12.2 G Protein–Coupled Receptors and Second Messengers
As their name implies, G protein–coupled receptors (GPCRs) are receptors that act through a member of the guanosine nucleotide–binding protein, or G protein, family. Three essential components define signal transduction through GPCRs: a plasma membrane receptor with seven transmembrane helical segments, a G protein that cycles between active (guanosine triphosphate (GTP)-bound) and inactive (guanosine diphosphate (GDP)-bound) forms, and an effector enzyme (or ion channel) in the plasma membrane that is regulated by the activated G protein. An extracellular signal such as a hormone, growth factor, or neurotransmitter is the “first messenger” that activates a receptor from outside the cell. Ligand binding to the receptor forces an allosteric transition that allows the receptor to interact with a G protein, causing it to exchange its bound GDP for a GTP from the cytosol. The G protein then dissociates from the activated receptor and binds to the nearby effector enzyme, altering its activity. The effector enzyme then causes a change in the cytosolic concentration of a low molecular weight metabolite (such as ,-cyclic AMP) or inorganic ion , which acts as a second messenger to activate or inhibit one or more downstream targets, often protein kinases.
The human genome encodes just over 800 GPCRs, about 350 for detecting hormones, growth factors, and other endogenous ligands, and up to 500 that serve as olfactory (smell) and gustatory (taste) receptors. The largest superfamily of proteins encoded in the human genome, GPCRs have been implicated in many common medical conditions, including allergies, depression, blindness, diabetes, and various cardiovascular defects. GPCR mutations are also found in 20% of all cancers. In the United States, more than a third of all pharmaceuticals on the market target a GPCR. For example, the β-adrenergic receptor, which mediates the effects of epinephrine, is the target of the “beta blockers,” prescribed for such diverse conditions as hypertension, cardiac arrhythmia, glaucoma, anxiety, and migraine headache. More than 100 of the GPCRs found in the human genome are still “orphan receptors,” meaning that their natural ligands are not yet identified, and so we know little about their biology. The β-adrenergic receptor, with well-understood biology and pharmacology, is the prototype for all GPCRs, and our discussion of signal-transducing systems begins there.
The β-Adrenergic Receptor System Acts through the Second Messenger cAMP
Epinephrine released from the adrenal glands sounds the alarm when a threat requires an animal to mobilize its energy-generating machinery; it signals the need to fight or flee. Epinephrine action begins when the hormone binds to a protein receptor in the plasma membrane of an epinephrine-sensitive cell (a myocyte in muscle, for example). Adrenergic receptors (“adrenergic” reflects the alternative name for epinephrine, adrenaline) are of four general types, , , , and , defined by differences in their affinities and responses to a group of agonists and antagonists. Agonists are molecules (natural ligands or their structural analogs) that bind a receptor and produce the effects of the natural ligand; antagonists are analogs that bind the receptor without triggering the normal effect and thereby block the effects of agonists, including the natural ligand. In some cases, the affinity of a synthetic agonist or antagonist for the receptor is greater than that of the natural agonist (Fig. 12-3). The four types of adrenergic receptors are found in different target tissues and mediate different responses to epinephrine. Here we focus on the β-adrenergic receptors of muscle, liver, and adipose tissue. These receptors mediate changes in fuel metabolism, as described in Chapter 23, including the increased breakdown of glycogen and fat. Adrenergic receptors of the and subtypes act through the same mechanism, so in our discussion, “β-adrenergic” applies to both types.
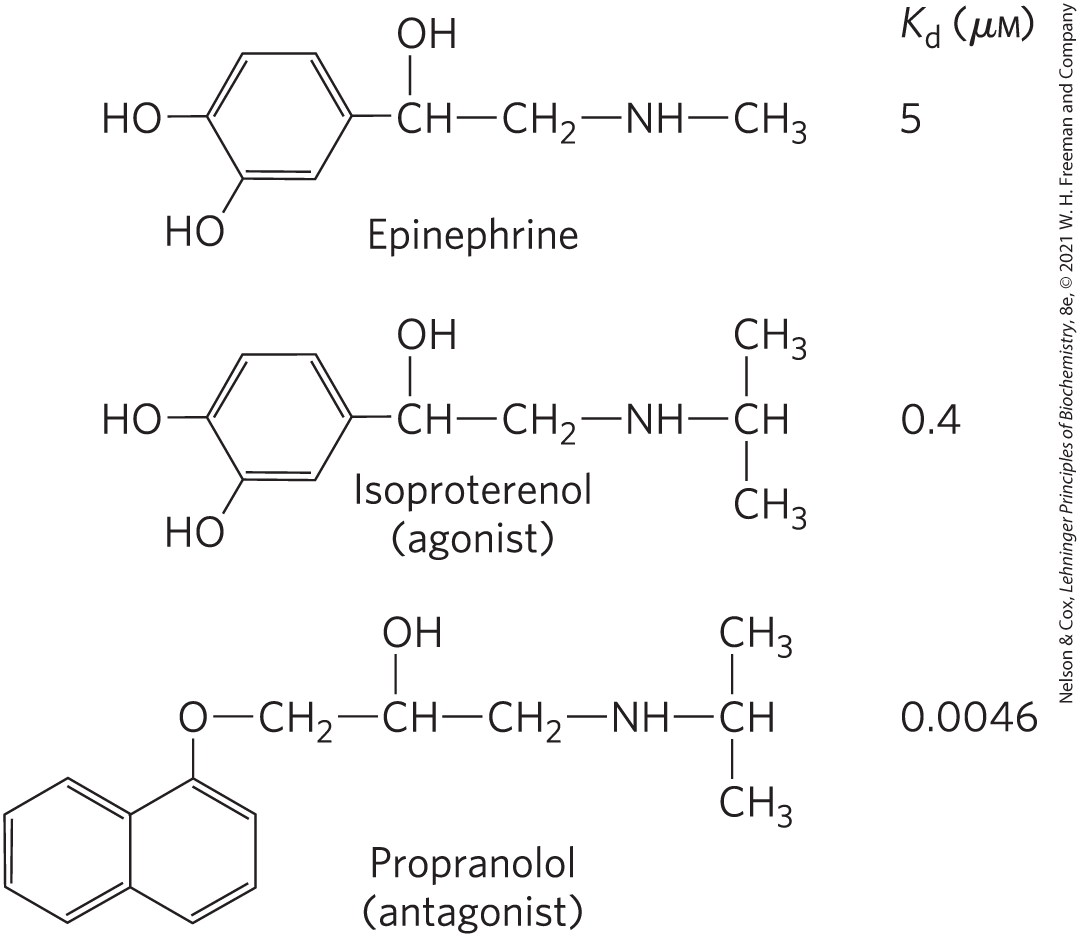
FIGURE 12-3 Epinephrine and its synthetic analogs. Epinephrine regulates energy-yielding metabolism in muscle, liver, and adipose tissue. Its affinity for its receptor is expressed as a dissociation constant for the receptor-ligand complex. Isoproterenol and propranolol are synthetic analogs, one an agonist with an affinity for the receptor that is higher than that of epinephrine, and the other an antagonist with extremely high affinity.
Like all GPCRs, the β-adrenergic receptor is an integral protein with seven hydrophobic, helical regions of 20 to 28 amino acid residues that span the plasma membrane seven times, thus the alternative names for GPCRs: seven-transmembrane (7tm) or heptahelical receptors. GPCRs effect signal transduction through interaction with heterotrimeric G proteins, a conserved family of signaling proteins with three subunits, α, β, and γ. The binding site for GDP or GTP is on the α subunit. When GDP is bound, the G protein is in its trimeric, inactive form.
GPCRs are allosteric proteins. When epinephrine binds to the β-adrenergic receptor (Fig. 12-4a, step ), allosteric transitions in the receptor and its associated G protein favor the replacement of GDP with GTP. Thus the hormone-bound GPCR acts as a guanosine nucleotide–exchange factor (GEF). In this active form, the G protein (step ) can transmit the signal from the activated receptor to the downstream effector protein, adenylyl cyclase. Because this G protein stimulates its effector, it is referred to as a stimulatory G protein, or . In the active form, the β and γ subunits of dissociate from the α subunit as a βγ dimer, and , with its bound GTP, moves in the plane of the membrane from the receptor to a nearby molecule of adenylyl cyclase (step ).
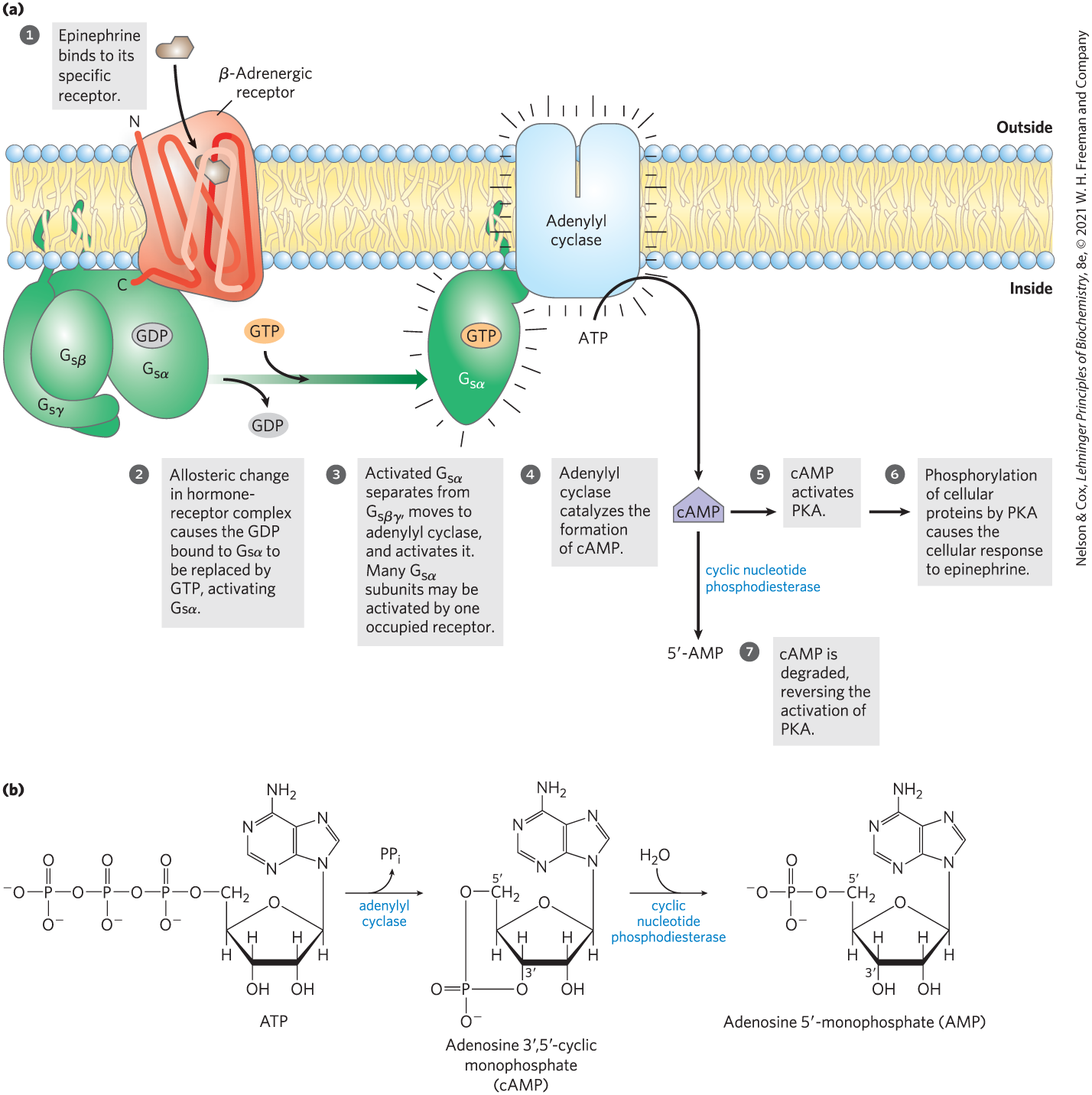
FIGURE 12-4 Transduction of the epinephrine signal: the β-adrenergic pathway. (a) The mechanism that couples binding of epinephrine to its receptor with activation of adenylyl cyclase; the seven steps are discussed in the text. The same adenylyl cyclase molecule in the plasma membrane may be regulated by a stimulatory G protein , as shown, or by an inhibitory G protein (, not shown). and are under the influence of different hormones. Hormones that induce GTP binding to cause inhibition of adenylyl cyclase, resulting in lower cellular [cAMP]. (b) The combined action of the enzymes that catalyze steps and , synthesis and hydrolysis of cAMP by adenylyl cyclase and cAMP phosphodiesterase, respectively.
Adenylyl cyclase is an integral protein of the plasma membrane, with its active site on the cytoplasmic face. The association of active with adenylyl cyclase stimulates the cyclase to catalyze the synthesis of second messenger cAMP from ATP (Fig. 12-4a, step ; Fig. 12-4b), raising the cytosolic [cAMP]. The interaction between and adenylyl cyclase occurs only when is bound to GTP.
The stimulation by is self-limiting; has intrinsic GTPase activity that switches to its inactive form by converting its bound GTP to GDP (Fig. 12-5). The inactive dissociates from adenylyl cyclase, rendering the cyclase inactive. reassociates with the βγ dimer , and inactive is again available to interact with a hormone-bound receptor.
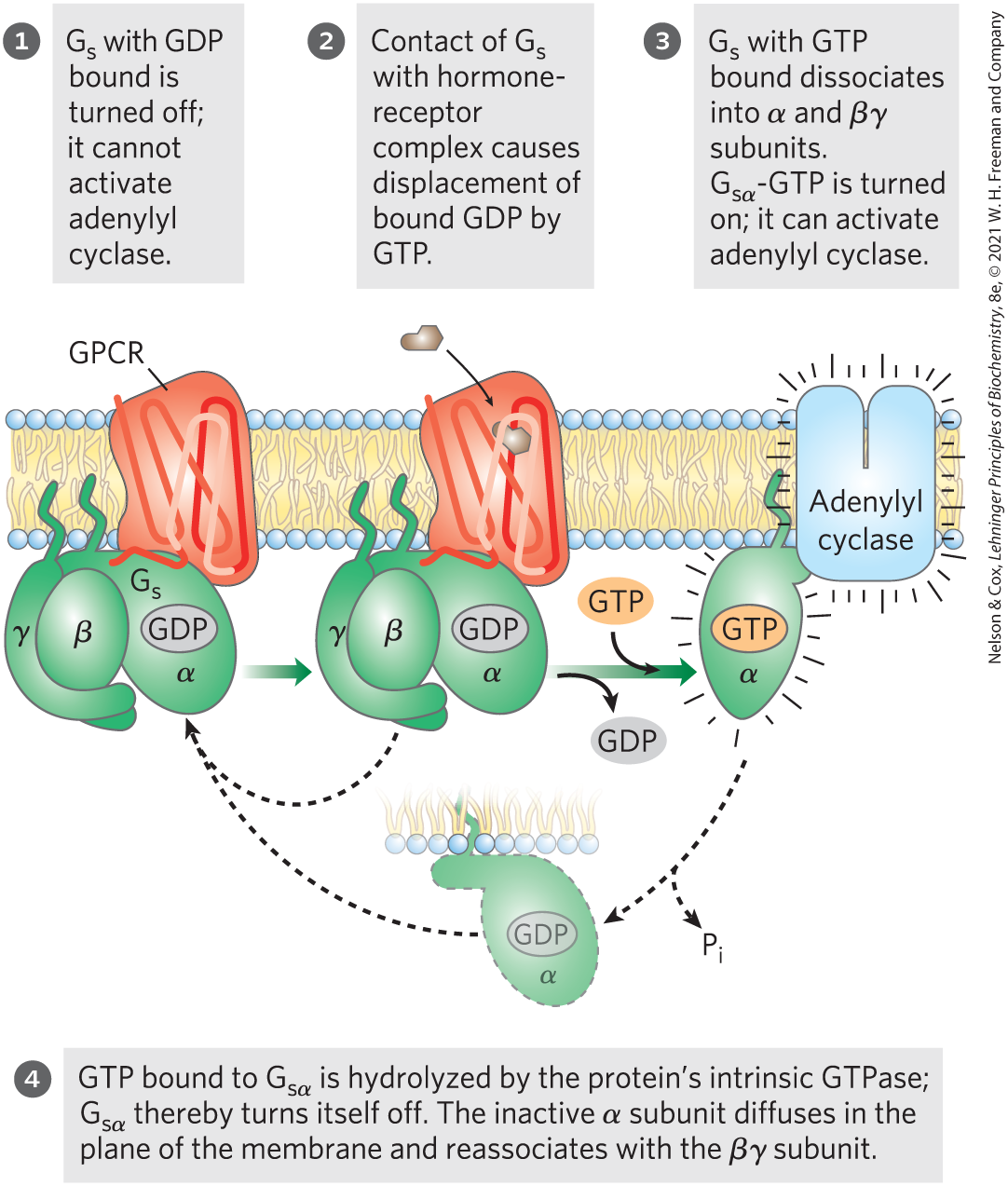
FIGURE 12-5 The GTPase switch. G proteins cycle between GDP-bound (off) and GTP-bound (on). The protein’s GTPase activity, in many cases stimulated by RGS proteins (regulators of G-protein signaling; see Fig. 12-8), determines how quickly bound GTP is hydrolyzed to GDP and thus how long the G protein remains active.
Cyclic AMP Activates Protein Kinase A
Epinephrine exerts its downstream effects through the increase in [cAMP] that results from activation of adenylyl cyclase. Cyclic AMP, the second messenger, allosterically activates cAMP-dependent protein kinase, also called protein kinase A or PKA (Fig. 12-4a, step ), which catalyzes the phosphorylation of specific Ser or Thr residues of targeted proteins, such as glycogen phosphorylase b kinase. The latter enzyme is active when phosphorylated and can begin the process of mobilizing glycogen stores in muscle and liver in anticipation of the need for energy, as signaled by epinephrine.
The inactive form of PKA has two identical catalytic subunits (C) and two identical regulatory subunits (R) (Fig. 12-6a). The tetrameric complex is catalytically inactive, because an autoinhibitory domain of each R subunit occupies the substrate-binding cleft of each C subunit. The autoinhibitory domain is an intrinsically disordered region that can fold to fit any of several protein partners. Cyclic AMP is an allosteric activator of PKA. When cAMP binds to the R subunits, they undergo a conformational change that moves the autoinhibitory domain of R out of the catalytic domain of C, and the complex dissociates to yield two free, catalytically active C subunits. This same basic mechanism — displacement of an autoinhibitory domain — mediates the allosteric activation of many types of protein kinases by their second messengers (as in Fig. 12-21 and 12-29, for example). The structure of the substrate-binding cleft in PKA is the prototype for all known protein kinases (Fig. 12-6b); certain residues in this cleft region have identical counterparts in all of the 544 protein kinases encoded in the human genome. The ATP-binding site of each catalytic subunit positions ATP perfectly for the transfer of its terminal phosphoryl group to the in the side chain of a Ser or Thr residue in the target protein.

FIGURE 12-6 Activation of cAMP-dependent protein kinase (PKA). (a) When [cAMP] is low, the two identical regulatory subunits (R; red) associate with the two identical catalytic subunits (C; blue). In this complex, the inhibitor sequences of the R subunits lie in the substrate-binding cleft of the C subunits and prevent binding of protein substrates; the complex is therefore catalytically inactive. The amino-terminal sequences of the R subunits interact to form an dimer, the site of binding to an A kinase anchoring protein (AKAP; Fig. 12-11). When [cAMP] rises in response to a hormonal signal, each R subunit binds two cAMP molecules and undergoes a dramatic reorganization that pulls its inhibitory sequence away from the C subunit, opening up the substrate-binding cleft and releasing each C subunit in its catalytically active form. (b) A crystal structure showing part of the complex — one C subunit and part of one R subunit. The amino-terminal dimerization region of the R subunit is omitted for simplicity. The small lobe of C contains the ATP-binding site, and the large lobe surrounds and defines the cleft where the protein substrate binds and undergoes phosphorylation at a Ser or Thr residue, with a phosphoryl group transferred from ATP. In this inactive form, the inhibitor sequence of R blocks the substrate-binding cleft of C, inactivating it. [(b) Data from PDB ID 3FHI, C. Kim et al., Science 307:690, 2005.]
As indicated in Figure 12-4a (step ), PKA regulates many enzymes downstream in the signaling pathway. Although these downstream targets have diverse functions, they share a region of sequence similarity around the Ser or Thr residue that undergoes phosphorylation, a sequence that marks them for regulation by PKA. The substrate-binding cleft of PKA recognizes these sequences and phosphorylates their Thr or Ser residue. Comparison of the sequences of various protein substrates for PKA has yielded the short consensus sequence in which the target for phosphorylation (Ser or Thr) is most commonly embedded. For PKA, the consensus sequence is xR[RK]x[ST]B, where x can be any residue, R is Arg, [RK] can be either Arg or Lys, [ST] is either Ser or Thr (which is the residue phosphorylated), and B is any basic residue. The consensus sequences of a number of protein kinases are shown in Table 6-10; they define the targets of the hundreds of protein kinases in the eukaryotic cell.
How do we know that two proteins interact, or where in the cell they interact? Changes in the state of association of two proteins (such as the R and C subunits of PKA) can be seen by measuring the nonradiative transfer of energy between fluorescent probes attached to each protein, a technique called fluorescence resonance energy transfer (FRET), which we describe in Box 12-1.
As in many signaling pathways, signal transduction by the β-adrenergic receptor and adenylyl cyclase entails several steps that amplify the original hormone signal (Fig. 12-7). First, the binding of one hormone molecule to one receptor molecule catalytically activates many molecules that associate with the activated receptor, one after the other. Next, by activating one molecule of adenylyl cyclase, each active molecule stimulates the catalytic synthesis of many molecules of cAMP. The second messenger cAMP now activates PKA, each molecule of which catalyzes the phosphorylation of many molecules of the target protein — phosphorylase b kinase in Figure 12-7. This kinase activates glycogen phosphorylase b, which leads to the rapid mobilization of glucose from glycogen. The net effect of the cascade is amplification of the hormonal signal by orders of magnitude, which accounts for the very low concentration of epinephrine (or any other hormone) required for hormone activity. This signaling pathway is also rapid: the signal leads to intracellular changes within fractions of a second.
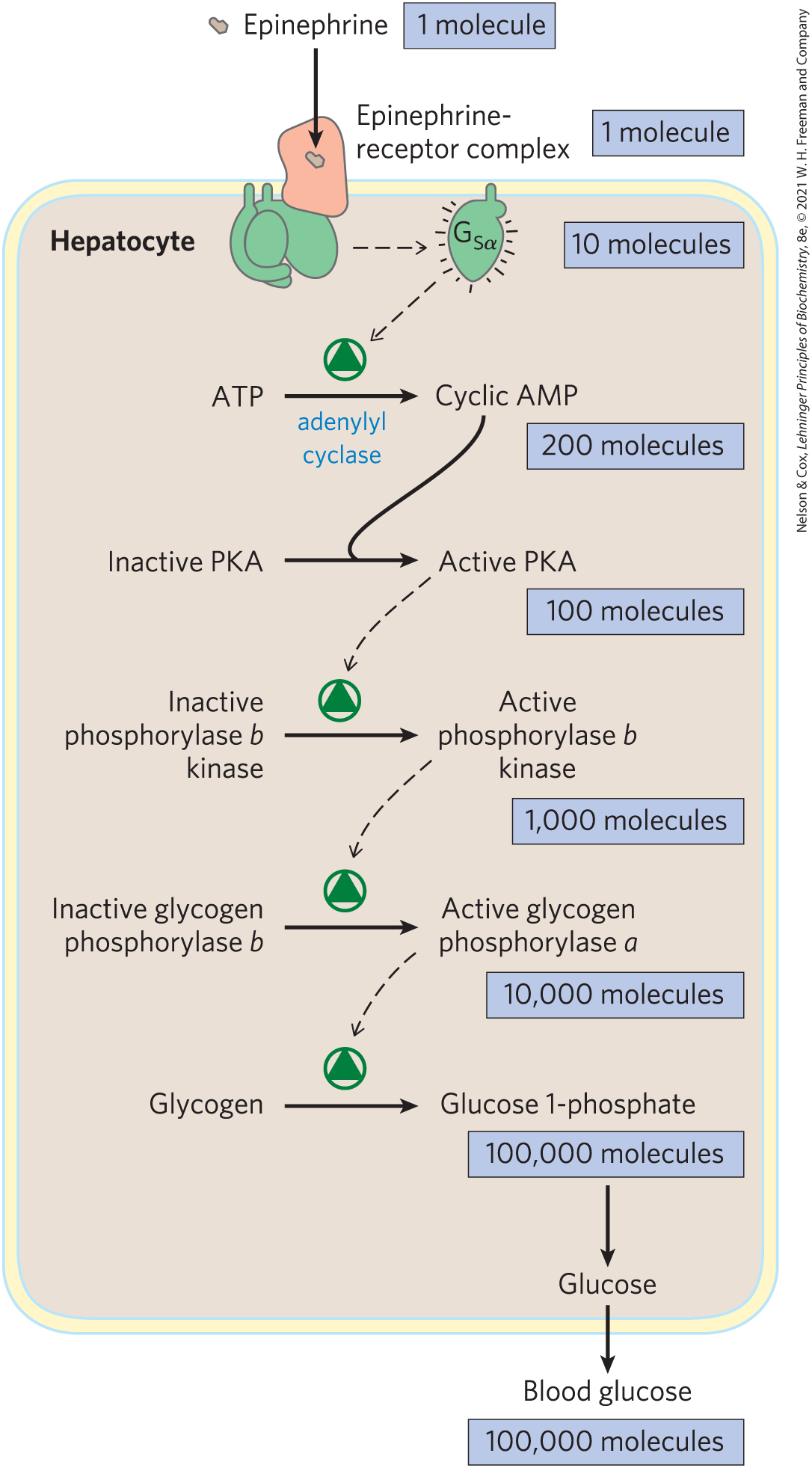
FIGURE 12-7 Epinephrine cascade. Epinephrine triggers a series of reactions in hepatocytes in which catalysts activate catalysts, resulting in great amplification of the original hormone signal. The numbers of molecules shown are simply to illustrate amplification and are almost certainly gross underestimates. Binding of one molecule of epinephrine to one β-adrenergic receptor on the cell surface activates many (possibly hundreds of) G proteins, one after another, each of which goes on to activate a molecule of the enzyme adenylyl cyclase. Adenylyl cyclase acts catalytically, producing many molecules of cAMP for each activated adenylyl cyclase. (Because two molecules of cAMP are required to activate one PKA catalytic subunit, this step does not amplify the signal.)
A small hexagonal molecule with a protrusion to the upper left is labeled epinephrine and is next to a rectangle labeled 1 molecule. An arrow points down to show the same molecule in a rectangle that extends across the membrane of a hepatocyte and that has a green oval below that curves to the upper left, where it protrudes into the membrane and meets a crescent-shaped green piece with a protrusion into the membrane that wraps around a green oval between it and the larger oval. The receptor with the bound epinephrine is labeled epinephrine-receptor complex with an adjacent rectangle labeled 1 molecule. A dashed arrow points right from the large green oval to a similar green oval that is almost vertical with a protrusion into the membrane, short lines radiating from all sides, and an adjacent rectangle labeled 10 molecules. A dashed arrow points diagonally down to the left to a green circle enclosing a green triangle above an arrow pointing from A T P to cyclic A T P and labeled blue highlighted adenylyl cyclase. An adjacent rectangle is labeled 200 molecules. An arrow points down to the lower left join an arrow from inactive P K A to active P K A. An adjacent rectangle is labeled 100 molecules. A dashed arrow points down to the lower left to a green circle enclosing a green triangle above an arrow that points from inactive phosphorylase italicized b end italics kinase to active phosphorylase italicized b end italics kinase. An adjacent rectangle is labeled 1,000 molecules. A dashed arrow points down to the lower left to a green circle enclosing a green triangle above and arrow that points from inactive glycogen phosphorylase italicized b end italics to active glycogen phosphorylase italicized a end italics. An adjacent rectangle is labeled 10,000 molecules. A dashed arrow points down to the lower left to a green circle enclosing a green triangle above and arrow that points from glycogen to active glucose 1-phosphate. An adjacent rectangle is labeled 100,000 molecules. An arrow points down to glucose, from which an arrow points out of the cell to blood glucose. An adjacent rectangle is labeled 100,000 molecules.
Several Mechanisms Cause Termination of the β-Adrenergic Response
To be useful, a signal-transducing system has to turn off after the hormonal or other stimulus has ended, and mechanisms for shutting off the signal are part of all signaling systems. Most systems also adapt to the continued presence of the signal by becoming less sensitive to it, by desensitizing. The β-adrenergic system illustrates both. Here, our focus is on termination.
The response to β-adrenergic stimulation will end when the concentration of the ligand (epinephrine) in the blood drops below the for its receptor. The epinephrine then dissociates from the receptor, and the latter reassumes its inactive conformation, in which it can no longer activate .
A second means of ending the response is the hydrolysis of GTP bound to the subunit, catalyzed by the GTPase activity of the G protein. Conversion of bound GTP to GDP favors the return of to the conformation in which it binds the subunits — the conformation in which the G protein is unable to interact with or stimulate adenylyl cyclase. This ends the production of cAMP. The rate of inactivation of depends on the GTPase activity, which for alone is very feeble. However, GTPase activator proteins (GAPs) strongly stimulate this GTPase activity, causing more-rapid inactivation of the G protein (Fig. 12-8). GAPs can themselves be regulated by other factors, providing a fine-tuning of the response to β-adrenergic stimulation. A third mechanism for terminating the response is to remove the second messenger: cAMP is hydrolyzed to -AMP (which is not active as a second messenger) by cyclic nucleotide phosphodiesterase (Fig. 12-4a, step ; 12-4b).
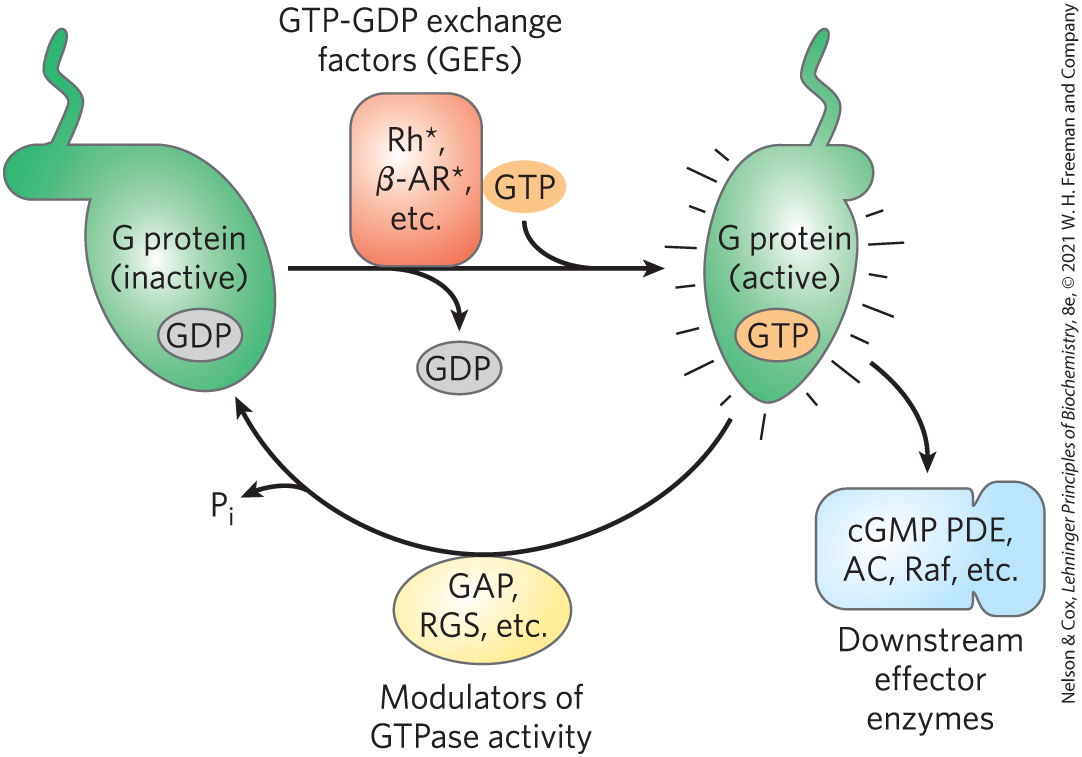
FIGURE 12-8 Factors that regulate G-protein activity. Inactive G proteins, both small G proteins such as Ras and heterotrimeric G proteins such as , interact with upstream GTP-GDP exchange factors (red). Often these exchange factors are activated (*) receptors such as rhodopsin (Rh) and β-adrenergic receptors (AR). The G proteins are activated by GTP binding, and in the GTP-bound form, activate downstream effector enzymes (blue), such as cGMP phosphodiesterase (PDE), adenylyl cyclase (AC), and Raf. By modulating the GTPase activity of G proteins, GTPase activator proteins (GAPs, in the case of small G proteins) and regulators of G-protein signaling (RGSs) (yellow), determine how long the G protein will remain active.
A green oval labeled G protein (inactive) has a horizontal protrusion to the upper left from which a thread extends upward and has a gray oval labeled G D P. An arrow pointing to the right has an arrow pointing away to show the loss of the G D P and is joined by an arrow from an orange oval labeled G T P. The G T P is attached to the right side of a vertical rectangle labeled R h asterisk, Greek letter beta-A R asterisk, etc. Text above the rectangle reads, G T P-G D P exchange factors (G E Fs). This produces an almost vertical green oval labeled G protein (active) with a small protrusion from its upper right, a thread extending upward, an orange oval labeled G T P, and short radiating lines all around it. Two arrows point away from this green oval. One arrow points to a rectangle with a vertical rectangle with rounded edges attached to its right side and labeled, c G M P P D E, A C, R a f, etc. Text below reads, Downstream effector enzymes. The second arrow curves clockwise above a yellow oval labeled G A P, R G S, etc. above text reading, Modulators of G T Pase activity. The arrow continues and splits to an arrow showing that P subscript I is lost and an arrow to the starting green oval labeled G protein (inactive).
Finally, at the end of the signaling pathway, the metabolic effects that result from phosphorylation of target enzymes by PKA are reversed by the action of phosphoprotein phosphatases, which hydrolyze phosphorylated Tyr, Ser, or Thr residues, releasing inorganic phosphate . About 190 genes in the human genome encode phosphoprotein phosphatases, fewer than the number (about 540) that encode protein kinases, reflecting the relative promiscuity of the phosphoprotein phosphatases. A single phosphoprotein phosphatase (PP1) dephosphorylates some 200 different phosphoprotein targets, including the β-adrenergic receptor and other GPCRs. Some phosphatases are known to be regulated; others may be constantly active. When [cAMP] drops and PKA returns to its inactive form (step in Fig. 12-4a), the balance between phosphorylation and dephosphorylation is tipped toward dephosphorylation by these phosphatases.
The β-Adrenergic Receptor Is Desensitized by Phosphorylation and by Association with Arrestin
The mechanisms for signal termination described above take effect when the stimulus ends. A different mechanism, desensitization, damps the response even while the signal persists. When the receptor remains occupied with epinephrine, β-adrenergic receptor kinase, or βARK, phosphorylates several Ser residues near the receptor’s carboxyl terminus, which is on the cytoplasmic side of the plasma membrane (Fig. 12-9). βARK is drawn to the plasma membrane by its association with the subunits and is thus positioned to phosphorylate the receptor. Receptor phosphorylation creates a binding site for the protein β-arrestin, or βarr (also called arrestin 2), and binding of β-arrestin blocks the sites in the receptor that interact with the G protein (Fig. 12-10). The binding of β-arrestin also facilitates the sequestration of receptor molecules and their removal from the plasma membrane by endocytosis into small intracellular vesicles (endosomes). The arrestin-receptor complex recruits clathrin and other proteins involved in vesicle formation, which initiate membrane invagination, leading to the formation of endosomes containing the adrenergic receptor. In this state, the receptors are inaccessible to epinephrine and therefore inactive. These receptor molecules are eventually dephosphorylated and returned to the plasma membrane, completing the circuit and resensitizing the system to epinephrine.
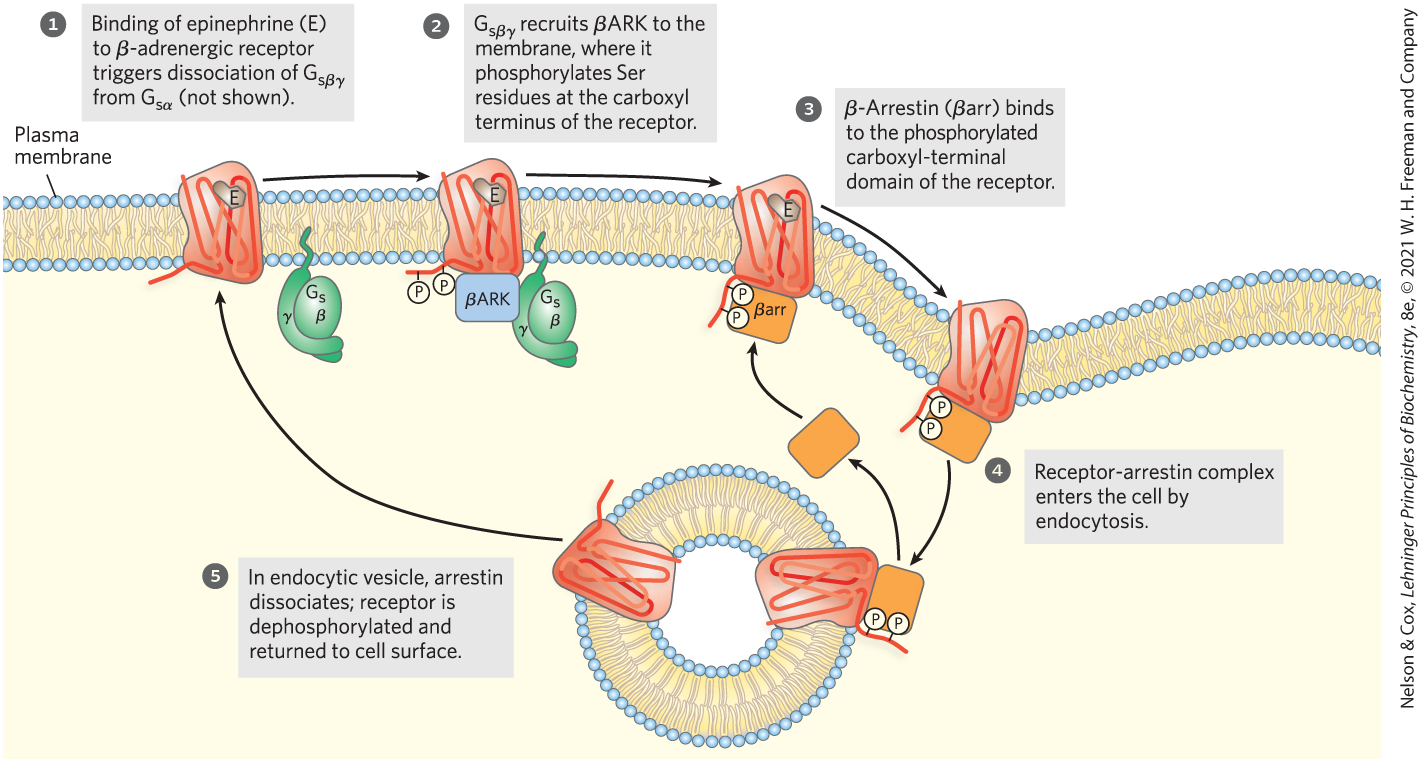
FIGURE 12-9 Desensitization of the β-adrenergic receptor in the continued presence of epinephrine. This process is mediated by two proteins: β-adrenergic protein kinase (βARK) and β-arrestin (βarr). Not shown here is the phosphorylation and activation of βARK by PKA. PKA is activated by the rise in [cAMP] in response to the initial signal (epinephrine).
A plasma membrane runs almost horizontally from left to right, with a slight dip on the right side. Step 1: Binding of epinephrine (E) to Greek letter beta-adrenergic receptor triggers dissociation of G subscript s Greek letter beta Greek letter gamma from G subscript s Greek letter alpha (not shown). A light red, roughly rectangular protein extends across the membrane. It contains a thread that begins at the upper right, runs up and down inside, and exits to the lower left. A hexagon labeled E with a protrusion from its lower left side is at the upper left side of the protein. A Greek oval labeled G subscript 2 and Greek letter beta has a small tail beneath that curves to the right and that runs behind the bottom right side of a crescent that has a small tail extending into the membrane from its upper right side. An arrow points to the right. Step 2: G subscript 2 Greek letter beta Greek letter gamma recruits Greek letter beta A R K to the membrane, where it phosphorylates S e r residues at the carboxyl terminus of the receptor. The rectangular protein in the membrane is still present with the molecule labeled E at its upper right. A blue rectangle labeled Greek letter beta uppercase A uppercase R uppercase K is against the bottom of the rectangular protein in the membrane. The thread extending from the lower left of the rectangular protein in the membrane has two circles labeled P extending from it. The green oval labeled G subscript s and Greek letter beta is still next to the green crescent labeled Greek letter gamma, which now has a piece at its middle left that runs slightly behind the blue rectangle labeled Greek letter beta uppercase A uppercase R uppercase K. An arrow points to the right. Step 3: Greek letter beta-arrestin (Greek letter beta a r r) binds to the phosphorylated carboxyl-terminal domain of the receptor. The rectangular protein in the membrane is still present with the molecule labeled E at its upper right. An orange rectangle labeled Greek letter beta lowercase a lowercase r lowercase r is against the bottom of the rectangular protein in the membrane. The thread extending from the lower left of the rectangular protein in the membrane still has two circles labeled P extending from it, but has now bent vertically so that the two circles labeled P are in the rectangle labeled Greek letter beta lowercase a lowercase r lowercase r. The membrane dips down at this point and an arrow points down to the inflection point before it begins to rise again. The rectangle in the membrane with the rectangle labeled Greek letter beta lowercase a lowercase r lowercase r is now in the inflection point. Step 4: Receptor-arrestin complex enters the cell by endocytosis. An arrow points down to an orange rectangle labeled Greek letter beta lowercase a lowercase r lowercase r that is on the outside of a vesicle and attached to a light red, rectangular protein to its left that extends across the membrane of the vesicle. A similar light red, rectangular protein with a thread extending from its top side extends across the membrane at the upper left side of the same vesicle. Step 5: In endocytic vesicle, arrestin dissociates; receptor is dephosphorylated and returned to cell surface.
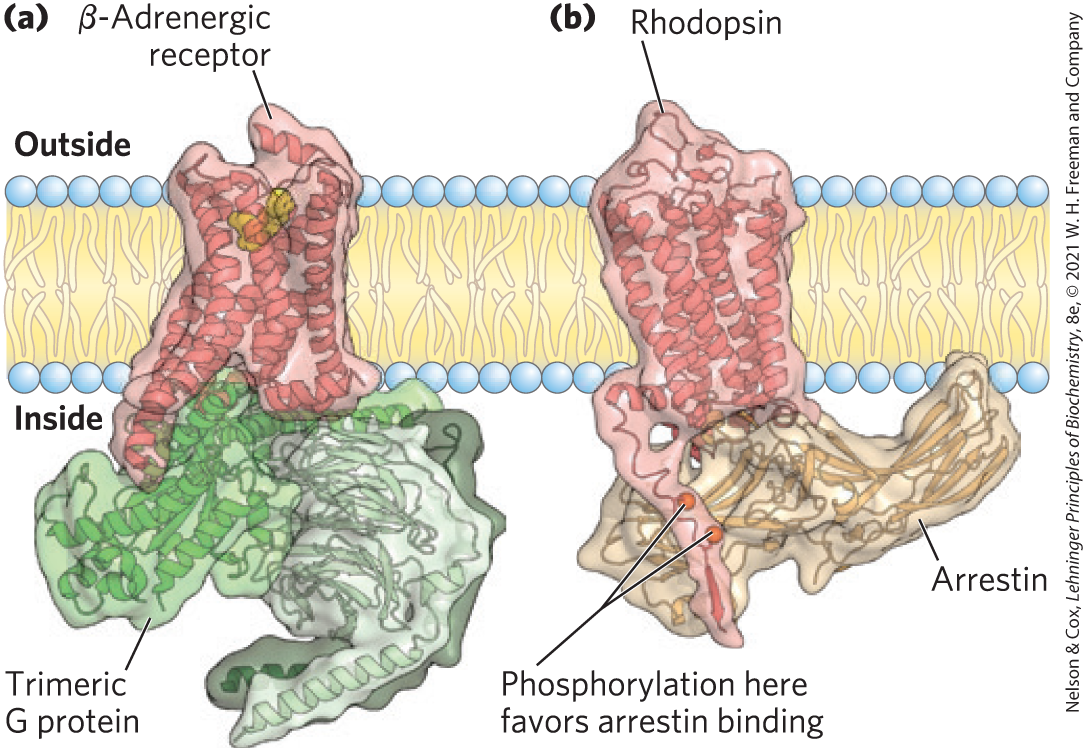
FIGURE 12-10 Mutual exclusion of trimeric G protein and arrestin in their interaction with a GPCR. (a) The β-adrenergic receptor complexed with its trimeric G protein, . (b) Another GPCR, the rhodopsin receptor, phosphorylated near its carboxyl terminus, and bound to arrestin. Binding of arrestin blocks binding and further activation of the G protein and ends the response to the initial signal. [Data from (a) PDB ID 3SN6, S. G. F. Rasmussen et al., Nature 477:549, 2011; (b) PDB ID 4ZWJ, Y. Kang et al., Nature 523:561, 2015.]
A horizontal piece of plasma membrane is shown with the area above labeled outside and the area below labeled inside. A roughly rectangular light red molecule labeled Greek letter beta-adrenergic receptor with mostly vertical helices inside extends across the membrane. A yellow molecule is visible near the top center. A bright green structure that resembles a thick rod angled slightly up to the right is beneath the light red protein and extends up higher in the middle than on the left and right sides of the bottom of the light red protein. It contains helices that are mostly horizontal or diagonal. A light green structure on the lower right is rounded and contains beta strands with a helix at the right side connected to a diagonal helix that forms a piece that extends to the lower left. A darker green piece is behind it and extends slightly above with a helix that extends slight above the helix of the light green piece to the lower left. The green structure is labeled trimeric green protein. Part b shows a similar light red protein with a piece labeled rhodopsin at the top. An irregular, roughly circular brown protein labeled arrestin is beneath the light red molecule and is wider to its left side with a thinner piece that extends diagonally up to the membrane to the right of the light red protein. The brown protein contains many beta strands. At the lower right of the light red protein, a strand extends down vertically over the brown molecule. Two red spheres in the center of this vertical piece are accompanied by text reading, phosphorylation here favors arrestin binding.
β-Adrenergic receptor kinase is a member of a family of G protein–coupled receptor kinases (GRKs), all of which phosphorylate GPCRs on their carboxyl-terminal cytoplasmic domains and play roles similar to that of βARK in desensitization and resensitization of their receptors. Seven GRKs and four different arrestins are encoded in the human genome; each GRK is capable of desensitizing a particular subset of GPCRs, and each arrestin can interact with many different types of phosphorylated receptors.
The receptor-arrestin complex has another important role: it initiates signaling by a second pathway, the MAPK cascade described below. Thus, acting through a single GPCR, epinephrine triggers two divergent signaling pathways. The two pathways, one initiated by the receptor’s interaction with a G protein and the other initiated by its interaction with arrestin, can be differentially affected by the agonist; in some cases, one agonist favors the G-protein pathway and another favors the arrestin pathway. This bias is an important consideration in the development of a medication that acts through a GPCR. For example, the most addictive of the opioid drugs of abuse act more strongly through G-protein signaling than through arrestin. An ideal opioid pain medication would act through the branch of the pathway that has therapeutic effects and not through the pathway that leads to addiction.
Cyclic AMP Acts as a Second Messenger for Many Regulatory Molecules
Epinephrine is just one of many hormones, growth factors, and other regulatory molecules that act by changing the intracellular [cAMP] and thus the activity of PKA. Table 12-3 lists a few examples. Glucagon binds to its receptors in the plasma membrane of adipocytes, activating (via a protein) adenylyl cyclase. PKA, stimulated by the resulting rise in [cAMP], phosphorylates and activates two proteins critical to the mobilization of the fatty acids of stored fats (see Fig. 17-2). Similarly, the peptide hormone ACTH (adrenocorticotropic hormone, also called corticotropin), produced by the anterior pituitary, binds to specific receptors in the adrenal cortex, activating adenylyl cyclase and raising the intracellular [cAMP]. PKA then phosphorylates and activates several of the enzymes required for the synthesis of cortisol and other steroid hormones. In many cell types, the catalytic subunit of PKA can also move into the nucleus, where it phosphorylates the cAMP response element binding protein (CREB), which alters the expression of specific genes regulated by cAMP.
| Corticotropin (ACTH) |
| Corticotropin-releasing hormone (CRH) |
| Dopamine [, ] |
| Epinephrine (β-adrenergic) |
| Follicle-stimulating hormone (FSH) |
| Glucagon |
| Histamine |
| Luteinizing hormone (LH) |
| Melanocyte-stimulating hormone (MSH) |
| Odorants (many) |
| Parathyroid hormone |
| Prostaglandins , |
| Serotonin |
| Somatostatin |
| Tastants (sweet, bitter) |
| Thyroid-stimulating hormone (TSH) |
| Note: Receptor subtypes in square brackets. Subtypes may have different transduction mechanisms. For example, serotonin is detected in some tissues by receptor subtypes and , which act through adenylyl cyclase and cAMP, and in other tissues by receptor subtype , acting through the phospholipase mechanism (see Table 12-4). |
Some hormones act by inhibiting adenylyl cyclase, thus lowering [cAMP] and suppressing protein phosphorylation by PKA. For example, the binding of somatostatin to its receptor in the pancreas leads to activation of an inhibitory G protein, or , structurally homologous to , that inhibits adenylyl cyclase and lowers [cAMP]. In this way, somatostatin inhibits the secretion of several hormones, including glucagon. In adipose tissue, prostaglandin (; see Fig. 10-17) inhibits adenylyl cyclase, thus lowering [cAMP] and slowing the mobilization of lipid reserves triggered by epinephrine and glucagon. In certain other tissues, stimulates cAMP synthesis: its receptors are coupled to adenylyl cyclase through a stimulatory G protein, . In tissues with -adrenergic receptors, epinephrine lowers [cAMP]; in this case, the receptors are coupled to adenylyl cyclase through an inhibitory G protein, . In short, an extracellular signal such as epinephrine or can have different effects on different tissues or cell types, depending on three factors: the type of receptor in the tissue, the type of G protein with which the receptor is coupled, and the set of PKA target enzymes in the cell. By summing the influences that tend to increase and decrease [cAMP], a cell achieves the integration of signals that is a general feature of signal-transducing mechanisms (Fig. 12-1f).
Another factor that explains how so many types of signals can be mediated by a single second messenger (cAMP) is the confinement of the signaling process to a specific region of the cell by adaptor proteins — noncatalytic proteins that hold together other protein molecules that function in concert (further described below). AKAPs (A kinase anchoring proteins) have multiple, distinct protein-binding domains, often intrinsically disordered regions; they are multivalent adaptor proteins. One domain binds to the R subunits of PKA and another binds to a specific structure in the cell, confining the PKA to the vicinity of that structure. For example, specific AKAPs bind PKA to microtubules, actin filaments, ion channels, mitochondria, or the nucleus. Different types of cells have different complements of AKAPs, so cAMP might stimulate phosphorylation of mitochondrial proteins in one cell and stimulate phosphorylation of actin filaments in another. In some cases, an AKAP connects PKA with the enzyme that triggers PKA activation (adenylyl cyclase) or terminates PKA action (cAMP phosphodiesterase or phosphoprotein phosphatase) (Fig. 12-11). The very close proximity of these activating and inactivating enzymes presumably achieves a highly localized, and very brief, response.
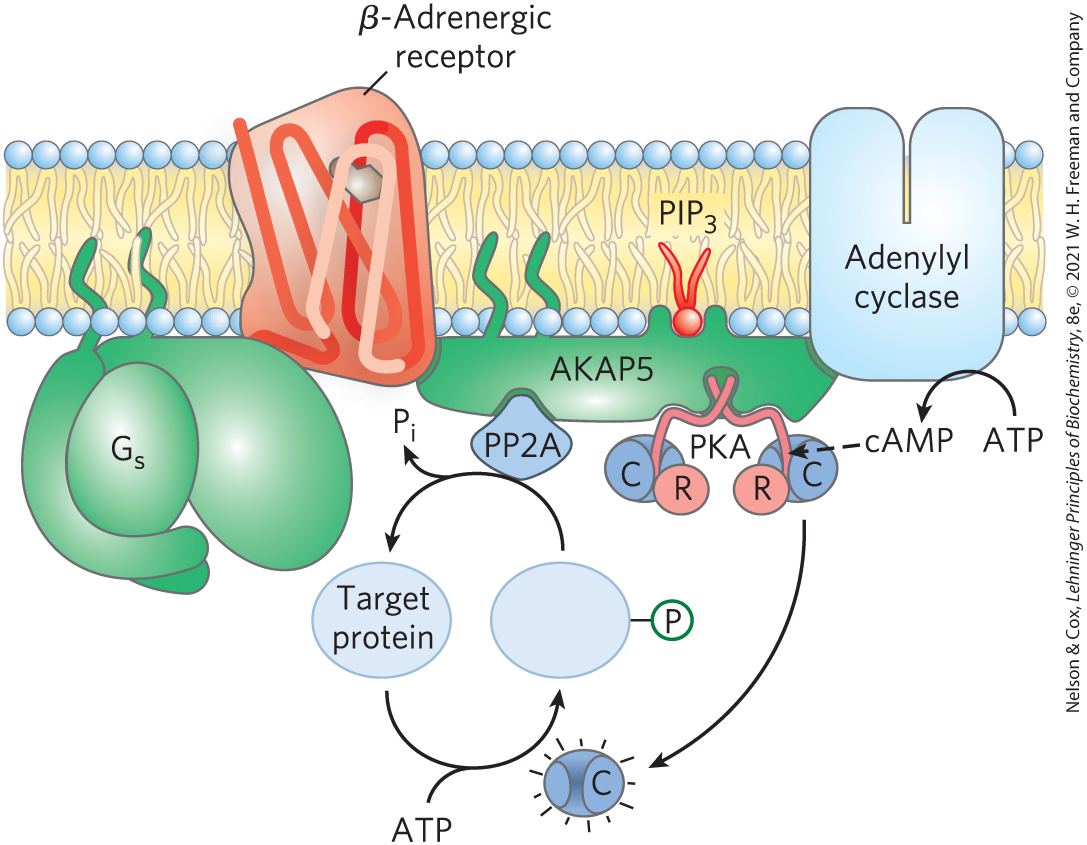
FIGURE 12-11 Nucleation of supramolecular complexes by A kinase anchoring proteins (AKAPs). AKAP5 is one of a family of proteins that act as multivalent scaffolds, holding PKA catalytic subunits — through interaction of the AKAP with the PKA regulatory subunits — in proximity to a particular region or structure in the cell. AKAP5 is targeted to rafts in the cytoplasmic face of the plasma membrane by two covalently attached palmitoyl groups and a site that binds phosphatidylinositol 3,4,5-trisphosphate in the membrane. AKAP5 also has binding sites for the β-adrenergic receptor, adenylyl cyclase, PKA, and a phosphoprotein phosphatase (PP2A), bringing them all together in the plane of the membrane. When epinephrine binds to the β-adrenergic receptor, adenylyl cyclase produces cAMP, which reaches the nearby PKA quickly and with very little dilution. PKA phosphorylates its target protein, altering its activity, until the phosphoprotein phosphatase removes the phosphoryl group and returns the target protein to its prestimulus state. The AKAPs in this and other cases bring about a high local concentration of enzymes and second messengers, so that the signaling circuit remains highly localized and the duration of the signal is limited.
A horizontal piece of membrane has a light red vertical rectangle labeled Greek letter beta-adrenergic receptor extending through its left side. This rectangle contains a thread that begins at its upper left and moves up and down. A hexagon with a protrusion on its left side is at the upper left of the rectangle. A large green oval extends almost horizontally under the left side of the light red rectangle and has a thread extending into the membrane from its upper left side, where it contacts a crescent-shaped green piece that has a thread extending into the membrane from its upper right and curves around an oval labeled G subscript s between it and the larger, almost horizontal oval to its right. On the right side of the membrane, there is a rectangle with its top half divided vertically in half labeled adenylyl cyclase. Between the Greek letter beta-adrenergic receptor and adenylyl cyclase, there is a long horizontal molecule labeled A K A P 5 that runs beneath the membrane with two threads extending into the membrane on its left side. On its right side, it has two protrusions between two membrane phosphate heads with a red sphere between them that has two red tails extending into the membrane that is labeled P I P subscript 3. Two threads labeled P K A cross in a cutout in the lower right side of A K A P 5. The left side runs horizontally and slightly up to the left against a cutout in A K A P 5, then bends vertically down to run through a groove in a blue sphere labeled C to end in a light red sphere labeled R. The right side runs horizontally and slightly up to the right against a cutout in A K A P 5, then bends vertically down to run through a groove in a blue sphere labeled C to end in a light red sphere labeled R. In a blunt triangular cutout in the lower left of A K A P 5, there is a blue protein labeled P P 2 A with an oval bottom with a blunt triangular protrusion. A light blue oval beneath P P 2 A has a green circle labeled P attached to its right side and a curved arrow above that runs against P P 2 A before splitting to show the loss of P subscript 1 and production of a light blue oval labeled target protein. A second curved arrow extends down from the target protein and is joined by A T P to produce the original light blue oval. A T P has a curved arrow through the lower right of adenylyl cyclase to produce c A M P, from which a dashed arrow points to the red thread of P K A. An arrow points down from the adjacent sphere labeled C to beneath the light blue oval bound to a green circle labeled P, where there is a similar blue sphere with short lines radiating from all sides.
In a process analogous with the cAMP-PKA pathway, a cyclic derivative of GTP (,-cyclic GMP; cGMP) is generated in response to an extracellular signal. The cGMP activates a cGMP-dependent protein kinase (PKG), which phosphorylates specific protein substrates, changing their activities in response to the initial signal (Box 12-2).
Box 12-2 MEDICINE
Receptor Guanylyl Cyclases, cGMP, and Protein Kinase G
Guanylyl cyclases (Fig. 1) are receptor enzymes that, when activated, convert GTP to the second messenger guanosine ,-cyclic monophosphate (cGMP) (Fig. 2). Many of the actions of cGMP in animals are mediated by cGMP-dependent protein kinase, also called protein kinase G (PKG). On activation by cGMP, PKG phosphorylates Ser and Thr residues in target proteins. The catalytic and regulatory domains of this enzyme are in a single polypeptide ( ~80,000). Part of the regulatory domain fits snugly in the substrate-binding cleft. Binding of cGMP forces this activation loop out of the binding site, opening the site to PKG target proteins.
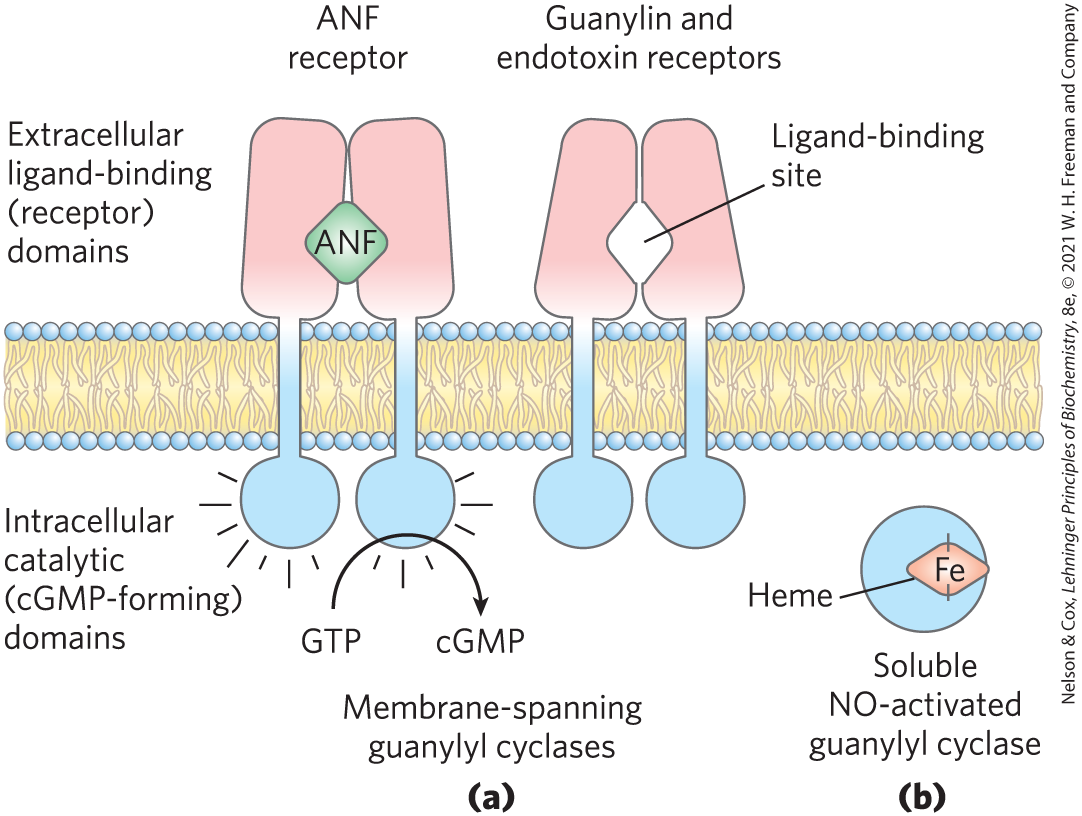
FIGURE 1 Two types of signal-transducing guanylyl cyclase. (a) Membrane-spanning guanylyl cyclases such as the ANF and guanylin receptors are homodimers with an extracellular ligand-binding domain and an intracellular guanylyl cyclase domain. (b) A soluble heme-containing guanylyl cyclase is activated by intracellular NO.
A horizontal piece of membrane shows the extracellular ligand-binding (receptor) domains of two receptors above the membrane and the intracellular catalytic (c G M P-forming) domains below the membrane. Part a is labeled membrane-spanning guanylyl cyclases. The left-hand structure is labeled A N F receptor. It has two light red rectangles above the membrane that each bend slightly toward the center, so they meet on top and are separate at the bottom. A green diamond labeled A N F is between them just below the midpoint of the structure. Each has a blue vertical line that extends down across the membrane. These blue lines each form a sphere beneath the membrane with short lines radiating from all sides. G T P is shown with a curved line running through the bottom of the right-hand blue circle to c G M P. The right-hand molecule is labeled guanylin and endotoxin receptors. It has a similar structure, except that the two light red halves each have cutouts to form a diamond-shaped opening just below the midpoint labeled ligand-binding site. Part b is labeled soluble N O-activated guanylyl cyclase. It shows a blue circle containing a light red diamond labeled F e inside the circle with its right side vertex joined to the right side of the circle. This light red diamond is labeled heme.
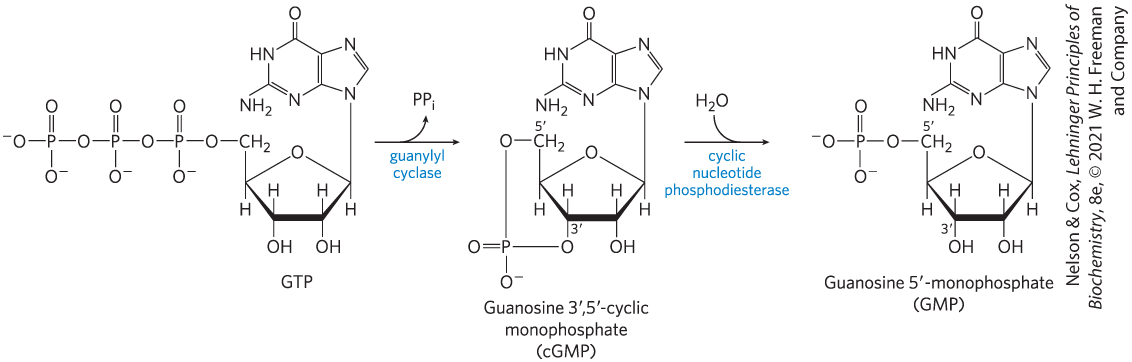
FIGURE 2 Synthesis of cGMP by guanylyl cyclase and its hydrolysis by cGMP phosphodiesterase.
G T P has a five-membered ring with O substituted for C at position 6 at the top vertex, C 1 prime bonded to N of the lower right vertex of a five-membered ring above and to H below, C 2 prime and C 3 prime bonded to H above and O H below, C 4 prime bonded to C 5 prime above and to H below, and C 5 prime bonded to 2 H and to O bonded to P double bonded to O above, bonded to O minus below, and bonded to P to the left double bonded to O above, bonded to O minus below, and bonded to P on the left double bonded to O above, bonded to O minus below, and bonded to O minus to the left. The five-membered ring shares its left side with a six-membered ring. It has N substituted for C at its upper right vertex at position 7, a double bond at its upper right side between positions 7 and 8, a double bond at its left side between positions 4 and 5, and N substituted for C at its lower right vertex at position 9 and further bonded to C 1 prime of the ring below. The six-membered ring has double bond at its right side between positions 4 and 5 shared with the five-membered ring, a double bond at its lower left side between positions 2 and 3, N substituted for C at its upper left vertex at position 1 and bonded to H, N substituted for C at its bottom vertex at position 3, its lower left vertex at position 2 bonded to N H 2, and its top vertex, C 6, double bonded to O. An arrow pointing right is labeled guanylyl cyclase and has an arrow from it showing that P P subscript 1 leaves. This yields guanosine 3 prime, 5 prime-cyclic monophosphate (c G M P). It has a similar structure to G T P except that C 3 prime is bonded to H above and to O below that is bonded to P to the left that is bonded to O minus below, double bonded to O to the left, and bonded to O above that is bonded to C 5 prime on the right that is bonded to 2 H and to C 4 prime of the ring below. An arrow pointing right is labeled cyclic nucleotide phosphodiesterase and is joined by an arrow representing the addition of H 2 O. This yields guanosine 5 prime-monophosphate. It has a similar structure except that C 3 prime is bonded to H above and O H below and C 5 prime is bonded to 2 H and to O bonded to P double bonded to O above and bonded to O minus below and to the left.
Cyclic GMP carries different messages in different tissues. In cardiac muscle (a type of smooth muscle), it signals relaxation. In the kidney and the intestine, it triggers changes in ion transport and water retention. Guanylyl cyclase in the kidney is activated by the peptide hormone atrial natriuretic factor (ANF; Fig. 1a), which is released by cells in the cardiac atrium when the heart is stretched by increased blood volume. Carried in the blood to the kidney, ANF activates guanylyl cyclase in cells of the collecting ducts. The resulting rise in [cGMP] triggers increased renal excretion of and consequently of water, driven by the change in osmotic pressure. Water loss reduces the blood volume, countering the stimulus that initially led to ANF secretion. Vascular smooth muscle also has an ANF receptor–guanylyl cyclase; on binding to this receptor, ANF causes relaxation (vasodilation) of the blood vessels, which increases blood flow while decreasing blood pressure.
A similar receptor guanylyl cyclase in the plasma membrane of epithelial cells lining the intestine is activated by the peptide guanylin (Fig. 1a), which regulates secretion in the intestine. This receptor is also the target of a heat-stable peptide endotoxin produced by Escherichia coli and other gram-negative bacteria. The elevation in [cGMP] caused by the endotoxin increases secretion and consequently decreases reabsorption of water by the intestinal epithelium, producing diarrhea.
A distinctly different type of guanylyl cyclase is a soluble cytosolic protein with a tightly associated heme group (Fig. 1b), an enzyme activated by nitric oxide (NO). Nitric oxide is produced from arginine by -dependent NO synthase, present in many mammalian tissues, and diffuses from its cell of origin into nearby cells (see Chapter 22).
In the target cell, NO binds to the heme group of guanylyl cyclase and activates cGMP production. In the heart, cGMP-dependent protein kinase reduces the forcefulness of contractions by stimulating the ion pump(s) that remove from the cytosol. NO-induced relaxation of cardiac muscle is the same response brought about by nitroglycerin and other nitrovasodilators taken to relieve angina pectoris, the pain caused by contraction of a heart deprived of because of blocked coronary arteries. Nitric oxide is unstable and its action is brief; within seconds of its formation, it undergoes oxidation to nitrite or nitrate. Nitrovasodilator drugs produce long-lasting relaxation of cardiac muscle because they break down over several hours, yielding a steady stream of NO. The value of nitroglycerin as a treatment for angina was discovered serendipitously in factories producing nitroglycerin as an explosive in the 1860s. Workers with angina reported that their condition was much improved during the workweek but worsened on weekends. The physicians treating these workers heard this story so often that they made the connection, and a drug was born.
The effects of increased cGMP synthesis diminish after the stimulus ceases, because a specific phosphodiesterase (cGMP PDE) converts cGMP to the inactive -GMP (see Fig. 2). Humans have several isoforms of cGMP PDE, with different tissue distributions. The isoform in the blood vessels of the penis is inhibited by the drugs sildenafil (Viagra) and tadalafil (Cialis), which therefore cause [cGMP] to remain elevated once raised by an appropriate stimulus, accounting for the usefulness of this drug in the treatment of erectile dysfunction.
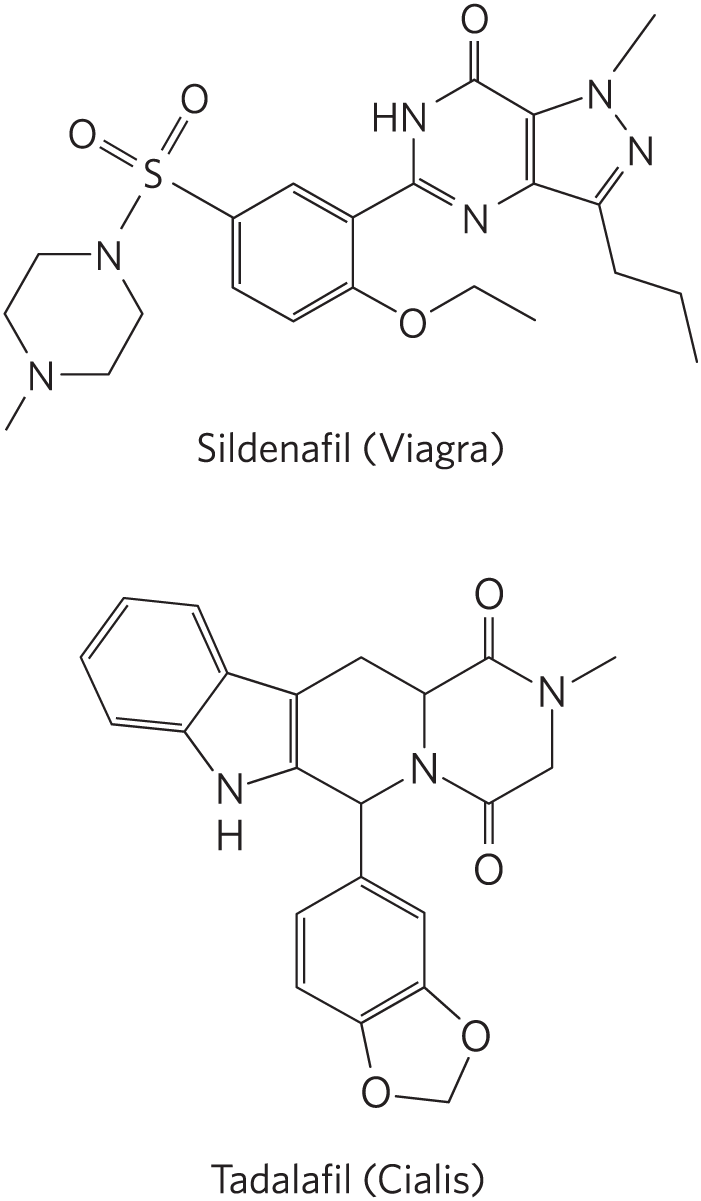
Sildenafil (Viagra) has a benzene ring with three substituents: the upper left vertex is bonded to S double bonded to O to the upper left and upper right and bonded to N to its lower left that is substituted for C at the top right vertex of a six-membered ring with another N substituted for C at its lower left vertex and bonded to C H 3; the lower right vertex is bonded to O further bonded to a two-carbon chain; and the upper right vertex is bonded to the lower left vertex of a six-membered ring that shares its right side with a five-membered ring. The six-membered ring has N substituted for C at its bottom vertex, N substituted for C at its upper left vertex and bonded to H, double bonds at its lower left side and right side, and its top vertex double bonded to O. The five-membered ring has a double bond on its left side shared with the six-membered ring, a double bond at its lower right side, N substituted for C at its upper right vertex and bonded to C H 3, N substituted for C at its right side vertex, and its lower right vertex bonded to a three-carbon chain. Tadalafil (Cialis) has a benzene ring that shares the double bond at its lower right side with a five-membered ring that has O at its right side and lower left vertices and C H 2 at its lower right vertex. The top vertex of the benzene ring is bonded to the bottom vertex of a six-membered ring above that shares its right side with a six-membered ring and a double bond on its left side with a five-membered ring and that has N substituted for C at its lower right vertex shared with the right-hand six-membered ring. The right-hand six-membered ring also has N substituted for C at its upper right vertex and bonded to C H 3 and its top and bottom vertices both double bonded to O. The five-membered ring on the left has N substituted for C at its bottom vertex and bonded to H and its upper left side shared with a benzene ring.
G Proteins Act as Self-Limiting Switches in Many Processes
Proteins sensitive to the binding of either GTP or GDP play critical roles in many cellular processes, including sensory perception, signaling for cell division, growth and differentiation, intracellular movements of proteins and membrane vesicles, and protein synthesis. The human genome encodes nearly 200 of these proteins, which differ in size and subunit structure, intracellular location, and function. But all G proteins share a common feature: they can become activated by binding GTP and then, after a brief period, can inactivate themselves with their GTPase activity, thereby serving as molecular binary switches with built-in timers. This superfamily of proteins includes the trimeric G proteins involved in β-adrenergic signaling ( or ) and vision (transducin); small, monomeric G proteins such as that involved in insulin signaling (Ras; see below) and others that function in vesicle trafficking (ARF, RAC1, and Rab), transport into and out of the nucleus (Ran), and timing of the cell cycle (Rho); and several proteins involved in protein synthesis (initiation factor IF2 and elongation factors EF-Tu and EF-G; see Chapter 27). Among the trimeric G proteins, subunits have covalently linked lipids: the amino terminus is palmitoylated, and some are also myristoylated. and the monomeric Ras protein have an isoprenyl lipid at their carboxyl termini. The attached lipids keep them anchored to the plasma membrane, restricting their action to that two-dimensional plane.
All G proteins have the same core structure and use the same mechanism for switching between an inactive conformation, favored when GDP is bound, and an active conformation, favored when GTP is bound. We can use Ras (~20 kDa, a minimal signaling unit) as a prototype for all members of this superfamily (Fig. 12-12).
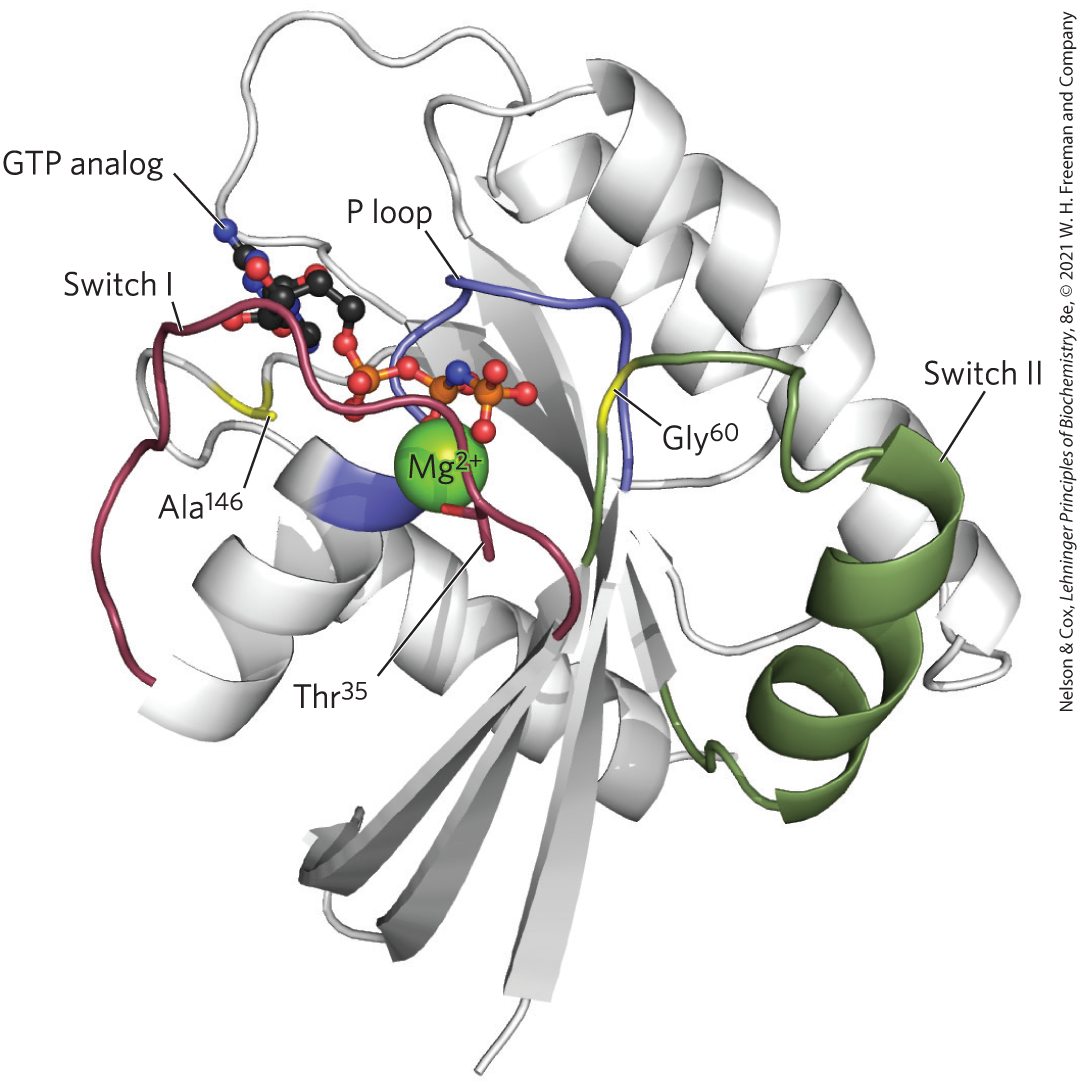
FIGURE 12-12 Ras, the G-protein prototype. -GTP is held by critical residues in the phosphate-binding P loop (blue) and by in the switch I region (red) and in the switch II region (green). gives specificity for GTP over ATP. In the structure shown here, the nonhydrolyzable GTP analog Gpp(NH)p is in the GTP-binding site. [Data from PDB ID 5P21, E. F. Pai et al., EMBO J. 9:2351, 1990.]
The ribbon structure shows a ball-and-stick model on the left side with highlighted strands around it that interact with a G T P analog and with M g 2 plus. The ribbon structure has a short white helix at the lower left that extends diagonally up to the right to a top coil that is purple and that contacts a green sphere labeled M g 2 plus at its upper right end. Another white helix extends diagonally down to the lower right behind it, running behind three beta strands. At the right side, a green thread leads to a short green helix that runs up and slightly to the right, where it overlaps with a piece near the bottom of a long white helix that runs up toward the left to end near the top center of the protein. The long white helix has a short helix that runs parallel and slightly behind it near its top. The green helix is labeled switch Roman numeral 2 and has a strand extending rom its top left into the center, where it becomes purple as it crosses a vertical purple strand behind and then bends down to the left with a purple region labeled G l y superscript 60 before becoming green again continuing down to meet the beta strands below. To the left of the strand with the G l y superscript 60, there are two beta strands running almost vertically up to the center and slightly to the left. Farther to the left, beneath the top ends of these two beta strands, there is a ball-and stick model labeled G T P analog that runs up and to the left with the green sphere labeled M g plus below its lower right. A wavy red strand runs across the M g 2 plus and curves down to meet one of the three beta strands at the bottom center to its lower right and bends upward to run along the lower half of the G T P analog before bending down to run almost vertically along the left side, where it is labeled switch Roman numeral 1. A white strand loops behind the red strand labeled switch Roman numeral 1 and has a yellow region labeled A l a superscript 1 46 that is beneath the G T P analog and about halfway between the left and right halves of the red strand that loops up along the G T P analog above. Where the red strand runs across M g 2 plus, there are two red sticks. The upper red stick runs along the bottom of the M g 2 plus and the lower one runs down and to the right and is labeled T h r superscript 35. The G T P analog has a chain of three phosphates running above M g 2 plus and a carbon chain on the left side.
In the nucleotide-binding site of Ras, hydrogen bonds to the guanine oxygen, allowing GTP, but not ATP, to bind. In the GTP-bound conformation, the G protein exposes previously buried regions (called switch I and switch II) that interact with proteins downstream in the signaling pathway until the G protein inactivates itself by hydrolyzing its bound GTP to GDP. The critical determinant of G-protein conformation is the γ phosphate of GTP, which interacts with a region called the P loop (phosphate-binding). In Ras, the γ phosphate of GTP binds to a Lys residue in the P loop and to two critical residues, in switch I and in switch II, that hydrogen-bond with the oxygens of the γ phosphate of GTP. These hydrogen bonds act like a pair of springs holding the protein in its active conformation (Fig. 12-13). When GTP is cleaved to GDP, and is released, these hydrogen bonds are lost; the protein then relaxes into its inactive conformation, burying switch I and II so they are no longer available to interact with other partners.
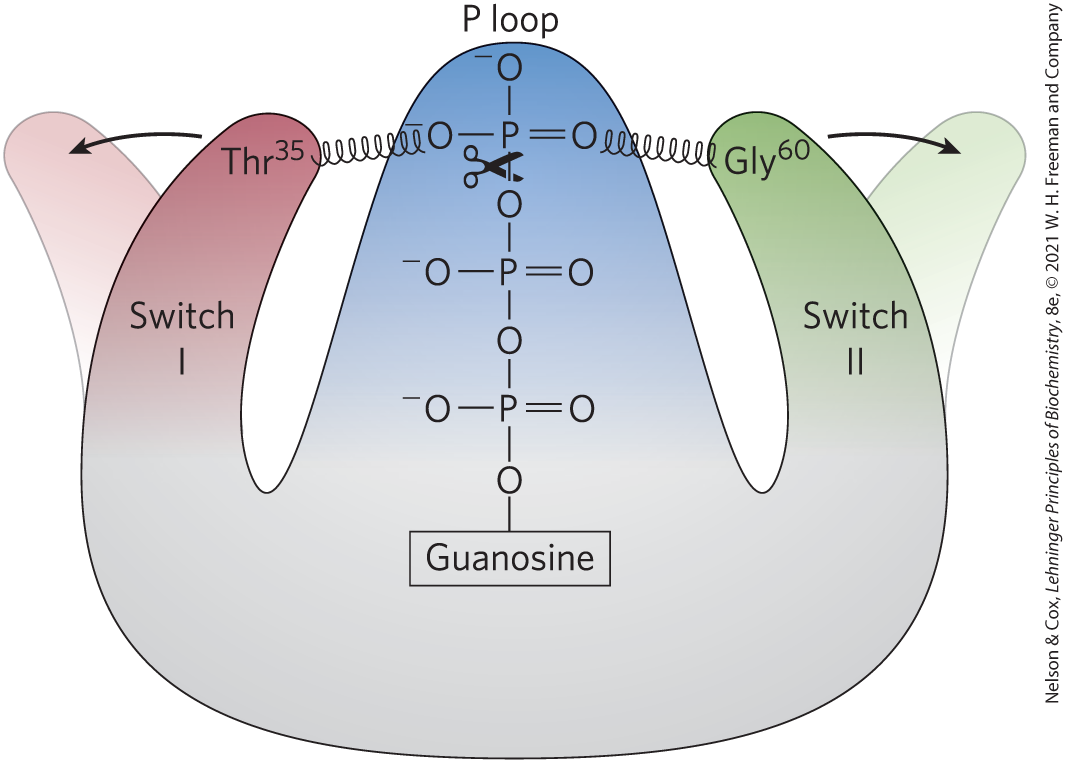
FIGURE 12-13 GTP hydrolysis flips the switches in Ras. When bound GTP is hydrolyzed by the GTPase activities of Ras and its GTPase activator protein (GAP), loss of hydrogen bonds to and allows the switch I and switch II regions to relax into a conformation in which they are no longer available to interact with downstream targets. [Information from I. R. Vetter and A. Wittinghofer, Science 294:1299, 2001, Fig. 3.]
A blunt pyramidal structure in blue is labeled P loop at its peak, which contains a phosphate group of G T P. The pyramidal structure has crescent-shaped pieces that curve up to the left and right. The pyramid is gray at the bottom and increasingly blue toward the top. The left-hand piece, labeled switch Roman numeral 1, curves to the left, where it is gray, and then curves back to the right as it becomes increasingly light red. It ends at T h r superscript 35 with a helix extending right to O minus of the top P of G T P in the peak of the pyramid. The right-hand piece, labeled switch Roman numeral 2, curves to the right, where it is gray, and then curves back to the left as it becomes increasingly green. It ends at G l y superscript 60 with a helix extending left to the right-hand O minus of the top P of G T P in the peak of the pyramid. A rectangle labeled guanosine in the gray region at the bottom center of the structure has three phosphates in a vertical chain above it. It is bonded to O bonded to P double bonded to O on the right, bonded to O minus on the left, and bonded to P above double bonded to O on the right, bonded to O minus on the left, and bonded to O above that is bonded to the final P by a bond with scissors across it. This P is double bonded to O on the left connected by a helix to T h r superscript 35 in the left-hand piece and to O on the right connected by a helix to G l y superscript 60 in the right-hand piece. The top of the left-hand and right hand pieces each have an arrow pointing to a faded piece showing how they bend outward when the bond between O and P is cut in the center piece.
The GTPase activity of most G proteins is very weak, but it is increased up to -fold by GTPase activator proteins (GAPs), also called, in the case of heterotrimeric G proteins, regulators of G-protein signaling (RGSs; Fig. 12-8). GAPs and RGSs thus determine how long the G-protein switch remains on. There are about 40 different RGS proteins associated with a variety of processes and expressed in most tissues. They have a critical Arg residue that reaches into the G-protein GTPase active site and contributes to catalysis. The intrinsically slow process of replacing bound GDP with GTP, switching the protein on, is catalyzed by guanosine nucleotide–exchange factors (GEFs, such as the β-adrenergic receptor) associated with the G protein. The ligand-bound β-adrenergic receptor is one of many GEFs, and a broad range of proteins act as GAPs. Their combined effects set the level of GTP-bound G proteins, and thus the strength of the response to signals that arrive at the receptors.
Because G proteins play crucial roles in so many signaling processes, it is not surprising that defects in G proteins lead to a variety of diseases. In about 25% of all human cancers (and in a much higher proportion of certain types of cancer), a mutation in a Ras protein — typically in one of the critical residues around the GTP-binding site or in the P loop — virtually eliminates its GTPase activity. Once activated by GTP binding, this Ras protein remains constantly active, promoting cell division in cells that should not divide. The tumor suppressor gene NF1 encodes a GAP that enhances the GTPase activity of normal Ras. Mutations in NF1 that result in a nonfunctioning GAP leave Ras with only its GTPase activity, which is very weak (that is, has a very low turnover number); once activated by GTP binding, Ras stays active for an extended period, continuing to send the signal: divide.
Defective heterotrimeric G proteins can also lead to disease. Mutations in the gene that encodes the α subunit of (which mediates changes in [cAMP] in response to hormonal stimuli) may result in a that is permanently active or permanently inactive. “Activating” mutations generally occur in residues crucial to GTPase activity; they lead to a continuously elevated [cAMP], with significant downstream consequences, including undesirable cell proliferation. Such mutations are found in about 40% of pituitary tumors (adenomas). Individuals with “inactivating” mutations in are unresponsive to hormones (such as thyroid hormone) that act through cAMP. Mutation in the gene for the transducin α subunit , which is involved in visual signaling, leads to a type of night blindness, apparently due to defective interaction between the activated subunit and the phosphodiesterase of the rod outer segment (see Fig. 12-19). A sequence variation in the gene encoding the β subunit of a heterotrimeric G protein is commonly found in individuals with hypertension (high blood pressure), and this variant gene is suspected of involvement in obesity and atherosclerosis.
The pathogenic bacterium that causes cholera produces a toxin that targets a G protein, interfering with normal signaling in host cells. Cholera toxin, secreted by Vibrio cholerae in the intestine of an infected person, is a heterodimeric protein. Subunit B recognizes and binds to specific gangliosides on the surface of intestinal epithelial cells and provides a route for subunit A to enter these cells. After entry, subunit A is broken into two fragments, A1 and A2. A1 associates with the host cell’s ADP-ribosylation factor ARF6, a small G protein, through residues in its switch I and switch II regions — which are accessible only when ARF6 is in its active (GTP-bound) form. This association with ARF6 activates A1, which catalyzes the transfer of ADP-ribose from to the critical Arg residue in the P loop of the α subunit of (Fig. 12-14). ADP-ribosylation blocks the GTPase activity of and thereby renders permanently active. This results in continuous activation of the adenylyl cyclase of intestinal epithelial cells, chronically high [cAMP], and chronically active PKA. PKA phosphorylates the CFTR channel (see Box 11-2) and a exchanger in the intestinal epithelial cells. The resultant efflux of NaCl triggers massive water loss through the intestine as cells respond to the ensuing osmotic imbalance. Severe dehydration and electrolyte loss are the major pathologies in cholera. These can be fatal in the absence of prompt rehydration therapy.
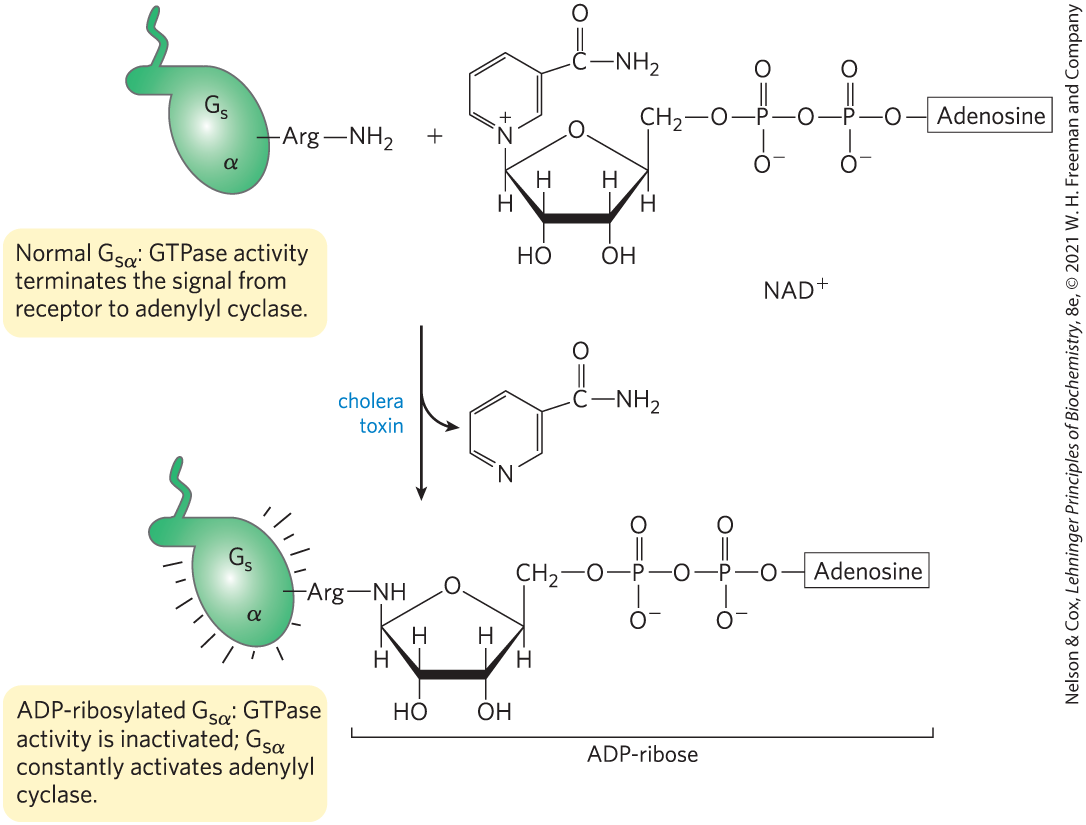
FIGURE 12-14 ADP-ribosylation locks in the active conformation. The bacterial toxin that causes cholera is an enzyme that catalyzes transfer of the ADP-ribose moiety of (nicotinamide adenine dinucleotide) to an Arg residue of . The G proteins thus modified fail to respond to normal hormonal stimuli. The pathology of cholera results from defective regulation of adenylyl cyclase and overproduction of cAMP.
The top part of the figure is accompanied by text reading, normal G subscript s Greek letter alpha: G T Pase activity terminates the signal from receptor to adenylyl cyclase. A green oval labeled G subscript s and Greek letter alpha has a protrusion to the upper left with a vertical tail extending up and a bond from its right side to A r g bonded to N H 2. It is added to a molecule labeled N A D plus that has the following structure. A five-membered ring has O substituted for C at position 6 at the top vertex, C 1 prime at its left side vertex bonded to N plus at the bottom vertex of a six-membered ring above and to H below, C 2 prime and C 3 prime bonded to H above and O H below, C 4 prime bonded to C 5 prime above and to H below, and C 5 prime bonded to 2 H and to O bonded to P double bonded to O above, bonded to O minus below, and bonded to P to the left double bonded to O above, bonded to O minus below, and bonded to O to the right that is further bonded to adenosine. The six-membered ring has double bonds at its upper left, lower left, and right sides, N plus substituted for C at its bottom vertex and further bonded to C 1 prime of the five-membered ring below, and its upper right vertex bonded to C double bonded to O and bonded to N H 2. A downward arrow labeled cholera toxin has an arrow branching off to show the loss of a six-membered ring that has double bonds at its upper left, lower left, and right sides, N substituted for C at its bottom vertex, and its upper right vertex bonded to C double bonded to O and bonded to N H 2. The bottom part showing the product of this reaction is accompanied by text reading, A D P-ribosylated G subscript s Greek letter alpha: G T Pase activity is inactivated; G subscript s Greek letter alpha constantly activates adenylyl cyclase. A green oval labeled G subscript s and Greek letter alpha has a protrusion to the upper left with a vertical tail extending up and a bond from its right side to A r g bonded to N bonded to C 1 prime of a five-membered ring below and to H. The rest of the molecule, beginning with the five-membered ring, is labeled A D P-ribose. It has the same structure as N A D plus above except that C 1 prime is now bonded to N H bonded to A r g bonded to the green oval instead of to N of a six-membered ring.
Diacylglycerol, Inositol Trisphosphate, and Have Related Roles as Second Messengers
A second broad class of GPCRs is coupled through a G protein to a plasma membrane phospholipase C (PLC) that catalyzes cleavage of the membrane phospholipid phosphatidylinositol 4,5-bisphosphate, or (see Fig. 10-14). When one of the agonists (hormone, neurotransmitter, growth factor; Table 12-4) that act by this mechanism binds its specific receptor in the plasma membrane (Fig. 12-15, step ), the receptor-hormone complex catalyzes GTP-GDP exchange on an associated trimeric G protein, (step ). This activates the proteins in much the same way that the β-adrenergic receptor activates (Fig. 12-4). The activated activates the -specific PLC (Fig. 12-15, step ), which catalyzes the production of two potent second messengers (step ), diacylglycerol and inositol 1,4,5-trisphosphate, or (not to be confused with , p. 435 or Fig. 12-24).
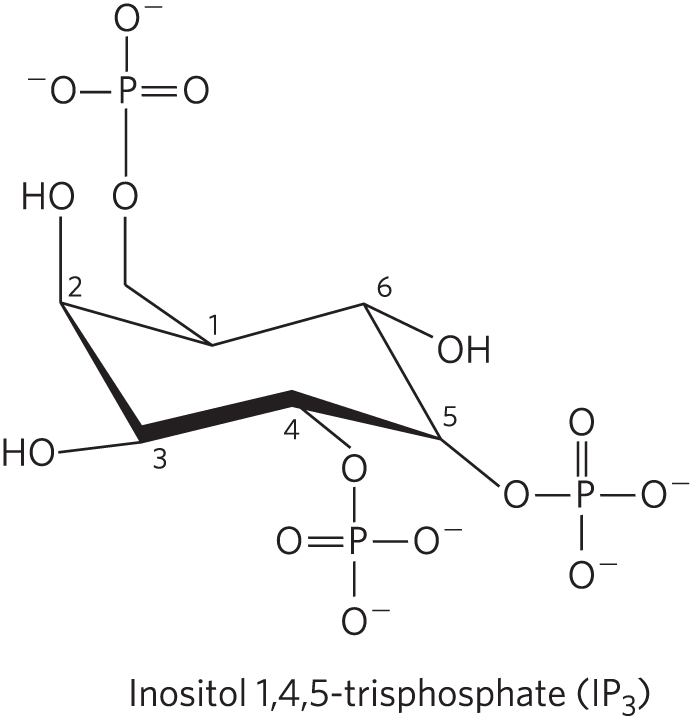
A chair conformation of a six-membered ring is shown with all of the carbons numbered and with C 1 at the upper middle vertex. C 1 has an up-equatorial bonded to O bonded to P double bonded to O to the right and bonded to O minus above and to the left; C 2 has an up-axial bond to O H; C 3 has an up-equatorial bond to O H; C 4 has a down-equatorial bond to O bonded to P double bonded to O to the left and bonded to O minus below and to the right; C 5 has a down-equatorial bond to O bonded to P double bonded to O to the left and bonded to O minus below and to the right; and C 6 has a down-equatorial bond to O H.
| Acetylcholine [muscarinic ] | Gastrin-releasing peptide | Platelet-derived growth factor (PDGF) |
| -Adrenergic agonists | Glutamate | Serotonin |
| Angiogenin | Gonadotropin-releasing hormone (GRH) | Thyrotropin-releasing hormone (TRH) |
| Angiotensin II | Histamine | Vasopressin |
| ATP | Light (Drosophila) | |
| Auxin | Oxytocin | |
| Note: Receptor subtypes are in square brackets; see footnote to Table 12-3. | ||
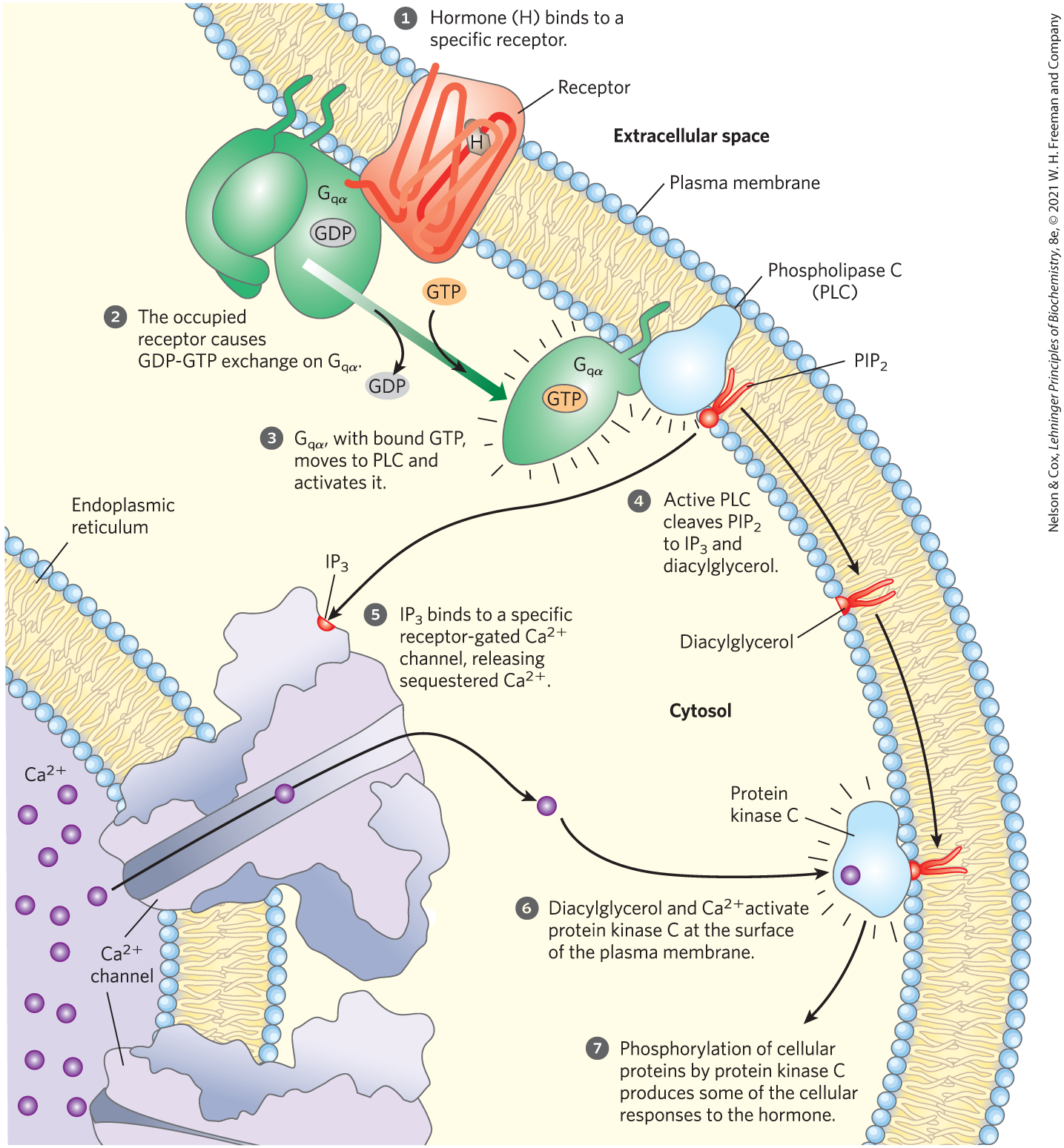
FIGURE 12-15 Hormone-activated phospholipase C and . Two intracellular second messengers are produced in the hormone-sensitive phosphatidylinositol system: inositol 1,4,5-trisphosphate and diacylglycerol are cleaved from phosphatidylinositol 4,5-bisphosphate . Both contribute to the activation of protein kinase C. By raising cytosolic , also activates other -dependent enzymes; thus also acts as a second messenger.
A piece of cell membrane curves down from the upper left to the lower right of the illustration. Step 1: Hormone (H) binds to a specific receptor. A roughly rectangular protein labeled receptor extends across the membrane. A strand begins at the upper left and bends up and down within the receptor, running above and below a small brown hexagon with a protrusion to the upper left labeled H, and then leaves the protein at the lower left to end at C within a green oval labeled G subscript q Greek letter alpha and with a small gray oval inside labeled G D P. The oval extends to the left from its top side, where a thread extends up halfway through the membrane, and it joins a green crescent-shaped piece labeled G subscript s Greek letter gamma that has a thread extending into the membrane at its upper right side, then curves to the left, and then wraps back to the right beneath a green oval piece that fits between it and the green oval labeled G subscript q subscript Greek letter alpha and that has a small tail extending below the lower right end of the crescent. Step 2: The occupied receptor causes G D P-G T P exchange on G subscript q Greek letter alpha. An arrow pointing to the right branches to an arrow showing the low of a gray oval labeled G D P and is joined by an arrow showing the addition of an orange oval labeled G T P. Step 3: G subscript q Greek letter alpha, with bound G T P, moves to P L C and activates it. This produces a green oval labeled G subscript q Greek letter alpha with a more pointed end away from the membrane that is almost perpendicular to the membrane with a protrusion extending to the right and a thread that extends into the membrane. It also contains an orange oval labeled G T P. The crescent-shaped piece and second oval are no longer present. The protrusion to the upper right along the membrane intersects with the lower left side of a light blue pear-shaped structure labeled phospholipase C (P L C). The narrow end of P L C ends just below the outer layer of phosphate heads in the membrane. There are short radiating lines extending out all around G subscript q Greek letter alpha and P L C. Most of the phospholipids in the membrane have a blue sphere with two yellow tails. Immediately to the right of P L C, there is a red molecule labeled P I P subscript 2 with a sphere aligned with those on the inner layer of phosphate heads in the membrane and with two red tails extending into the membrane. Two arrows point away from this red molecule, one pointing to the left into the cytosol of the cell and one pointing to the right within the membrane. Step 4: Active P L C cleaves P I P subscript 2 to I P subscript 3 and diacylglycerol. The arrow running through the membrane points to a red half-circle aligned with the phosphate heads on the inner side of the membrane with two red tails extending into the membrane. This red molecule is labeled diacylglycerol. Another arrow points along the membrane to show the same red molecule with its half-circle against a blue irregular oval labeled protein kinase C with a purple C a 2 plus sphere inside and short lines radiating out from all sides. Step 5: I P subscript 3 binds to a specific receptor-gated C a 2 plus channel, releasing sequestered C a 2 plus. The arrow pointing from the red molecule of P I P 2 into the cytosol indicates a red half-circle labeled I P subscript 3 at the top of a large protein labeled C a 2 plus channel that runs through a membrane to the left labeled endoplasmic reticulum. This membrane curves through the left side of the illustration, from the center left to lower left. Part of a second C a 2 plus channel extends across the membrane as it leaves the frame. The C a 2 plus with bound I P subscript 3 is wider above the membrane and narrower within the membrane. It contains a channel that contains a purple sphere showing that C a 2 plus ions, which are abundant to the left of the channel within the endoplasmic reticulum, can move through the channel into the cytosol. An arrow points from C a 2 plus in the channel to C a 2 plus in the cytosol, from which another arrow points to the C a 2 plus within the protein kinase C molecule that is bound to diacylglycerol in the membrane. Step 6: Diacylglycerol and C a 2 plus activate protein kinase C at the surface of the plasma membrane. An arrow points from protein kinase C down to the left. Step 7: Phosphorylation of cellular proteins by protein kinase C produces some of the cellular responses to the hormone.
Inositol trisphosphate, a water-soluble compound, diffuses from the plasma membrane to the endoplasmic reticulum (ER), where it binds to the -gated channel, causing the channel to open. The action of the SERCA pump (pp. 393–394) ensures that in the ER is orders of magnitude higher than that in the cytosol, so when these gated channels open, rushes into the cytosol (Fig. 12-15, step ), and the cytosolic rises sharply to about . One effect of elevated is the activation of protein kinase C (PKC; C for ). Diacylglycerol cooperates with in activating PKC, thus also acting as a second messenger (step ). Activation involves the movement of a PKC domain (the pseudosubstrate domain) away from its location in the substrate-binding region of the enzyme, allowing the enzyme to bind and phosphorylate proteins that contain a PKC consensus sequence — Ser or Thr residues embedded in an amino acid sequence recognized by PKC (step ). Figure 12-16 shows the structure of the receptor and a proposed mechanism of its action as a gated channel.
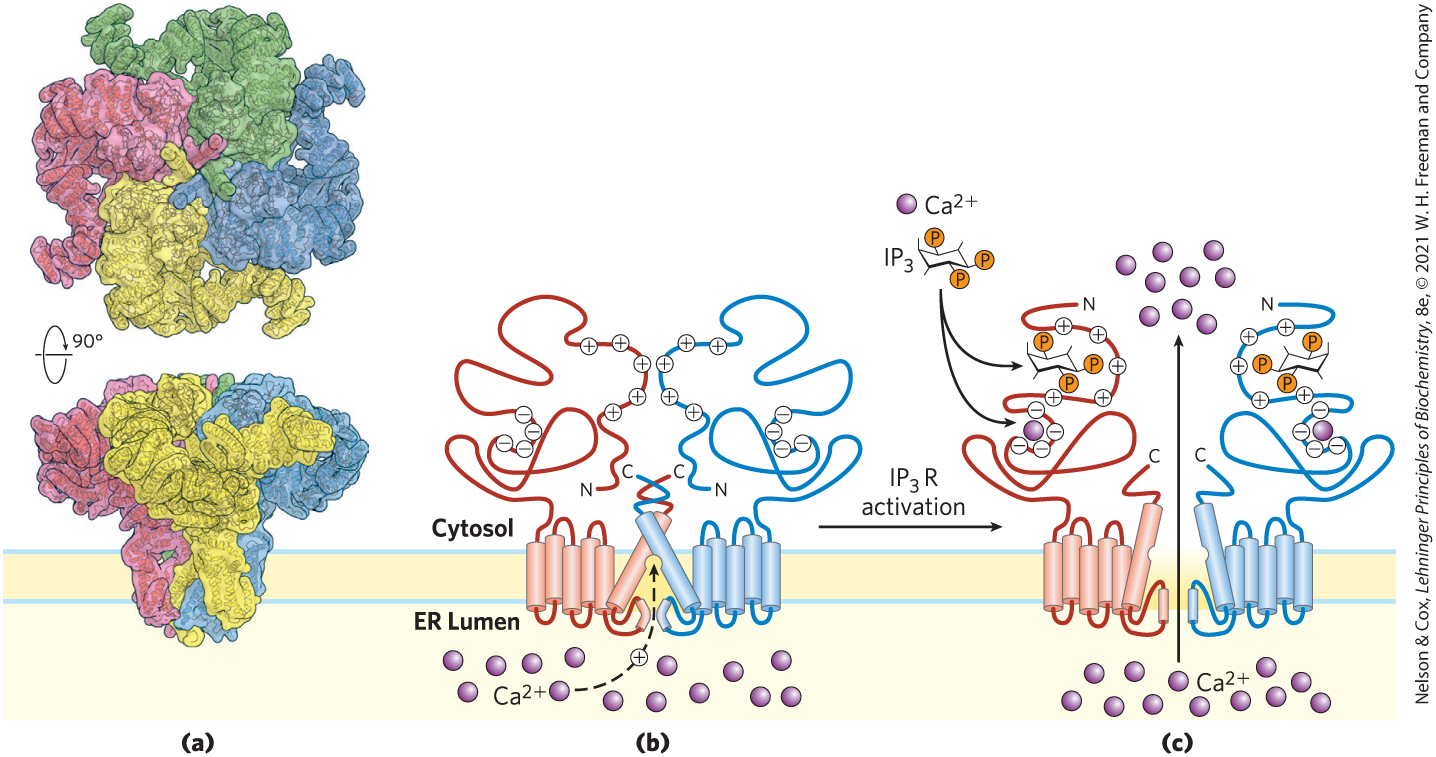
FIGURE 12-16 Proposed mechanism of action of the -gated channel. (a) The receptor in its closed conformation, determined by cryo-EM. The 1.3 MDa tetramer has 24 transmembrane helices that surround a central pore. The bulk of the protein protrudes out of the ER into the cytosol and contains the -binding sites. The central pore is the channel for movement, which does not conduct in the absence of . (b, c) Model for receptor activation by , showing only two of the four identical subunits (b) in the absence of , and (c) with one bound to each subunit. According to this model, binding near the amino-terminal end of a subunit causes a major rearrangement of the α helix at the carboxyl-terminal end, opening the channel. [(a) Data from PDB ID 6MU2, G. Fan et al., Cell Res. 28:1158, 2018. (b, c) Information from M. J. Berridge, Physiol. Rev. 96:1261, 2016, Fig. 3.]
A horizontal membrane is shown with the area above labeled as cytosol and the area below labeled as lumen. Part a shows two views of the I P subscript 3-gated C a 2 plus channel. The first view shows four similar subunits that each contain many helices in a spherical region that thins on one side to produce a curved tail. The red subunit on the left has its spherical region to the right with its tail to the left. A small piece of yellow extends up into its lower right side, where it has a small red protrusion into the green sphere to its right. Each of the pieces is similar with green at the top, blue at the right side, and yellow below. This is shown rotated 90 degrees with the yellow subunit in front. The entire structure is much wider above the membrane in the cytosol than in the part that crosses the membrane into the E R lumen. The yellow subunit has a “C”-shaped piece with a horizontal piece extending from its left side across the open region of the “C” and with a vertical piece extending down across the membrane with a small piece of the blue subunit from the right separating the yellow piece at the bottom as it emerges from the membrane. The red subunit is on the left, the green subunit is barely visible at the back, and the blue subunit is on the right. Part b shows a set of five vertical cylinders that extend through the membrane and that alternate being slightly higher or lower. The left-hand cylinder has a thread extending above with many loops. One loop above and slightly to the left has four circles labeled minus and another loop, in the center above the right side of the five vertical light red cylinders, has four circles labeled plus just above where the strand ends at N. The left-hand cylinder has a loop to the bottom of the second cylinder, which has a loop from its top to the top of the third cylinder, which has a loop from its bottom to the bottom of the fourth cylinder, which has a loop from its top to the top of the fifth cylinder, which has a loop from its bottom to a short bent light red vertical cylinder that crosses the very bottom of the membrane with a dashed arrow between it and a similar blue cylinder bent in the opposite direction. The light red bent vertical cylinder has a strand connecting it to a longer cylinder that crosses the membrane at a diagonal to emerge just above the dashed arrow below. Its top crosses the top of a similar blue cylinder extending up from the lower right. Strands from the blue and light red cylinders intertwine above them to end at C. This blue cylinder has a loop connecting to the small bent blue cylinder below, which connects to the first of five vertical cylinders arranged similarly to the light red cylinders on the left and also connected by loops above and below. The right-hand blue cylinder has a strand extending from its top that loops like the one from the left-hand light red cylinder, with similar patterns of circles with minus and plus signs. Many purple spheres labeled C a 2 plus are in the E R lumen and a dashed arrow from one points between the two small bent cylinders and into a rounded opening formed when the long light red and blue cylinders intersect. An arrow labeled I P subscript 3 end subscript R activation points right to part c. Part c shows a similar structure to the one in part c except that the small bent cylinders are now straight and vertical and the long diagonal cylinders that had intersected are now almost vertical. The strands above the long diagonal cylinders are now separate instead of intertwined. The left and right regions with circles with minus signs now each wrap around a purple C a 2 plus sphere and the regions with circles with minus signs now wrap around I P subscript 3 molecules. Each I P subscript 3 molecule has a chair conformation of a six-membered ring with an up-axial bond from the upper left vertex, an up-equatorial bond to P from the upper middle vertex, an up-equatorial bond from the upper right vertex, an up-equatorial bond to P from the lower right vertex, a down-equatorial bond to P from the lower middle vertex, and a down-equatorial bond from the lower left vertex.
Several isozymes of PKC are known, each with a characteristic tissue distribution, target protein specificity, and role. PLCβ is activated by GPCRs; another isoform, PLCγ, is activated by receptor tyrosine kinases, as described below. The ultimate PKC targets include cytoskeletal proteins, enzymes, and nuclear proteins that regulate gene expression. Taken together, this family of enzymes has a wide range of cellular actions, affecting neuronal and immune function and the regulation of cell division. Compounds that lead to overexpression of PKC or that increase its activity to abnormal levels act as tumor promoters; animals exposed to these substances have increased rates of cancer.
Calcium Is a Second Messenger That Is Limited in Space and Time
There are many variations on this basic scheme for signaling. In many cell types that respond to extracellular signals, serves as a second messenger that triggers intracellular responses, such as exocytosis in neurons and endocrine cells, contraction in skeletal muscle, and cytoskeletal rearrangements during amoeboid movement. In unstimulated cells, cytosolic is kept very low by the action of pumps in the ER, mitochondria, and plasma membrane (as further discussed below). Hormonal, neural, or other stimuli cause either an influx of into the cell through specific channels in the plasma membrane or the release of sequestered from the ER or mitochondria, in either case raising the cytosolic and triggering a cellular response.
Changes in intracellular are detected by -binding proteins that regulate a variety of -dependent enzymes. Calmodulin (CaM; 17,000) is an acidic protein with four high-affinity -binding sites ( to 1 μm) (Fig. 12-17). When intracellular rises to about (1 μm), the binding of to calmodulin drives a conformational change in the protein. Calmodulin associates with a variety of proteins and, in its -bound state, modulates their activities. It is a member of a family of -binding proteins that also includes troponin (see Fig. 5-30), which triggers skeletal muscle contraction in response to increased . Members of this family share a characteristic -binding structure, the EF hand.
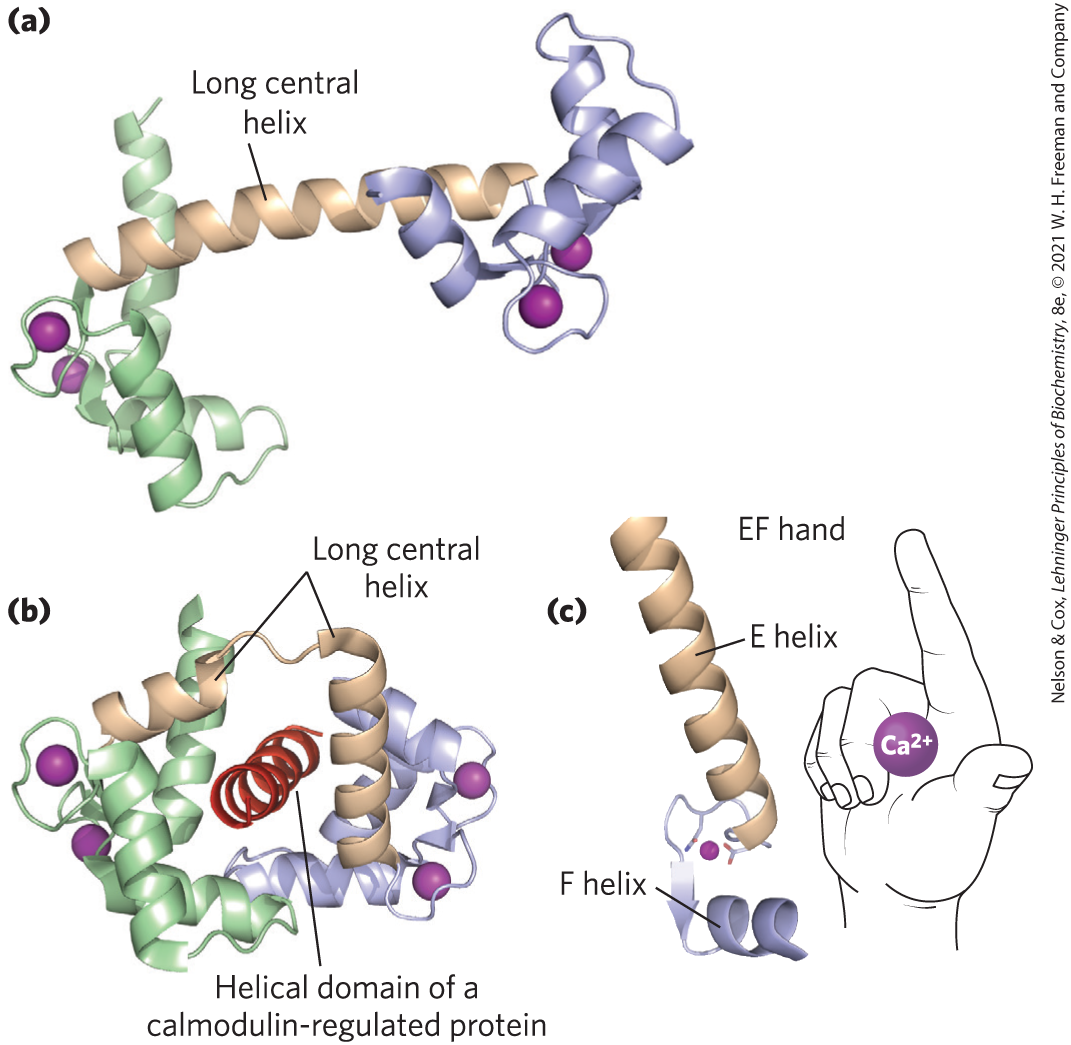
FIGURE 12-17 Calmodulin, the protein mediator of many -stimulated enzymatic reactions. (a) In this ribbon model of the crystal structure of calmodulin, the four high-affinity -binding sites are occupied by (purple). The amino-terminal domain is on the left; the carboxyl-terminal domain on the right. (b) Calmodulin associated with a helical domain of one of the many enzymes it regulates, calmodulin-dependent protein kinase II. Notice that the long central α helix of calmodulin visible in (a) has bent back on itself in binding to the helical substrate domain. The central helix of calmodulin is clearly more flexible in solution than in the crystal. (c) Each of the four -binding sites occurs in a helix-loop-helix motif called the EF hand, found in many -binding proteins. [Data from (a) PDB ID 1CLL, R. Chattopadhyaya et al., J. Mol. Biol. 228:1177, 1992; (b, c) PDB ID 1CDL, W. E. Meador et al., Science 257:1251, 1992.]
Part a shows a ribbon structure with a long brown helix labeled long central helix that runs almost horizontally. A vertical green helix runs behind the left side of the long brown helix and a short green helix runs in front of it slower half. A horizontal helix behind this short green helix runs to the left, where there are beta strands and threads that loop around two purple spheres to the lower left of the long central helix. A short purple helix runs from below the left end long central helix to intersect the long central helix slightly to the left. It does not extend above the long central helix. A second purple helix begins below the right end of the long central helix and extends up to the right, where it runs in front of another short purple helix that extends above the long central helix. To the lower right of the long central helix, in loops extending down from the purple helices, there are two purple spheres. Part b shows the same helices but the long central helix has bent and the overall structure is now more spherical. The green helices form the left side and a small piece of the long central helix runs across the upper part. The two purple spheres associated with the green helices are more spread out amongst the threads on the center of the left side. The purple helices form the right side except for one that runs horizontally across the bottom of the structure to meet the green helices. A longer piece of the long central helix runs vertically across the left of this purple side and has a strand connecting it to the shorter piece of the long central helix on the left. The two purple spheres associated with the purple helices are more spread out amongst the threads on the right side. A helix in the open region in the center of this structure extends away from the viewer and is labeled, helical domain of a calmodulin-regulated protein. Part c shows an E F hand. A brown piece of long central helix that runs from the lower right to the upper right is labeled E helix. At its bottom end, there is a strand with several sticks extending from it to show the piece that interact with a purple sphere. Two sticks on the left are blue and orange and two on the right are orange. The stand connected to a beta strand pointing down that joins to a short horizontal helix labeled F helix. A right hand is shown with the thumb and forefinger extended and the other fingers folded down and with a purple sphere labeled C a 2 plus shown at the base of the forefinger.
Calmodulin is a subunit of the /calmodulin-dependent protein kinases (CaM kinases, types I through IV). When intracellular increases in response to a stimulus, calmodulin binds , undergoes a change in conformation, and activates the CaM kinase. The kinase then phosphorylates target enzymes, regulating their activities. Calmodulin is also a regulatory subunit of phosphorylase b kinase of muscle, which is activated by . Thus triggers ATP-requiring muscle contractions while also activating glycogen breakdown, providing fuel for ATP synthesis. Many other enzymes are known to be modulated by through calmodulin (Table 12-5). The activity of the second messenger , like that of cAMP, can be spatially restricted; after its release triggers a local response, is generally removed before it can diffuse to distant parts of the cell.
| Adenylyl cyclase (brain) |
| /calmodulin-dependent protein kinases (CaM kinases I to IV) |
| -dependent channel (Paramecium) |
| -release channel of sarcoplasmic reticulum |
| Calcineurin (phosphoprotein phosphatase 2B) |
| cAMP phosphodiesterase |
| cAMP-gated olfactory channel |
| cGMP-gated , channels (rod and cone cells) |
| Glutamate decarboxylase |
| Myosin light-chain kinases |
| kinase |
| Nitric oxide synthase |
| Phosphatidylinositol 3-kinase |
| Plasma membrane ATPase ( pump) |
| RNA helicase (p68) |
Commonly, level does not simply rise and then fall, but rather oscillates with a period of a few seconds (Fig. 12-18) — even when the extracellular concentration of the triggering hormone remains constant. The mechanism underlying oscillations presumably entails feedback regulation by on some part of the -release process. Whatever the mechanism, the effect is that one kind of signal (hormone concentration, for example) is converted into another (frequency and amplitude of intracellular “spikes”). The signal diminishes as diffuses away from the initial source (the channel), is sequestered in the ER, or is pumped out of the cell.
![A two-part figure shows the appearance of thymus cells stimulated with extracellular A T P, dyed with fura, and viewed using fluorescence microscopy in part a and a graph of cytosolic [C a 2 plus] (n M) plotted against time (s) in part b.](../images/8e_12_18ab_277038.png)
FIGURE 12-18 Triggering of oscillations in intracellular by extracellular signals. (a) The dye fura, which undergoes fluorescence changes when it binds , can be used with fluorescence microscopy to measure the instantaneous in cells. The color scale relates fluorescence intensity to . Here, thymus cells have been stimulated with extracellular ATP, which raises their internal . The cells are heterogeneous in their responses: some have high intracellular (red), others have much lower (blue). (b) When such a probe is used in a single hepatocyte, the agonist norepinephrine (added at the arrow) causes oscillations of from 200 to 500 nm. Similar oscillations are induced in other cell types by other extracellular signals. [(a) Courtesy Michael D. Cahalan, University of California, Irvine, Department of Physiology and Biophysics. (b) Data from T. A. Rooney et al., J. Biol. Chem. 264:17,131, 1989.]
Part a shows many brightly colored spheres against a black background. A scale below shows colored associated with [C a 2 plus] (mu M) ranging from 0 to 1.0. The colors change from dark blue representing 0 to a narrow band of light blue at 0.3, to a green region, to a narrow yellow band at 0.7, to a red region. The picture shows many dark blue spheres with 7 light blue spheres, about 4 greenish spheres, and 24 spheres with grains of red, orange, yellow, and green. Part b shows a graph that plots cytosolic [C a 2 plus] (n M) on the vertical axis, ranging from 100 to 600 and labeled in increments of 100, against time (s) on the horizontal axis, ranging from 0 to 400 and labeled in increments of 100. An irregular horizontal line begins at (0, 200) and waves slightly as it runs past a downward arrow at (70, 204) before forming a sharp peak at (60, 540). It drops back to (70, 215), then rises sharply from (115, 204), to peak at (120, 535), then drops back to (135, 205), runs horizontally, then rises sharply from (175, 205) to peak at (200, 530), then drops to (210, 205), runs horizontally, then rises sharply from (255, 205) to peak at (260, 525), then drops back to (275, 205), runs horizontally, then rises sharply from (320, 205) to peak at (325, 520), before dropping back to (330, 520) to run almost horizontally to end at (380, 205). All data are approximate.
There is significant cross talk between the and cAMP signaling systems. In some tissues, both the enzyme that produces cAMP (adenylyl cyclase) and the enzyme that degrades cAMP (phosphodiesterase) are stimulated by . Temporal and spatial changes in can therefore produce transient, localized changes in [cAMP]. We have noted already that PKA, the enzyme that responds to cAMP, is often part of a highly localized supramolecular complex assembled on scaffold proteins such as AKAPs. This subcellular localization of target enzymes, combined with temporal and spatial gradients in and [cAMP], allows a cell to respond to one or several signals with subtly nuanced metabolic changes.
SUMMARY 12.2 G Protein–Coupled Receptors and Second Messengers
- G protein–coupled receptors (GPCRs) have seven transmembrane helices and act through heterotrimeric G proteins. Ligand binding activates the G protein, which then stimulates or inhibits the activity of an effector enzyme, changing the local concentration of its second-messenger product cAMP.
- Epinephrine, acting through its GPCR and the protein, stimulates adenylyl cyclase, which produces cAMP. Cyclic AMP activates protein kinase A (PKA), which then phosphorylates target proteins on a Ser or Thr residue, changing their biological activity.
- To end the response to epinephrine, a phosphodiesterase breaks down cAMP, and the G protein is inactivated by its own GTPase activity. Phosphoprotein phosphatases reverse the effects of PKA.
- When the epinephrine signal persists, β-adrenergic receptor–specific protein kinase phosphorylates the GPCR, creating a binding site for the protein β-arrestin, which prevents interaction between the GPCR and its G protein. Arrestin triggers desensitization by movement of the receptor into intracellular vesicles.
- GPCRs typically have their effects through the G protein-cAMP-PKA pathway; some act through , raising [cAMP], others through , lowering [cAMP]. Adaptor proteins such as AKAPs tether PKA and limit its area of action and its target proteins.
- Trimeric G proteins coupled to GPCRs are activated when their bound GDP is exchanged for GTP, and they remain active until their GTPase converts bound GTP to GDP. Their GTPase activity is modulated by GTPase activator proteins (GAPs) and regulators of G-protein signaling (RGSs).
- Monomeric (small) G proteins such as Ras, Rab, and Ran also serve as self-limiting switches. Defective G-protein signaling is common in individuals with some types of cancer.
- Some GPCRs are coupled to a G protein that acts through a plasma membrane phospholipase C that cleaves to diacylglycerol and . By opening channels in the endoplasmic reticulum, raises cytosolic . Diacylglycerol and act together to activate protein kinase C, which phosphorylates and changes the activity of specific cellular proteins.
- Cellular also regulates (often through calmodulin) many other enzymes and proteins involved in secretion, cytoskeletal rearrangements, or contraction. Many of the target enzymes are in the family of -activated protein kinases (PKCs).
 Three essential components define signal transduction through GPCRs: a plasma membrane receptor with seven transmembrane helical segments, a G protein that cycles between active (guanosine triphosphate (GTP)-bound) and inactive (guanosine diphosphate (GDP)-bound) forms, and an effector enzyme (or ion channel) in the plasma membrane that is regulated by the activated G protein. An extracellular signal such as a hormone, growth factor, or neurotransmitter is the “first messenger” that activates a receptor from outside the cell. Ligand binding to the receptor forces an allosteric transition that allows the receptor to interact with a G protein, causing it to exchange its bound GDP for a GTP from the cytosol. The G protein then dissociates from the activated receptor and binds to the nearby effector enzyme, altering its activity. The effector enzyme then causes a change in the cytosolic concentration of a low molecular weight metabolite (such as ,-cyclic AMP) or inorganic ion , which acts as a
Three essential components define signal transduction through GPCRs: a plasma membrane receptor with seven transmembrane helical segments, a G protein that cycles between active (guanosine triphosphate (GTP)-bound) and inactive (guanosine diphosphate (GDP)-bound) forms, and an effector enzyme (or ion channel) in the plasma membrane that is regulated by the activated G protein. An extracellular signal such as a hormone, growth factor, or neurotransmitter is the “first messenger” that activates a receptor from outside the cell. Ligand binding to the receptor forces an allosteric transition that allows the receptor to interact with a G protein, causing it to exchange its bound GDP for a GTP from the cytosol. The G protein then dissociates from the activated receptor and binds to the nearby effector enzyme, altering its activity. The effector enzyme then causes a change in the cytosolic concentration of a low molecular weight metabolite (such as ,-cyclic AMP) or inorganic ion , which acts as a  The human genome encodes just over 800 GPCRs, about 350 for detecting hormones, growth factors, and other endogenous ligands, and up to 500 that serve as olfactory (smell) and gustatory (taste) receptors. The largest superfamily of proteins encoded in the human genome, GPCRs have been implicated in many common medical conditions, including allergies, depression, blindness, diabetes, and various cardiovascular defects. GPCR mutations are also found in 20% of all cancers. In the United States, more than a third of all pharmaceuticals on the market target a GPCR. For example, the β-adrenergic receptor, which mediates the effects of epinephrine, is the target of the “beta blockers,” prescribed for such diverse conditions as hypertension, cardiac arrhythmia, glaucoma, anxiety, and migraine headache. More than 100 of the GPCRs found in the human genome are still “orphan receptors,” meaning that their natural ligands are not yet identified, and so we know little about their biology. The β-adrenergic receptor, with well-understood biology and pharmacology, is the prototype for all GPCRs, and our discussion of signal-transducing systems begins there.
The human genome encodes just over 800 GPCRs, about 350 for detecting hormones, growth factors, and other endogenous ligands, and up to 500 that serve as olfactory (smell) and gustatory (taste) receptors. The largest superfamily of proteins encoded in the human genome, GPCRs have been implicated in many common medical conditions, including allergies, depression, blindness, diabetes, and various cardiovascular defects. GPCR mutations are also found in 20% of all cancers. In the United States, more than a third of all pharmaceuticals on the market target a GPCR. For example, the β-adrenergic receptor, which mediates the effects of epinephrine, is the target of the “beta blockers,” prescribed for such diverse conditions as hypertension, cardiac arrhythmia, glaucoma, anxiety, and migraine headache. More than 100 of the GPCRs found in the human genome are still “orphan receptors,” meaning that their natural ligands are not yet identified, and so we know little about their biology. The β-adrenergic receptor, with well-understood biology and pharmacology, is the prototype for all GPCRs, and our discussion of signal-transducing systems begins there. 
 ), allosteric transitions in the receptor and its associated G protein favor the replacement of GDP with GTP. Thus the hormone-bound GPCR acts as a
), allosteric transitions in the receptor and its associated G protein favor the replacement of GDP with GTP. Thus the hormone-bound GPCR acts as a  ) can transmit the signal from the activated receptor to the downstream effector protein, adenylyl cyclase. Because this G protein stimulates its effector, it is referred to as a
) can transmit the signal from the activated receptor to the downstream effector protein, adenylyl cyclase. Because this G protein stimulates its effector, it is referred to as a  ).
). and
and  , synthesis and hydrolysis of cAMP by adenylyl cyclase and cAMP phosphodiesterase, respectively.
, synthesis and hydrolysis of cAMP by adenylyl cyclase and cAMP phosphodiesterase, respectively. ), which catalyzes the phosphorylation of specific Ser or Thr residues of targeted proteins, such as glycogen phosphorylase b kinase. The latter enzyme is active when phosphorylated and can begin the process of mobilizing glycogen stores in muscle and liver in anticipation of the need for energy, as signaled by epinephrine.
), which catalyzes the phosphorylation of specific Ser or Thr residues of targeted proteins, such as glycogen phosphorylase b kinase. The latter enzyme is active when phosphorylated and can begin the process of mobilizing glycogen stores in muscle and liver in anticipation of the need for energy, as signaled by epinephrine. This same basic mechanism — displacement of an autoinhibitory domain — mediates the allosteric activation of many types of protein kinases by their second messengers (as in
This same basic mechanism — displacement of an autoinhibitory domain — mediates the allosteric activation of many types of protein kinases by their second messengers (as in  ), PKA regulates many enzymes downstream in the signaling pathway. Although these downstream targets have diverse functions, they share a region of sequence similarity around the Ser or Thr residue that undergoes phosphorylation, a sequence that marks them for regulation by PKA. The substrate-binding cleft of PKA recognizes these sequences and phosphorylates their Thr or Ser residue. Comparison of the sequences of various protein substrates for PKA has yielded the short
), PKA regulates many enzymes downstream in the signaling pathway. Although these downstream targets have diverse functions, they share a region of sequence similarity around the Ser or Thr residue that undergoes phosphorylation, a sequence that marks them for regulation by PKA. The substrate-binding cleft of PKA recognizes these sequences and phosphorylates their Thr or Ser residue. Comparison of the sequences of various protein substrates for PKA has yielded the short  As in many signaling pathways, signal transduction by the β-adrenergic receptor and adenylyl cyclase entails several steps that amplify the original hormone signal (
As in many signaling pathways, signal transduction by the β-adrenergic receptor and adenylyl cyclase entails several steps that amplify the original hormone signal ( Epinephrine is just one of many hormones, growth factors, and other regulatory molecules that act by changing the intracellular [cAMP] and thus the activity of PKA.
Epinephrine is just one of many hormones, growth factors, and other regulatory molecules that act by changing the intracellular [cAMP] and thus the activity of PKA.  MEDICINE
MEDICINE Because G proteins play crucial roles in so many signaling processes, it is not surprising that defects in G proteins lead to a variety of diseases. In about 25% of all human cancers (and in a much higher proportion of certain types of cancer), a mutation in a Ras protein — typically in one of the critical residues around the GTP-binding site or in the P loop — virtually eliminates its GTPase activity. Once activated by GTP binding, this Ras protein remains constantly active, promoting cell division in cells that should not divide. The tumor suppressor gene NF1 encodes a GAP that enhances the GTPase activity of normal Ras. Mutations in NF1 that result in a nonfunctioning GAP leave Ras with only its GTPase activity, which is very weak (that is, has a very low turnover number); once activated by GTP binding, Ras stays active for an extended period, continuing to send the signal: divide.
Because G proteins play crucial roles in so many signaling processes, it is not surprising that defects in G proteins lead to a variety of diseases. In about 25% of all human cancers (and in a much higher proportion of certain types of cancer), a mutation in a Ras protein — typically in one of the critical residues around the GTP-binding site or in the P loop — virtually eliminates its GTPase activity. Once activated by GTP binding, this Ras protein remains constantly active, promoting cell division in cells that should not divide. The tumor suppressor gene NF1 encodes a GAP that enhances the GTPase activity of normal Ras. Mutations in NF1 that result in a nonfunctioning GAP leave Ras with only its GTPase activity, which is very weak (that is, has a very low turnover number); once activated by GTP binding, Ras stays active for an extended period, continuing to send the signal: divide. Hormonal, neural, or other stimuli cause either an influx of into the cell through specific channels in the plasma membrane or the release of sequestered from the ER or mitochondria, in either case raising the cytosolic and triggering a cellular response.
Hormonal, neural, or other stimuli cause either an influx of into the cell through specific channels in the plasma membrane or the release of sequestered from the ER or mitochondria, in either case raising the cytosolic and triggering a cellular response. G protein–coupled receptors (GPCRs) have seven transmembrane helices and act through heterotrimeric G proteins. Ligand binding activates the G protein, which then stimulates or inhibits the activity of an effector enzyme, changing the local concentration of its second-messenger product cAMP.
G protein–coupled receptors (GPCRs) have seven transmembrane helices and act through heterotrimeric G proteins. Ligand binding activates the G protein, which then stimulates or inhibits the activity of an effector enzyme, changing the local concentration of its second-messenger product cAMP.