12.3 GPCRs in Vision, Olfaction, and Gustation
The detection of light, odors, and tastes (vision, olfaction, and gustation, respectively) in animals is accomplished by specialized sensory neurons that use signal-transduction mechanisms fundamentally similar to those that detect hormones, neurotransmitters, and growth factors. An initial sensory signal is greatly amplified by mechanisms that include gated ion channels and intracellular second messengers; the system adapts to continued stimulation by changing its sensitivity to the stimulus (desensitization); and sensory input from several receptors is integrated before the final signal goes to the brain.
The Vertebrate Eye Uses Classic GPCR Mechanisms
Visual transduction (Fig. 12-19) begins when light falls on rhodopsin, a GPCR in the disk membranes of rod cells of the vertebrate eye. (Rod cells do not detect colors; cone cells do, as we shall see in Box 12-3.) The light-absorbing pigment (chromophore) 11-cis-retinal is covalently attached to opsin, the protein component of rhodopsin, which lies near the middle of the disk membrane bilayer. When a photon is absorbed by the retinal component of rhodopsin (step ), the energy causes a photochemical change; 11-cis-retinal is converted to all-trans-retinal (see Fig. 10-20). This change in the structure of the chromophore forces conformational changes in the rhodopsin molecule, allowing it to interact with and thus activate its trimeric G protein, transducin. Rhodopsin now stimulates the exchange of bound GDP on transducin for GTP from the cytosol (Fig. 12-19, step ), and activated transducin stimulates the membrane protein cyclic GMP (cGMP) phosphodiesterase (PDE) by removing an inhibitory subunit (step ). The activated cGMP PDE degrades the second messenger ,-cGMP to -GMP, lowering [cGMP] (step ). A cGMP-dependent or channel in the plasma membrane closes (step ), while a active antiporter continues to pump outward across the plasma membrane (step ), making the transmembrane electrical potential more negative inside (that is, hyperpolarizing the rod cell). This electrical change passes through a series of specialized nerve cells to the visual cortex of the brain.
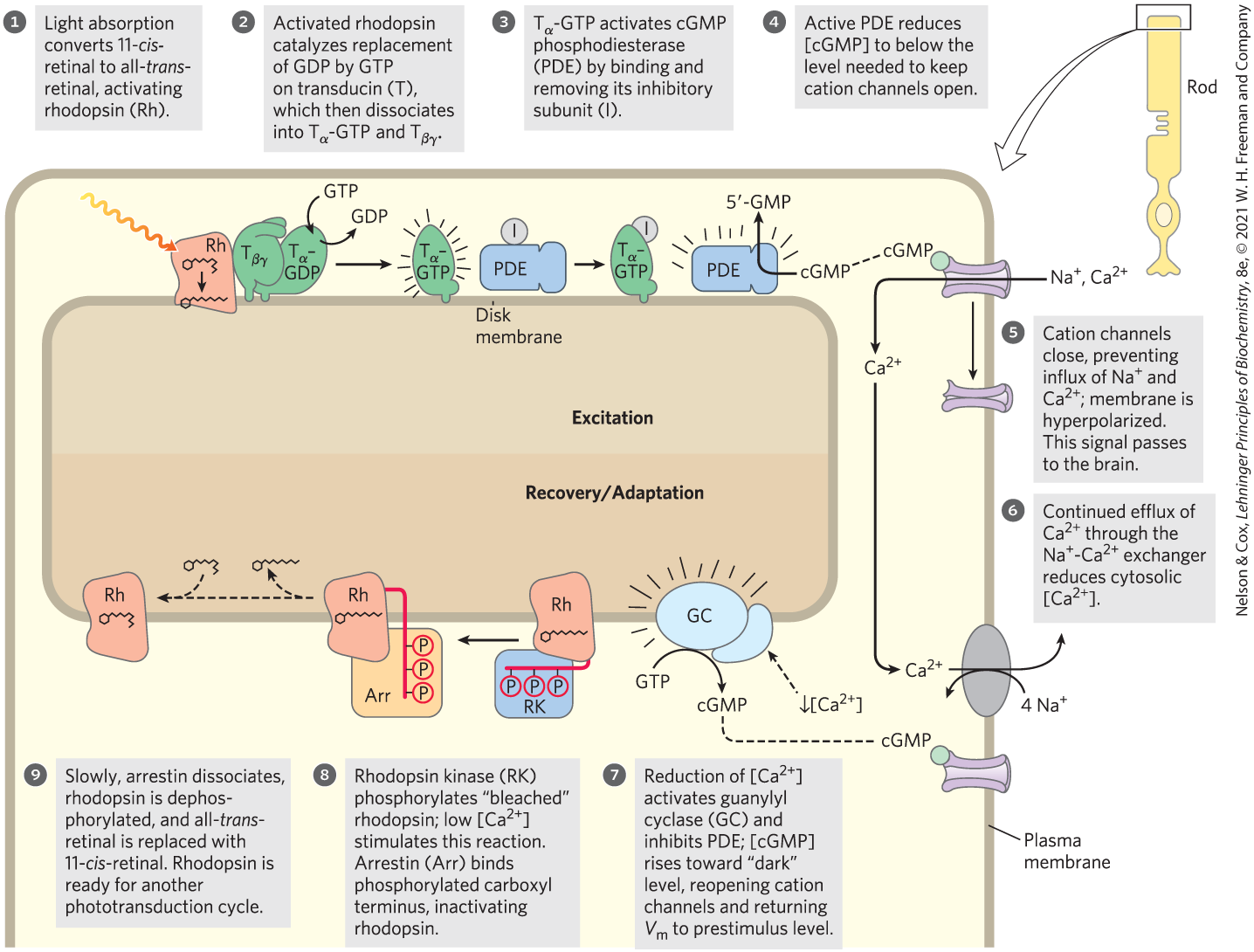
FIGURE 12-19 Molecular consequences of photon absorption by rhodopsin in the rod outer segment. The top half of the figure (steps to ) describes excitation; the bottom shows post-illumination steps: recovery (steps and ) and adaptation (steps and ).
Several steps in the visual-transduction process result in a huge amplification of the signal. Each excited rhodopsin molecule activates at least 500 molecules of transducin, and each transducin molecule can activate a molecule of cGMP PDE. This phosphodiesterase has a remarkably high turnover number: each activated molecule hydrolyzes 4,200 molecules of cGMP per second. The binding of cGMP to cGMP-gated ion channels is cooperative, and a relatively small change in [cGMP] therefore registers as a large change in ion conductance. The result of these amplifications is exquisite sensitivity to light. Absorption of a single photon closes 1,000 or more ion channels for and , hyperpolarizing the cell’s membrane by about 1 mV.
As your eyes move across this line of type, the retinal images of the first words disappear rapidly — before you see the next series of words. In that short interval, a great deal of biochemistry has taken place. Very soon after illumination of the rod or cone cells stops, the photosensory system shuts off. The α subunit of transducin (, with bound GTP) has GTPase activity. Within milliseconds after the decrease in light intensity, GTP is hydrolyzed and reassociates with . The inhibitory subunit of PDE, which had been bound to -GTP, is released and reassociates with PDE, strongly inhibiting its activity and thus slowing cGMP breakdown.
At the same time, a second factor that helps to end the response to light is the reduction of intracellular that results from continued efflux through the exchanger (Fig. 12-19, step ). High inhibits the enzyme that makes cGMP (guanylyl cyclase; step ), so cGMP production rises when falls, quickly reaching its prestimulus level.
In response to prolonged illumination, rhodopsin itself undergoes changes that limit the duration of its signaling activity. The conformational change induced in rhodopsin by light absorption exposes several Thr and Ser residues in its carboxyl-terminal domain, and these residues are phosphorylated by rhodopsin kinase (step ), which is functionally and structurally homologous to the β-adrenergic kinase (βARK) that desensitizes the β-adrenergic receptor. The phosphorylated carboxyl-terminal domain of rhodopsin is bound by the protein arrestin 1, preventing further interaction between activated rhodopsin and transducin (see Fig. 12-10b). Arrestin 1 is a close homolog of arrestin 2 (βarr) of the β-adrenergic system. On a much longer time scale (step ), the all-trans-retinal bound to light-bleached rhodopsin is removed and replaced with 11-cis-retinal, making rhodopsin ready to detect another photon.
Vertebrate Olfaction and Gustation Use Mechanisms Similar to the Visual System
The sensory cells that detect odors and tastes have much in common with the visual receptor system. Binding of an odorant molecule to one of its specific GPCRs (humans have about 800 different GPCRs; rodents have about 1,200) triggers a change in receptor conformation, activating a G protein, , analogous to transducin and to of the β-adrenergic system. The activated activates adenylyl cyclase, raising the local [cAMP]. The cAMP-gated and channels of the plasma membrane open, and the influx of and produces a small depolarization called the receptor potential. If a sufficient number of odorant molecules encounter receptors, the receptor potential is strong enough to cause the neuron to fire an action potential. This signal is relayed to the brain in several stages and registers as a specific smell. All these events occur within 100 to 200 ms. When the olfactory stimulus is no longer present, the transducing machinery shuts itself off in several ways. A cAMP phosphodiesterase returns [cAMP] to the prestimulus level. hydrolyzes its bound GTP to GDP, thereby inactivating itself. Phosphorylation of the receptor by a specific kinase prevents its interaction with , by a mechanism analogous to that used to desensitize the β-adrenergic receptor and rhodopsin. Some odorants are detected by another mechanism we have seen in other signal transductions: activation of a phospholipase and production of , leading to a rise in intracellular .
The sense of taste in vertebrates reflects the activity of gustatory neurons clustered in taste buds on the surface of the tongue. In taste sensory neurons, GPCRs are coupled to the heterotrimeric G protein gustducin. When the tastant molecule binds its receptor, gustducin is activated and stimulates cAMP production by adenylyl cyclase. The resulting elevation of [cAMP] activates PKA, which phosphorylates channels in the plasma membrane, causing them to close and sending an electrical signal to the brain. Other taste buds specialize in detecting bitter, sour, salty, or umami (savory) tastants, using various combinations of second messengers and ion channels in the transduction mechanisms.
All GPCR Systems Share Universal Features
We have now looked at several types of signaling systems (hormone signaling, vision, olfaction, and gustation) in which membrane receptors are coupled to second messenger–generating enzymes through G proteins. As we have intimated, signaling mechanisms must have arisen early in evolution; genomic studies have revealed hundreds of genes encoding GPCRs in vertebrates, arthropods (Drosophila and mosquito), and the roundworm Caenorhabditis elegans. Even the common budding yeast Saccharomyces uses GPCRs and G proteins to detect the opposite mating type. Overall patterns have been conserved, and the introduction of variety has given modern organisms the ability to respond to a wide range of stimuli (Table 12-6). Of the approximately 20,000 genes in the human genome, as many as 800 encode GPCRs, including hundreds for olfactory stimuli and many orphan receptors, for which the natural ligand is not yet known.
Amines
Peptides
|
Protein hormones
Prostanoids
Others
|
All well-studied signal-transducing systems that act through heterotrimeric G proteins share some common features that reflect their evolutionary relatedness (Fig. 12-20). The receptors have seven transmembrane segments, a domain (generally the loop between transmembrane helices 6 and 7) that interacts with a G protein, and a carboxyl-terminal cytoplasmic domain that undergoes reversible phosphorylation on several Ser or Thr residues. The ligand-binding site (or, in the case of light reception, the light receptor) is buried deep in the membrane and includes residues from several of the transmembrane segments. Ligand binding (or light) induces a conformational change in the receptor, exposing a domain that can interact with a G protein. Heterotrimeric G proteins activate or inhibit effector enzymes (adenylyl cyclase, PDE, or PLC), which change the concentration of a second messenger (cAMP, cGMP, , or ). In the hormone-detecting systems, the final output is an activated protein kinase that regulates some cellular process by phosphorylating a protein critical to that process. In sensory neurons, the output is a change in membrane potential and a consequent electrical signal that passes to another neuron in the pathway connecting the sensory cell to the brain.
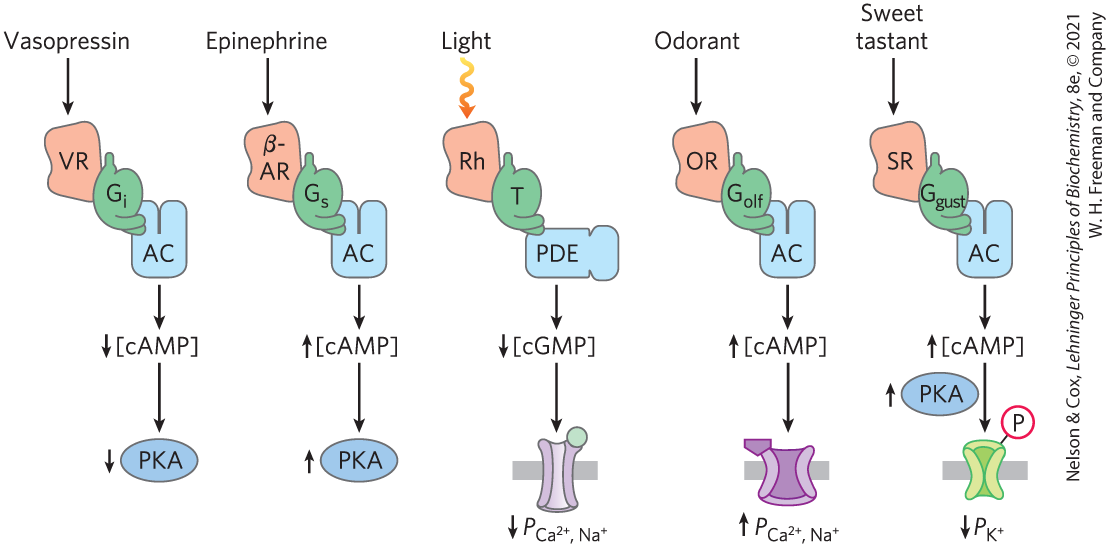
FIGURE 12-20 Common features of signaling systems that detect hormones, light, smells, and tastes. GPCRs provide signal specificity, and their interaction with G proteins provides signal amplification. Heterotrimeric G proteins activate or inhibit effector enzymes: adenylyl cyclase (AC) and phosphodiesterases (PDEs) that degrade cAMP or cGMP. Changes in concentration of the second messengers (cAMP, cGMP) result in alterations in enzymatic activities via phosphorylation or alterations in the permeability (P) of surface membranes to , , and . The resulting depolarization or hyperpolarization of the sensory cell (the signal) passes through relay neurons to sensory centers in the brain. In the best-studied cases, desensitization includes phosphorylation of the receptor and binding of a protein (arrestin) that interrupts receptor–G protein interactions. (The path of odorant detection by production of and increase in intracellular , mentioned in the text, is not shown here.) VR is the vasopressin receptor; β-AR, the β-adrenergic receptor; Rh, rhodopsin; OR, olfactory receptor; SR, sweet-taste receptor.
The first system has an irregular light red rectangle labeled V R with a green rounded structure labeled G subscript i attached to its lower right that has a protrusion to its upper left and two curved protrusions to its lower right, where it overlaps with a light blue rectangle labeled A C that has its top half divided vertically into two halves. An arrow points down from vasopressin to the light red rectangle labeled V R. An arrow points down from the blue rectangle labeled A C to text reading downward arrow symbol [c A M P], from which an arrow points down to a blue oval labeled P K A with a downward arrow symbol to its left. The second system has an irregular light red rectangle labeled Greek letter beta-A R with a green rounded structure labeled G subscript s attached to its lower right that has a protrusion to its upper left and two curved protrusions to its lower right, where it overlaps with a light blue rectangle labeled A C that has its top half divided vertically into two halves. An arrow points down from epinephrine to the light red rectangle labeled Greek letter beta-A R. An arrow points down from the blue rectangle labeled A C to text reading upward arrow symbol [c A M P], from which an arrow points down to a blue oval labeled P K A with an upward arrow symbol to its left. The third system has an irregular light red rectangle labeled R h with a green rounded structure labeled T attached to its lower right that has a protrusion to its upper left and two curved protrusions to its lower right, where it overlaps with a light blue rectangle labeled P D E that has a thin vertical rectangular piece on its right side. A wavy arrow points down from light to the light red rectangle labeled R h. An arrow points down from the blue rectangle labeled P D E to text reading downward arrow symbol [c G M P], from which an arrow points down to a vertical channel in a membrane with a green sphere bonded to its upper right and with text below reading, downward arrow symbol P subscript C a 2 plus, N a plus. The fourth system has an irregular light red rectangle labeled O R with a green rounded structure labeled G subscript o l f attached to its lower right that has a protrusion to its upper left and two curved protrusions to its lower right, where it overlaps with a light blue rectangle labeled A C that has its top half divided vertically into two halves. An arrow points down from odorant to the light red rectangle labeled O R. An arrow points down from the blue rectangle labeled A C to text reading upward arrow symbol [c A M P], from which an arrow points down to a vertical channel in a membrane with a piece attached to its upper left side that resembles a rectangle with a triangle attached that points downward and with text below that reads, upward arrow symbol P subscript C a 2 plus, N a plus. The fifth system has an irregular light red rectangle labeled S R with a green rounded structure labeled G subscript g u s t attached to its lower right that has a protrusion to its upper left and two curved protrusions to its lower right, where it overlaps with a light blue rectangle labeled A C that has its top half divided vertically into two halves. An arrow points down from tastant to the light red rectangle labeled S R. An arrow points down from the blue rectangle labeled A C to text reading upward arrow symbol [c A M P], from which an arrow next to a blue oval labeled P K A with an upward arrow symbol to its left points down to a vertical channel in a membrane that has its sides pinched in in the center, a bond to a red circle labeled P at the upper right, and text below reading, downward arrow symbol P subscript K plus.
All these systems self-inactivate. Bound GTP is converted to GDP by the GTPase activity of G proteins, often augmented by GTPase-activating proteins (GAPs) or regulators of G-protein signaling (RGS). In some cases, the effector enzymes that are the targets of modulation by G proteins also serve as GAPs. The desensitization mechanism involving phosphorylation of the carboxyl-terminal region followed by arrestin binding is widespread and may be universal.
SUMMARY 12.3 GPCRs in Vision, Olfaction, and Gustation
- Vision, olfaction, and gustation in vertebrates employ GPCRs, which act through heterotrimeric G proteins to change the membrane potential of a sensory neuron.
- Light activates the GPCR rhodopsin, which allosterically activates the trimeric G protein transducin. activates a cGMP phosphodiesterase, lowering [cGMP] and closing cGMP-dependent ion channels. The resulting electrical impulse carries the signal to the brain.
- In olfactory neurons, olfactory stimuli, acting through GPCRs and G proteins, trigger an increase in [cAMP] (by activating adenylyl cyclase) or (by activating PLC). These second messengers affect ion channels and thus the . Gustatory neurons have GPCRs that respond to tastants by altering levels of cAMP, which changes by gating ion channels.
- There is a high degree of conservation of signaling proteins and transduction mechanisms across signaling systems and across species. GPCRs with seven transmembrane helices, G proteins with intrinsic GTPase activities, cyclic nucleotides, and protein kinases are central to signaling.
 ), the energy causes a photochemical change; 11-cis-retinal is converted
), the energy causes a photochemical change; 11-cis-retinal is converted  ), and activated transducin stimulates the membrane protein cyclic GMP (cGMP) phosphodiesterase (PDE) by removing an inhibitory subunit (step
), and activated transducin stimulates the membrane protein cyclic GMP (cGMP) phosphodiesterase (PDE) by removing an inhibitory subunit (step  ). The activated cGMP PDE degrades the second messenger ,-cGMP to -GMP, lowering [cGMP] (step
). The activated cGMP PDE degrades the second messenger ,-cGMP to -GMP, lowering [cGMP] (step  ). A cGMP-dependent or channel in the plasma membrane closes (step
). A cGMP-dependent or channel in the plasma membrane closes (step  ), while a active antiporter continues to pump outward across the
), while a active antiporter continues to pump outward across the  ), making the transmembrane electrical potential more negative inside (that is, hyperpolarizing the rod cell). This electrical change passes through a series of specialized nerve cells to the visual cortex of the brain.
), making the transmembrane electrical potential more negative inside (that is, hyperpolarizing the rod cell). This electrical change passes through a series of specialized nerve cells to the visual cortex of the brain. ) and adaptation (steps
) and adaptation (steps  and
and  ).
). The cAMP-gated and channels of the plasma membrane open, and the influx of and produces a small depolarization called the receptor potential. If a sufficient number of odorant molecules encounter receptors, the receptor potential is strong enough to cause the neuron to fire an action potential. This signal is relayed to the brain in several stages and registers as a specific smell. All these events occur within 100 to 200 ms. When the olfactory stimulus is no longer present, the transducing machinery shuts itself off in several ways. A cAMP phosphodiesterase returns [cAMP] to the prestimulus level. hydrolyzes its bound GTP to GDP, thereby inactivating itself. Phosphorylation of the receptor by a specific kinase prevents its interaction with , by a mechanism analogous to that used to desensitize the β-adrenergic receptor and rhodopsin. Some odorants are detected by another mechanism we have seen in other signal transductions: activation of a phospholipase and production of , leading to a rise in intracellular .
The cAMP-gated and channels of the plasma membrane open, and the influx of and produces a small depolarization called the receptor potential. If a sufficient number of odorant molecules encounter receptors, the receptor potential is strong enough to cause the neuron to fire an action potential. This signal is relayed to the brain in several stages and registers as a specific smell. All these events occur within 100 to 200 ms. When the olfactory stimulus is no longer present, the transducing machinery shuts itself off in several ways. A cAMP phosphodiesterase returns [cAMP] to the prestimulus level. hydrolyzes its bound GTP to GDP, thereby inactivating itself. Phosphorylation of the receptor by a specific kinase prevents its interaction with , by a mechanism analogous to that used to desensitize the β-adrenergic receptor and rhodopsin. Some odorants are detected by another mechanism we have seen in other signal transductions: activation of a phospholipase and production of , leading to a rise in intracellular . All well-studied signal-transducing systems that act through heterotrimeric G proteins share some common features that reflect their evolutionary relatedness (
All well-studied signal-transducing systems that act through heterotrimeric G proteins share some common features that reflect their evolutionary relatedness ( Vision, olfaction, and gustation in vertebrates employ GPCRs, which act through heterotrimeric G proteins to change the membrane potential of a sensory neuron.
Vision, olfaction, and gustation in vertebrates employ GPCRs, which act through heterotrimeric G proteins to change the membrane potential of a sensory neuron.