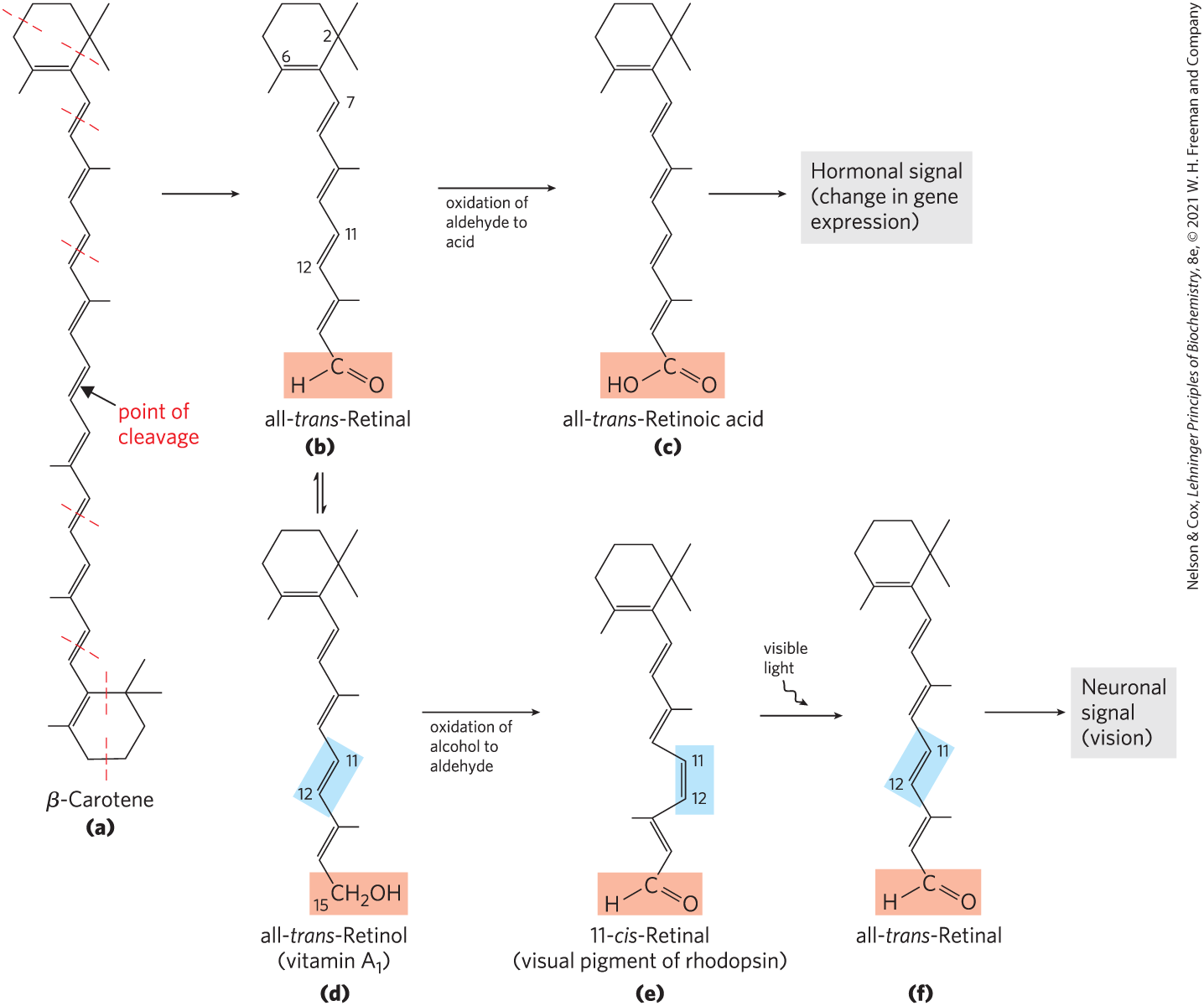
FIGURE 10-20 Dietary β-carotene and vitamin as precursors of the retinoids. (a) β-Carotene is shown with its isoprene structural units set off by dashed red lines. Symmetric cleavage of β-carotene yields two molecules of all-trans-retinal (b), which can be either further oxidized to all-trans-retinoic acid, a retinoid hormone (c), or reduced to all-trans-retinol, vitamin (d). In the visual pathway, all-trans-retinol from this reaction, or obtained directly through the diet, can be converted to the aldehyde 11-cis-retinal (e). This product combines with the protein opsin to form rhodopsin (not shown), a visual pigment widespread in nature. In the dark, the retinal of rhodopsin is in the 11-cis form. When a rhodopsin molecule is excited by visible light, the 11-cis-retinal undergoes a series of photochemical reactions that convert it to all-trans-retinal (f), forcing a change in the shape of the entire rhodopsin molecule. This transformation in the rod cell of the vertebrate retina sends an electrical signal to the brain that is the basis of visual transduction.
Unlike most vitamins, vitamin A can be stored for some time in the body (primarily as its ester with palmitic acid, in the liver). Vitamin A was first isolated from fish liver oils; eggs, whole milk, and butter are also good dietary sources. Another source is β-carotene (Fig. 10-20), the pigment that gives carrots, sweet potatoes, and other yellow vegetables their characteristic color. Carotene is one of a very large number (>700) of carotenoids, natural products with a characteristic extensive system of conjugated double bonds, which makes possible their strong absorption of visible light (450–470 nm).
Vitamin A deficiency in a pregnant woman can lead to congenital malformations and growth retardation in the infant. In adults, vitamin A is also essential to vision, immunity, and reproduction. Deficiency of vitamin A leads to a variety of symptoms, including dryness of the skin, eyes, and mucous membranes, and night blindness, an early symptom commonly used in diagnosing vitamin A deficiency. In the developing world, vitamin A deficiency causes an estimated 250,000 or more cases of blindness or death in children each year. One effective strategy for providing vitamin A is the metabolic engineering of rice strains to overproduce β-carotene. Rice has all the enzymatic machinery to produce β-carotene in its leaves, but these enzymes are less active in the grain. Introduction of two genes into the rice has resulted in “golden rice” with grains enriched in β-carotene (Fig. 10-21).

FIGURE 10-21 Carotene-enriched rice. Worldwide, more than 250 million children and pregnant women suffer from vitamin A deficiency, which causes at least 250,000 cases of irreversible blindness each year in children. Half of these children end up dying within a year of losing their sight. This deficiency is particularly prevalent where rice is a staple food. An international humanitarian effort — the Golden Rice Project — has made great strides in addressing this health crisis. Wild-type rice grains (left) do not produce β-carotene, the metabolic precursor of vitamin A. Rice plants have been genetically engineered to produce β-carotene in the grain, which takes on the yellow color of the carotene (right). A diet supplemented with Golden Rice provides enough β-carotene to prevent vitamin A deficiency and its tragic health consequences.
Vitamins E and K and the Lipid Quinones Are Oxidation-Reduction Cofactors
Vitamin E is the collective name for a group of closely related lipids called tocopherols, all of which contain a substituted aromatic ring and a long isoprenoid side chain (Fig. 10-22a). Because they are hydrophobic, tocopherols associate with cell membranes, lipid deposits, and lipoproteins in the blood. Tocopherols are biological antioxidants. The aromatic ring reacts with and destroys the most reactive forms of oxygen radicals and other free radicals, protecting unsaturated fatty acids from oxidation and preventing oxidative damage to membrane lipids, which can cause cell fragility. Tocopherols are found in eggs and vegetable oils and are especially abundant in wheat germ. Laboratory animals fed diets depleted of vitamin E develop scaly skin, muscular weakness and wasting, and sterility. Vitamin E deficiency in humans is very rare; the principal symptom is fragile erythrocytes.

FIGURE 10-22 Some other biologically active isoprenoid compounds or derivatives. Units derived from isoprene are set off by dashed red lines. In most mammalian tissues, ubiquinone (also called coenzyme Q) has 10 isoprene units. Dolichols of animals have 17 to 21 isoprene units (85 to 105 carbon atoms), bacterial dolichols have 11, and dolichols of plants and fungi have 14 to 24.
The aromatic ring of vitamin K (Fig. 10-22b) undergoes a cycle of oxidation and reduction during the formation of active prothrombin, a blood plasma protein essential in blood clotting. Prothrombin is a proteolytic enzyme that splits peptide bonds in the blood protein fibrinogen to convert it to fibrin, the insoluble fibrous protein that holds blood clots together (see Fig. 6-43). Vitamin K deficiency, which slows blood clotting, is extremely uncommon in humans, aside from a small percentage of infants who suffer from hemorrhagic disease of the newborn, a potentially fatal disorder. In the United States, newborns are routinely given a 1 mg injection of vitamin K. Vitamin (phylloquinone) is found in green plant leaves; a related form, vitamin (menaquinone), is formed by bacteria living in the vertebrate intestine.
Warfarin (Fig. 10-22c) is a synthetic compound that inhibits the formation of active prothrombin. It is particularly poisonous to rats, causing death by internal bleeding. Ironically, this potent rodenticide is also an invaluable anticoagulant drug for treating humans at risk for excessive blood clotting, such as surgical patients and those with coronary thrombosis.
Ubiquinone (also called coenzyme Q) and plastoquinone (Fig. 10-22d, e) are isoprenoids that function as lipophilic electron carriers in the oxidation-reduction reactions that drive ATP synthesis in mitochondria and chloroplasts, respectively. Both ubiquinone and plastoquinone can accept either one or two electrons and either one or two protons (see Fig. 19-3).
Dolichols Activate Sugar Precursors for Biosynthesis
During assembly of the complex carbohydrates of bacterial cell walls, and during the addition of polysaccharide units to certain proteins (glycoproteins) and lipids (glycolipids) in eukaryotes, the sugar units to be added are chemically activated by attachment to isoprenoid alcohols called dolichols (Fig. 10-22f). These compounds have strong hydrophobic interactions with membrane lipids, anchoring the attached sugars to the membrane, where they participate in sugar-transfer reactions.
Many Natural Pigments Are Lipidic Conjugated Dienes
Conjugated dienes have carbon chains with alternating single and double bonds. Because this structural arrangement allows the delocalization of electrons, the compounds can be excited by low-energy electromagnetic radiation (visible light), giving them colors visible to humans and other animals. Carotene (Fig. 10-20) is yellow-orange; similar compounds give bird feathers their striking reds, oranges, and yellows (Fig. 10-23). Like sterols, steroids, dolichols, vitamins A, E, D, and K, ubiquinone, and plastoquinone, these pigments are synthesized from five-carbon isoprene derivatives; the biosynthetic pathway is described in detail in Chapter 21.
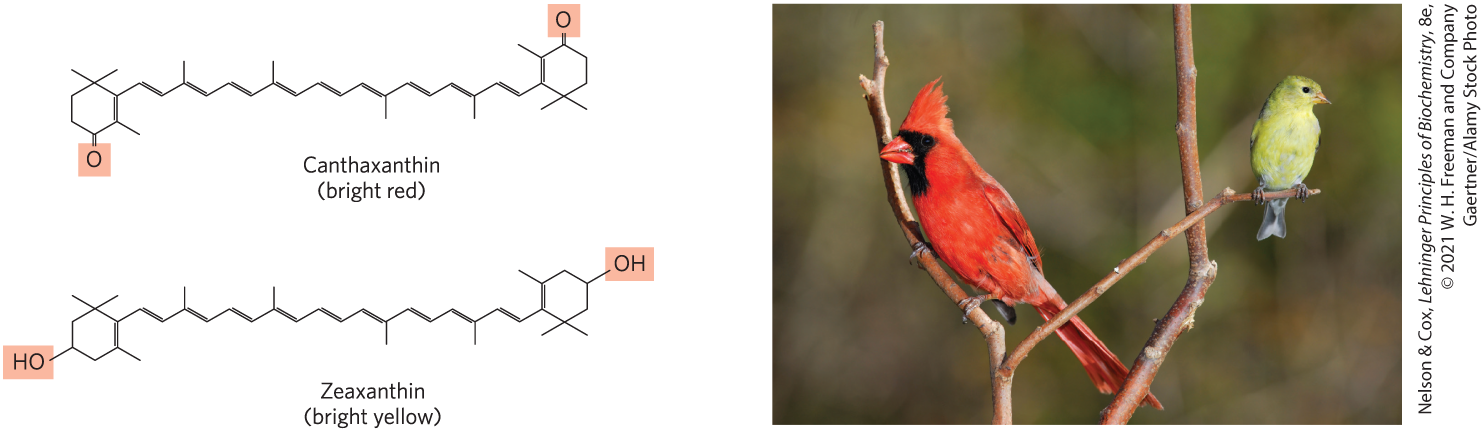
FIGURE 10-23 Lipids as pigments in plants and bird feathers. Compounds with long conjugated systems absorb light in the visible region of the spectrum. Subtle differences in the chemistry of these compounds produce pigments of strikingly different colors. Birds acquire the pigments that color their feathers red or yellow by eating plant materials that contain carotenoid pigments, such as canthaxanthin and zeaxanthin. The differences in pigmentation between male and female birds are the result of differences in intestinal uptake and processing of carotenoids.
Polyketides Are Natural Products with Potent Biological Activities
Polyketides are a diverse group of lipids with biosynthetic pathways (Claisen condensations) similar to those for fatty acids. They are secondary metabolites, compounds that are not central to an organism’s metabolism but serve some subsidiary function that gives the organism an advantage in some ecological niche. Many polyketides find use in medicine as antibiotics (erythromycin), antifungals (amphotericin B), or inhibitors of cholesterol synthesis (lovastatin) (Fig. 10-24).
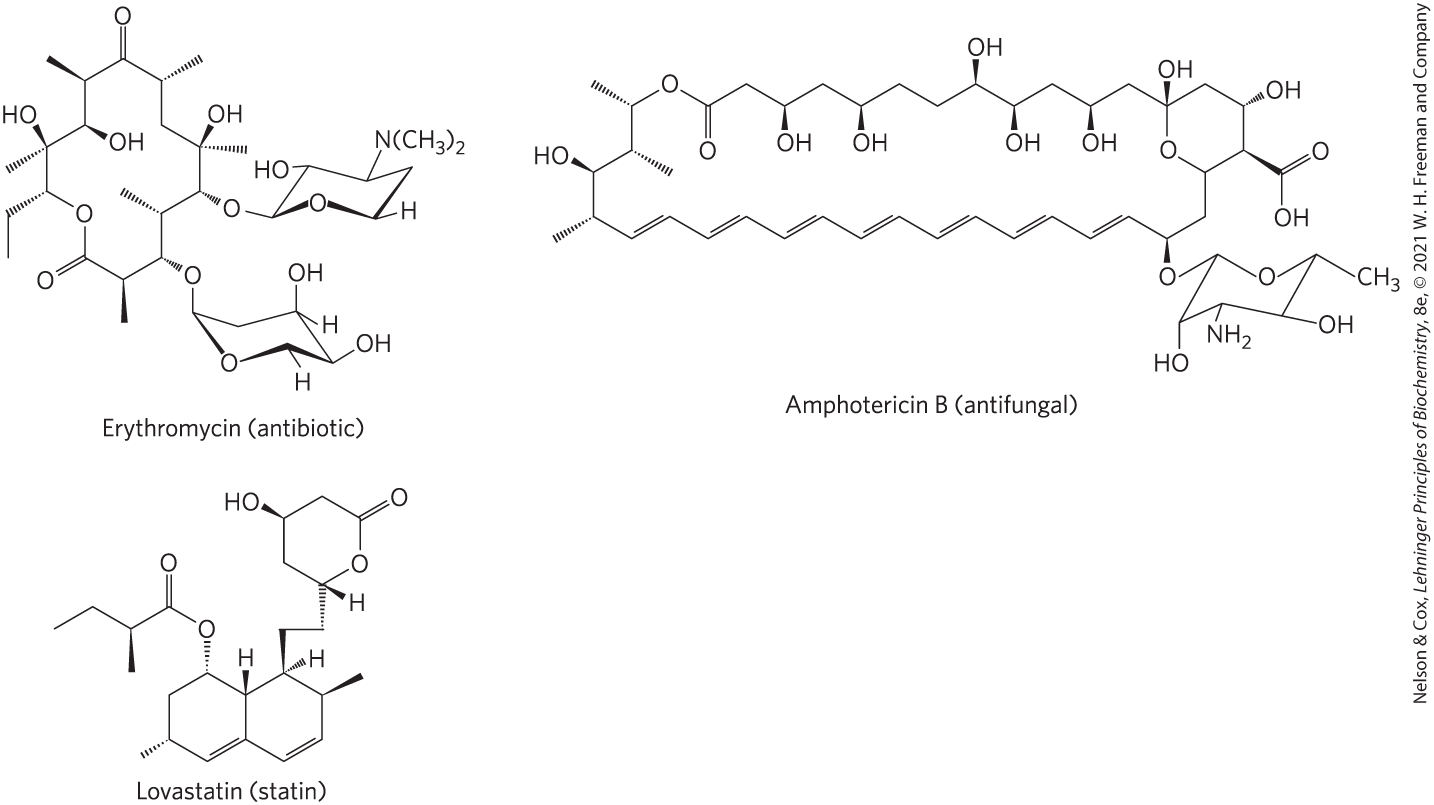
FIGURE 10-24 Three polyketide natural products used in human medicine.
SUMMARY 10.3 Lipids as Signals, Cofactors, and Pigments
- Some types of lipids, although present in relatively small quantities, play critical roles as cofactors or signals.
- Membrane sphingolipids and derivatives of phosphatidylinositol such as and are used as signaling molecules or for nucleating formation of multiprotein complexes.
- Prostaglandins, thromboxanes, leukotrienes, and lipoxins, all of which are eicosanoids derived from arachidonate, are extremely potent hormones involved in reproduction, inflammation, regulating blood pressure, and other bodily processes. NSAIDs inhibit formation of some prostaglandins and thromboxanes.
- Steroid hormones, such as the sex hormones, are derived from sterols. They serve as powerful biological signals and move through the bloodstream to alter gene expression in target cells.
- Plants produce volatile lipids to attract or repel other organisms and for communication. Many of these lipids are used as fragrances in perfumes.
- Vitamins D, A, E, and K are fat-soluble compounds made up of isoprene units. All play essential roles in the metabolism or physiology of animals. Vitamin D is precursor to a hormone that regulates calcium metabolism. Vitamin A furnishes the visual pigment of the vertebrate eye and is a regulator of gene expression during epithelial cell growth.
- Vitamins E and K and quinones can be oxidized or reduced by cells. Vitamin E functions in the protection of membrane lipids from oxidative damage, and vitamin K is essential for blood clotting. Quinones are essential for carrying electrons during the reactions that drive ATP synthesis in mitochondria and chloroplasts.
- Dolichols activate and anchor sugars to cellular membranes; the sugar groups are then used in the synthesis of complex carbohydrates, glycolipids, and glycoproteins.
- Lipidic conjugated dienes serve as pigments in flowers and fruits and give bird feathers their striking colors.
- Polyketides are natural products widely used in medicine.
 Another group
Another group

 Some types of lipids, although present in relatively small quantities, play critical roles as cofactors or signals.
Some types of lipids, although present in relatively small quantities, play critical roles as cofactors or signals.