21.1 Biosynthesis of Fatty Acids and Eicosanoids
Even when compared to other major classes of metabolites, the division between fatty acid biosynthesis and breakdown is particularly striking. The two processes occur by different pathways, catalyzed by different sets of enzymes, and, in eukaryotes, occur in different cellular compartments. Fatty acid breakdown occurs in the mitochondria, whereas biosynthesis occurs in the cytosol. Moreover, biosynthesis requires the participation of a three-carbon intermediate, malonyl-CoA, that does not appear in the path of fatty acid breakdown.

The general pathway of fatty acid synthesis and its regulation now take center stage. We consider the biosynthesis of longer-chain fatty acids, unsaturated fatty acids, and their eicosanoid derivatives at the end of this section.
Malonyl-CoA Is Formed from Acetyl-CoA and Bicarbonate
The formation of malonyl-CoA from acetyl-CoA is an irreversible three-step process, catalyzed by acetyl-CoA carboxylase. In animal cells, all three steps are catalyzed in the cytoplasm by a single multifunctional polypeptide (Fig. 21-1). The enzyme contains a biotin prosthetic group covalently bound in amide linkage to the ε-amino group of a Lys residue in one of the domains of the enzyme molecule. The reaction catalyzed by this enzyme is very similar to other biotin-dependent carboxylation reactions, such as those catalyzed by pyruvate carboxylase (see Fig. 16-16) and propionyl-CoA carboxylase (see Fig. 17-12). A carboxyl group, derived from bicarbonate , is first transferred to biotin in an ATP-dependent reaction. In a second step, the carboxyl group is carried by the biotin to a different active site, where the is transferred to acetyl-CoA in the third and final step to yield malonyl-CoA. As we will see, this carboxylation has the same function as the carboxylation of pyruvate by pyruvate carboxylase — it renders the next step in the reaction sequence much more favorable thermodynamically.

FIGURE 21-1 The acetyl-CoA carboxylase reaction. The mammalian acetyl-CoA carboxylase of the cytoplasm has three functional domains with distinct functions: biotin carrier protein; biotin carboxylase, which activates by attaching it to a nitrogen in the biotin ring in an ATP-dependent reaction; and transcarboxylase, which transfers activated (shaded green) from biotin to acetyl-CoA, producing malonyl-CoA. Part of the biotin carrier protein domain and the long, flexible biotin arm rotate to carry the activated from the biotin carboxylase active site to the transcarboxylase active site. The active domain in each step is shaded in blue.
The bacterial version of acetyl-CoA carboxylase is similar but has three separate polypeptide subunits (including a separate biotin carrier protein) that catalyze the three steps. Plant cells contain both types of acetyl-CoA carboxylase.
Fatty Acid Synthesis Proceeds in a Repeating Reaction Sequence
The long carbon chains of fatty acids are assembled in the cytosol in a repeating four-step sequence (Fig. 21-2), catalyzed by a system collectively referred to as fatty acid synthase. A saturated acyl group produced by each four-step series of reactions becomes the substrate for subsequent condensation with an activated malonyl group. With each passage through the cycle, the fatty acyl chain is extended by two carbons.
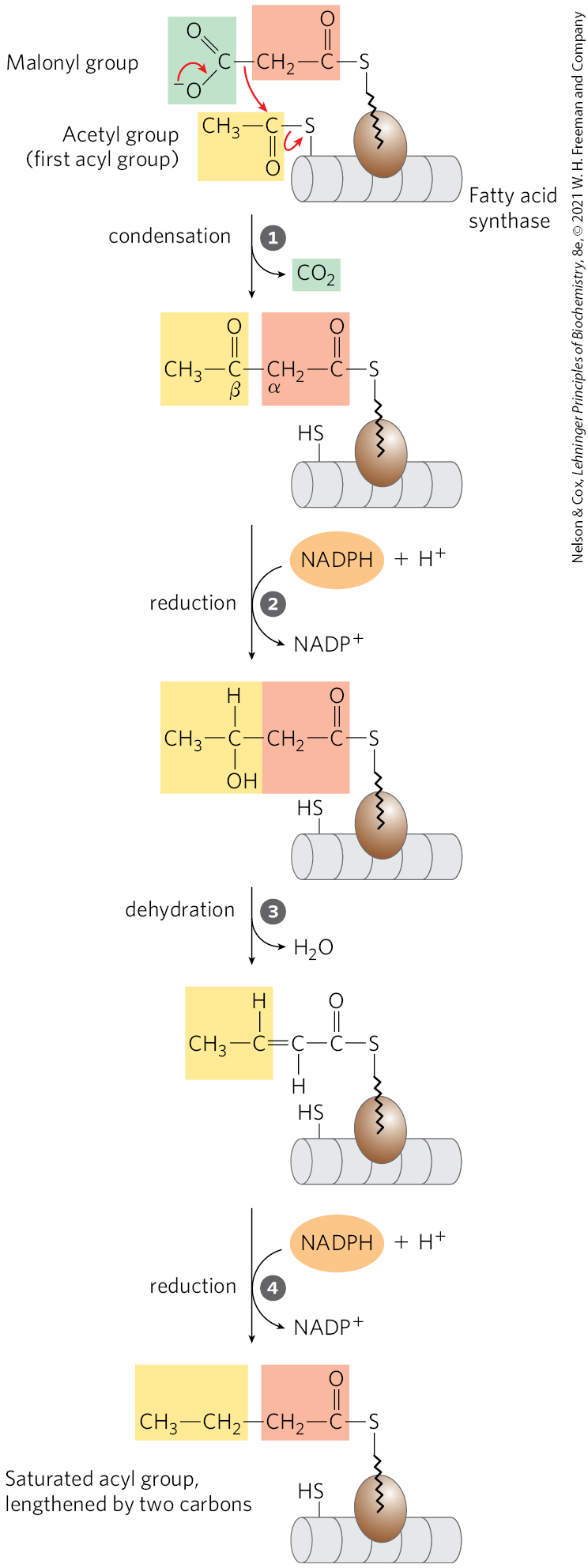
FIGURE 21-2 Addition of two carbons to a growing fatty acyl chain: a four-step sequence. Each malonyl group and acetyl (or longer acyl) group is activated by a thioester that links it to fatty acid synthase, a multienzyme system. Condensation of an activated acyl group (an acetyl group from acetyl-CoA is the first acyl group) and two carbons derived from malonyl-CoA, with elimination of from the malonyl group, extends the acyl chain by two carbons. The mechanism of the first step of this reaction is given to illustrate the role of decarboxylation in facilitating condensation. The β-keto product of the condensation is then reduced in three more steps nearly identical to the reactions of β oxidation, but in the reverse sequence: the β-keto group is reduced to an alcohol, elimination of creates a double bond, and the double bond is reduced to form the corresponding saturated fatty acyl group.
In the four-step pathway, a condensation reaction is followed by a reduction-dehydration-reduction sequence to convert the C-3 carbonyl to a methylene. The latter three steps are the chemical reverse of the oxidation-hydration-oxidation sequence in the β oxidation of fatty acids (Fig. 17-8a). However, both the electron-carrying cofactor and the activating groups in the reductive anabolic sequence differ from those in the oxidative catabolic process. Recall that in β oxidation, and FAD serve as electron acceptors and the activating group is the thiol group of coenzyme A. By contrast, the reducing agent in the synthetic sequence is NADPH and the activating groups are two different enzyme-bound groups, as described below.
In mammals, this synthetic system is called fatty acid synthase I (FAS I). There are seven active sites to catalyze the four-step cycle plus the charging steps described below. The active sites for different reactions lie in separate domains (Fig. 21-3a), all within a single multifunctional polypeptide chain . Two of these multifunctional polypeptides function as a homodimer (; Fig. 21-3b). The two subunits seem to function independently. When all the active sites in one subunit are inactivated by mutation, fatty acid synthesis is only moderately reduced.
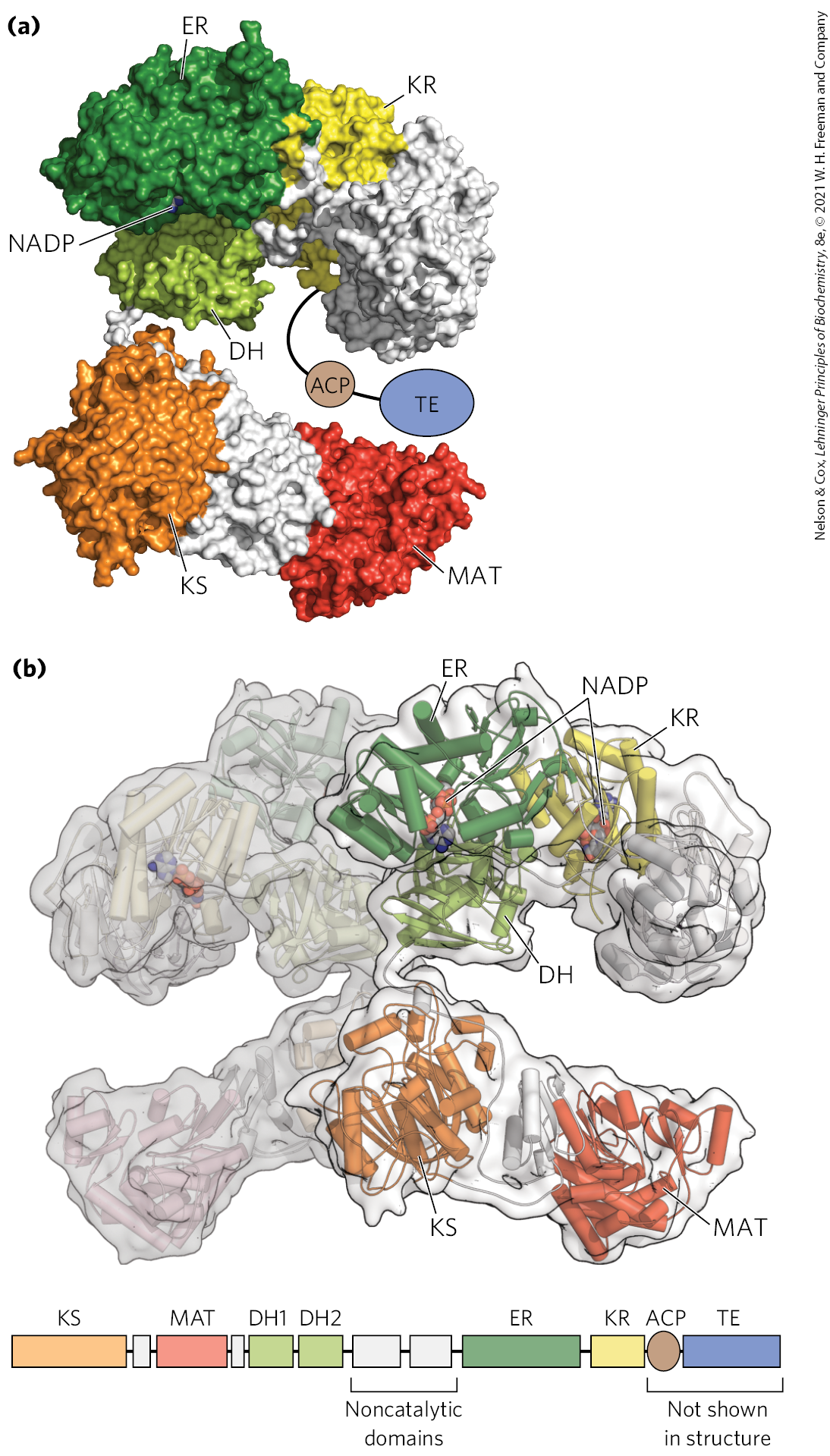
FIGURE 21-3 The structure of a fatty acid synthase type I system. Shown here is the structure of a single (monomeric) polypeptide chain of the mammalian (porcine) enzyme system. (a) All of the active sites in the mammalian system are located in different domains within a single large polypeptide chain. The different enzymatic activities are β-ketoacyl-ACP synthase (KS), malonyl/acetyl-CoA–ACP transferase (MAT), β-hydroxyacyl-ACP dehydratase (DH), enoyl-ACP reductase (ER), and β-ketoacyl-ACP reductase (KR). ACP is the acyl carrier protein. The seventh domain is a thioesterase (TE) that releases the palmitate product from ACP when synthesis is completed. The ACP and TE domains are disordered in the crystal and are therefore not shown in the structure. (b) The native dimeric structure is shown, with one polypeptide transparent to show how the two independently operating subunits come together. The linear arrangement of the domains in the polypeptide is shown below the structure. [Data from PDB ID 2CF2, T. Maier et al., Science 311:1258, 2006.]
With FAS I systems, fatty acid synthesis leads to a single product. As it goes through the cycle, the acyl group is covalently linked to acyl carrier protein (ACP), which shuttles it from one active site to another in sequence. Acyl carrier protein is yet another contiguous part of the single FAS I polypeptide. No intermediates are released. When the chain length reaches 16 carbons, that product (palmitate, 16:0; see Table 10-1) leaves the cycle. Carbons C-16 and C-15 of the palmitate are derived from the methyl and carboxyl carbon atoms, respectively, of an acetyl-CoA used directly to prime the system at the outset (Fig. 21-4); the rest of the carbon atoms in the chain are derived from acetyl-CoA via malonyl-CoA.
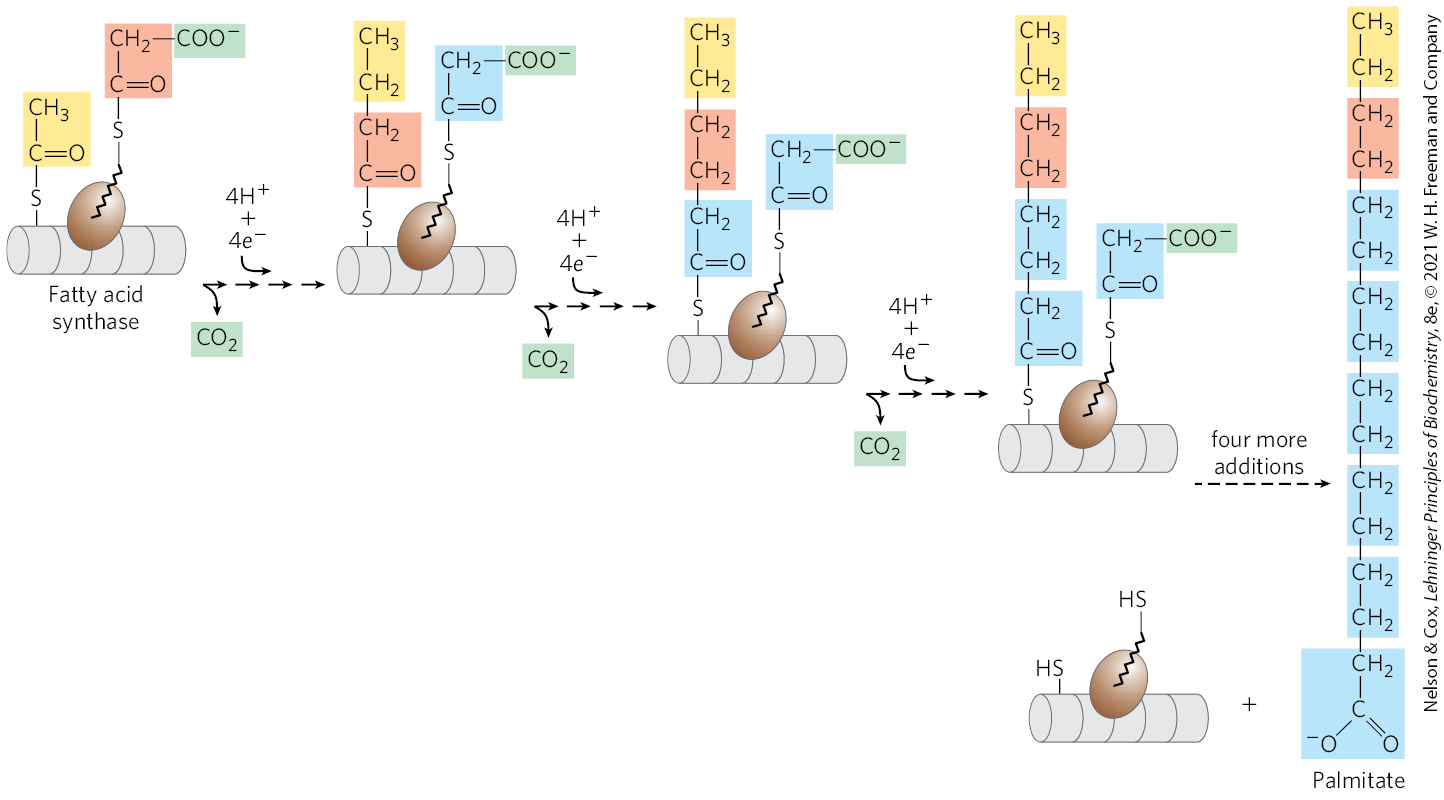
FIGURE 21-4 The overall process of palmitate synthesis. The fatty acyl chain grows by two-carbon units donated by activated malonate, with loss of at each step. The initial acetyl group is shaded yellow; C-1 and C-2 of malonate, light red; and the carbon released as , green. After each two-carbon addition, reductions convert the growing chain to a saturated fatty acid of four, then six, then eight carbons, and so on. The final product is palmitate (16:0).
A somewhat different FAS I is found in yeast and other fungi, and is made up of two multifunctional polypeptides that form a complex with an architecture distinct from that of the vertebrate systems. Three of the seven required active sites are found on the α subunit and four on the β subunit. A different system, called FAS II, is found in plants and most bacteria. FAS II is a dissociated system; each step in the synthesis is catalyzed by a separate enzyme. Unlike FAS I, FAS II generates a variety of products, including saturated fatty acids of several lengths, as well as unsaturated, branched, and hydroxy fatty acids. An FAS II system is also found in vertebrate mitochondria, yet another indication of the bacterial origins of mitochondria in evolution.
The Mammalian Fatty Acid Synthase Has Multiple Active Sites
The multiple domains of mammalian FAS I function as distinct but linked enzymes. The active site for each enzyme is found in a separate domain within the larger polypeptide. Throughout the process of fatty acid synthesis, the intermediates remain covalently attached as thioesters to one of two thiol groups. One point of attachment is the group of a Cys residue in one of the synthase domains (β-ketoacyl-ACP synthase; KS); the other is the group of acyl carrier protein, a separate domain of the same polypeptide. Hydrolysis of thioesters is highly exergonic, and the energy released helps to make two steps ( and in Fig. 21-6) in fatty acid synthesis thermodynamically favorable.
Acyl carrier protein is the shuttle that holds the system together, containing the prosthetic group -phosphopantetheine, also found in coenzyme A (Fig. 21-5). The -phosphopantetheine serves as a flexible arm, tethering the growing fatty acyl chain to the surface of the fatty acid synthase complex while carrying the reaction intermediates from one enzyme active site to the next. The same prosthetic group is used in FAS II systems.
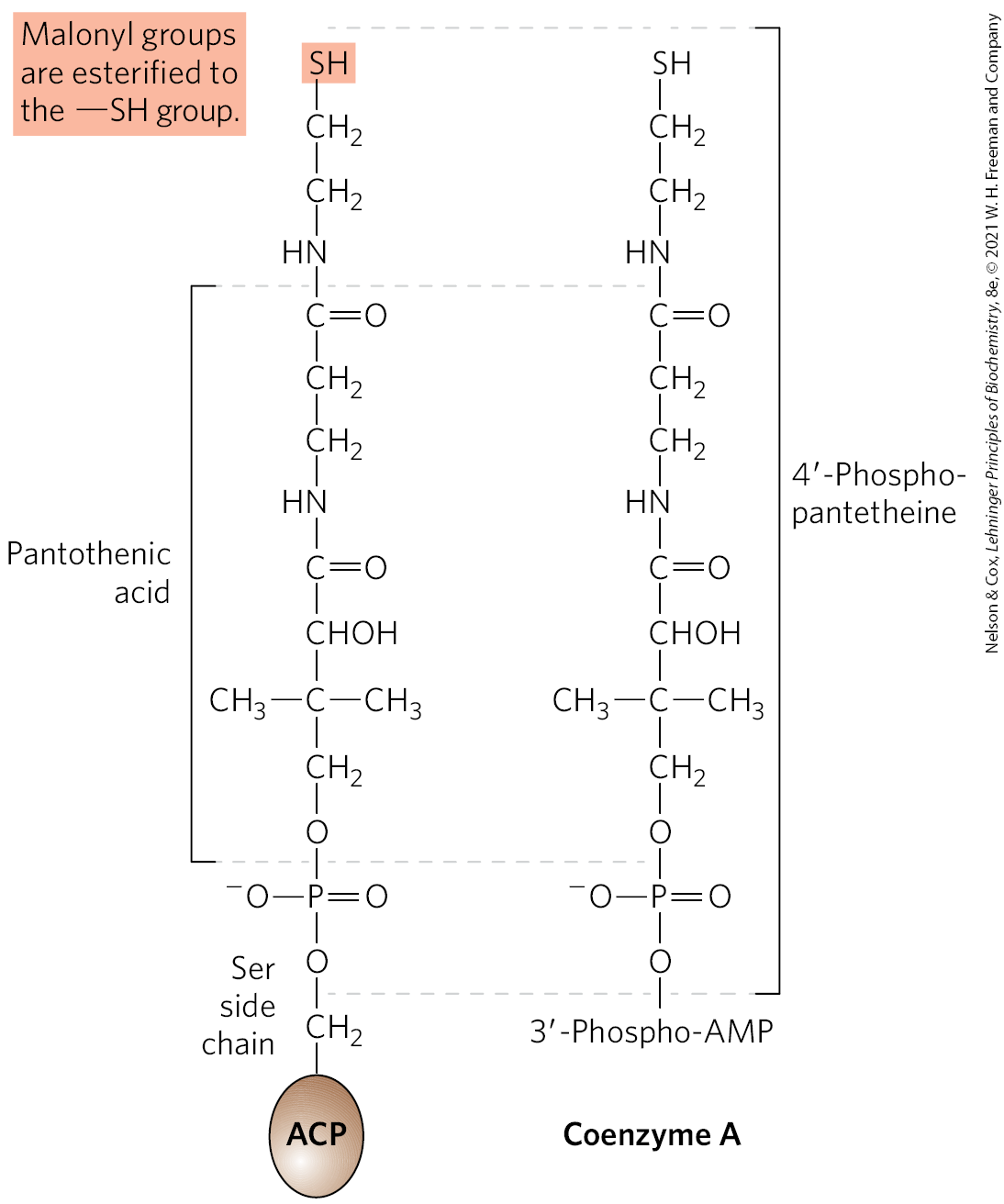
FIGURE 21-5 Acyl carrier protein (ACP). The prosthetic group is -phosphopantetheine, which is covalently attached to the hydroxyl group of a Ser residue in ACP. Phosphopantetheine, also found in the coenzyme A molecule, contains the B vitamin pantothenic acid. Its group is the site of entry of malonyl groups during fatty acid synthesis. Coenzyme A, shown here for comparison, serves a similar chemical purpose in general metabolism.
Fatty Acid Synthase Receives the Acetyl and Malonyl Groups
Before the condensation reactions that build up the fatty acid chain can begin, the two thiol groups on the enzyme complex must be charged with the correct acyl groups ( Fig. 21-6a). First, the acetyl group of acetyl-CoA is transferred to ACP in a reaction catalyzed by the malonyl/acetyl-CoA–ACP transferase (MAT) domain of the multifunctional polypeptide. The acetyl group is then transferred to the Cys group of the β-ketoacyl-ACP synthase (KS). The second reaction, transfer of the malonyl group from malonyl-CoA to the group of ACP, is also catalyzed by malonyl/acetyl-CoA–ACP transferase. In the charged synthase complex, the acetyl and malonyl groups are activated for the chain-lengthening process. We now consider the first four steps of this process in some detail; all step numbers refer to Figure 21-6.
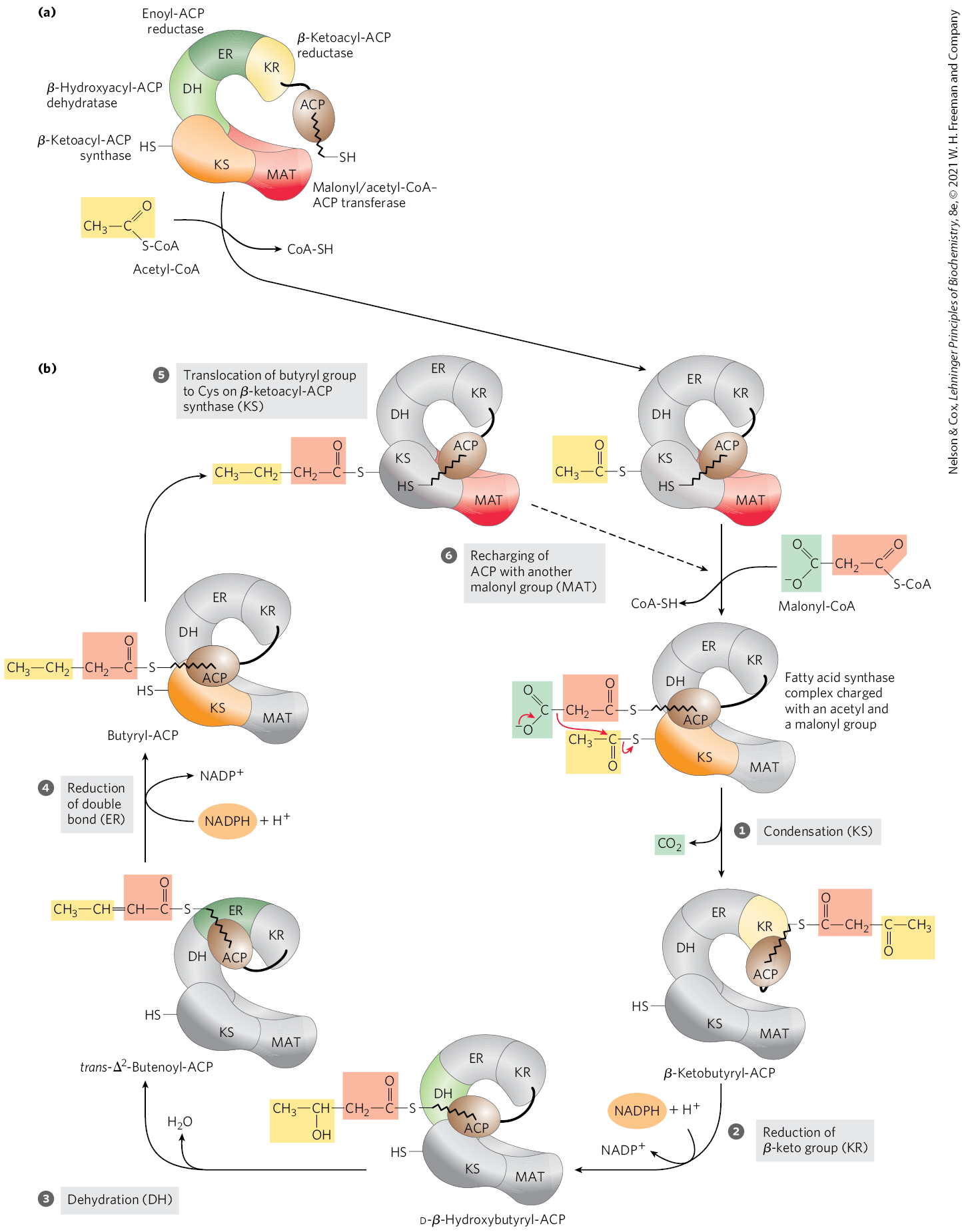
FIGURE 21-6 Sequence of events during synthesis of a fatty acid. (a) The mammalian FAS I complex is shown schematically, with catalytic domains colored as in Figure 21-3. Each domain of the larger polypeptide represents one of the six enzymatic activities of the complex, arranged in a large, tight S shape. The acyl carrier protein (ACP) is not resolved in the crystal structure shown in Figure 21-3, but it is attached to the KR domain. The phosphopantetheine arm of ACP ends in an . (b) The enzyme shown in color is the one that will act in the next step. As in Figure 21-4, the initial acetyl group is shaded yellow; C-1 and C-2 of malonate, light red; and the carbon released as , green. Steps to are described in the text.
Step Condensation
The first reaction in the formation of a fatty acid chain is a formal Claisen condensation (see reaction class in Fig. 13-4) of the activated acetyl and malonyl groups to form acetoacetyl-ACP, an acetoacetyl group bound to ACP through the phosphopantetheine group; simultaneously, a molecule of is produced. In this reaction, catalyzed by β-ketoacyl-ACP synthase, the acetyl group is transferred from the Cys group of the enzyme to the malonyl group on the of ACP, becoming the methyl-terminal two-carbon unit of the new acetoacetyl group.
The carbon atom of the formed in this reaction is the same carbon originally introduced into malonyl-CoA from in the acetyl-CoA carboxylase reaction (Fig. 21-1). Thus, is only transiently in covalent linkage during fatty acid biosynthesis; it is removed as each two-carbon unit is added.
Why do cells go to the trouble of adding to make a malonyl group from an acetyl group, only to lose the during the formation of acetoacetate? The use of activated malonyl groups rather than acetyl groups makes the condensation reactions thermodynamically favorable. The methylene carbon (C-2) of the malonyl group, sandwiched between carbonyl and carboxyl carbons, forms a good nucleophile. In the condensation step, decarboxylation of the malonyl group facilitates nucleophilic attack of the methylene carbon on the thioester linking the acetyl group to β-ketoacyl-ACP synthase, displacing the enzyme’s group. Coupling the condensation to the decarboxylation of the malonyl group renders the overall process highly exergonic. A similar carboxylation-decarboxylation sequence facilitates the formation of phosphoenolpyruvate from pyruvate in gluconeogenesis (see Figs. 14-17 and 14-18).
By using activated malonyl groups in the synthesis of fatty acids and activated acetate in their degradation, the cell makes both processes energetically favorable, although one is effectively the reversal of the other. The extra energy required to make fatty acid synthesis favorable is provided by the ATP used to synthesize malonyl-CoA from acetyl-CoA and (Fig. 21-1).
Step Reduction of the Carbonyl Group
The acetoacetyl-ACP formed in the condensation step now undergoes reduction of the carbonyl group at C-3 to form d-β-hydroxybutyryl-ACP. This reaction is catalyzed by β-ketoacyl-ACP reductase (KR), and the electron donor is NADPH. Notice that the d-β-hydroxybutyryl group does not have the same stereoisomeric form as the l-β-hydroxyacyl intermediate in fatty acid oxidation (see Fig. 17-8).
Step Dehydration
The elements of water are now removed from C-2 and C-3 of d-β-hydroxybutyryl-ACP to yield a double bond in the product, trans--butenoyl-ACP. The enzyme that catalyzes this dehydration is β-hydroxyacyl-ACP dehydratase (DH).
Step Reduction of the Double Bond
Finally, the double bond of trans--butenoyl-ACP is reduced (saturated) to form butyryl-ACP by the action of enoyl-ACP reductase (ER); again, NADPH is the electron donor.
The Fatty Acid Synthase Reactions Are Repeated to Form Palmitate
Production of the four-carbon, saturated fatty acyl–ACP marks completion of one pass through the fatty acid synthase complex. In step , the butyryl group is transferred from the phosphopantetheine group of ACP to the Cys group of β-ketoacyl-ACP synthase, which initially bore the acetyl group (Fig. 21-6). To start the next cycle of four reactions that lengthens the chain by two more carbons (step ), another malonyl group is linked to the now unoccupied phosphopantetheine group of ACP (Fig. 21-7). Condensation occurs as the butyryl group, acting like the acetyl group in the first cycle, is linked to two carbons of the malonyl-ACP group with concurrent loss of . The product of this condensation is a six-carbon acyl group, covalently bound to the phosphopantetheine group. Its β-keto group is reduced in the next three steps of the synthase cycle to yield the saturated acyl group, exactly as in the first round of reactions — in this case forming the six-carbon product.
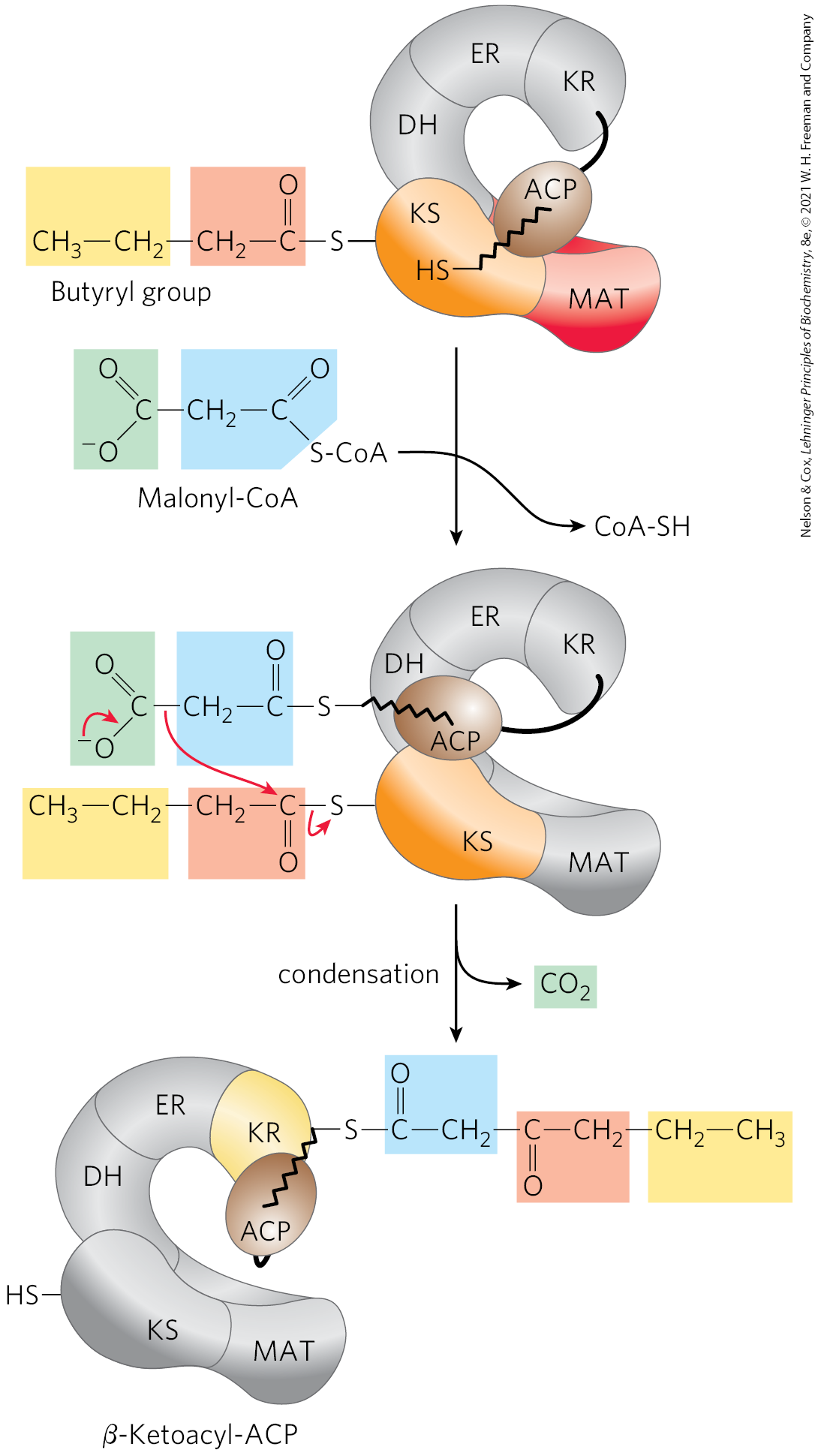
FIGURE 21-7 Beginning of the second round of the fatty acid synthesis cycle. The butyryl group is on the Cys group. The incoming malonyl group is first attached to the phosphopantetheine group. Then, in the condensation step, the entire butyryl group on the Cys is exchanged for the carboxyl group of the malonyl residue, which is lost as (green). This step is analogous to step in Figure 21-6. The product, a six-carbon β-ketoacyl group, now contains four carbons derived from malonyl-CoA and two derived from the acetyl-CoA that started the reaction. The β-ketoacyl group then undergoes steps through in Figure 21-6.
Seven cycles of condensation and reduction produce the 16-carbon saturated palmitoyl group, still bound to ACP. For reasons not well understood, chain elongation by the synthase complex generally stops at this point, and free palmitate is released from the ACP by a hydrolytic activity (thioesterase; TE) in the multifunctional protein.
We can consider the overall reaction for the synthesis of palmitate from acetyl-CoA in two parts. First, the formation of seven malonyl-CoA molecules:
(21-1)
then seven cycles of condensation and reduction:
(21-2)
Notice that only six net water molecules are produced, because one is used to hydrolyze the thioester linking the palmitate product to the enzyme. The overall process (the sum of Eqns 21-1 and 21-2) is
(21-3)
The biosynthesis of fatty acids such as palmitate thus requires acetyl-CoA and the input of chemical energy in two forms: the group transfer potential of ATP and the reducing power of NADPH. The ATP is required to attach to acetyl-CoA to make malonyl-CoA; the NADPH molecules are required to reduce the β-keto group and the double bond.
In nonphotosynthetic eukaryotes there is an additional cost to fatty acid synthesis, because acetyl-CoA is generated in the mitochondria and must be transported to the cytosol. As we will see, this extra step consumes two ATP per molecule of acetyl-CoA transported, increasing the energetic cost of fatty acid synthesis to three ATP per two-carbon unit.
Fatty Acid Synthesis Is a Cytosolic Process in Most Eukaryotes but Takes Place in the Chloroplasts in Plants
In most eukaryotes, the fatty acid synthase complex (FAS I) is found in the cytosol, as are the biosynthetic enzymes for nucleotides, amino acids, and glucose. This location segregates synthetic processes from degradative reactions, many of which take place in the mitochondrial matrix. There is a corresponding segregation of the electron-carrying cofactors used in anabolism (generally a reductive process) and those used in catabolism (generally oxidative).
Usually, NADPH is the electron carrier for anabolic reactions, and serves in catabolic reactions. The liver, the largest mammalian internal organ and a key metabolic center responding to large changes during feasting and fasting, provides a useful focus for this discussion. In hepatocytes, the ratio is very high (~75) in the cytosol, furnishing a strongly reducing environment for the reductive synthesis of fatty acids and other biomolecules. The cytosolic ratio is much lower , so the -dependent oxidative catabolism of glucose can take place in the same compartment, and at the same time, as fatty acid synthesis. The ratio in the mitochondrion is much higher than that in the cytosol, because of the flow of electrons to from the oxidation of fatty acids, amino acids, pyruvate, and acetyl-CoA. This high mitochondrial ratio favors the reduction of oxygen via the respiratory chain.
In hepatocytes and adipocytes, cytosolic NADPH is largely generated by the pentose phosphate pathway (Fig. 21-8a; also see Fig. 14-30) and by malic enzyme (Fig. 21-8b). The pyruvate produced by the action of malic enzyme reenters the mitochondrion.
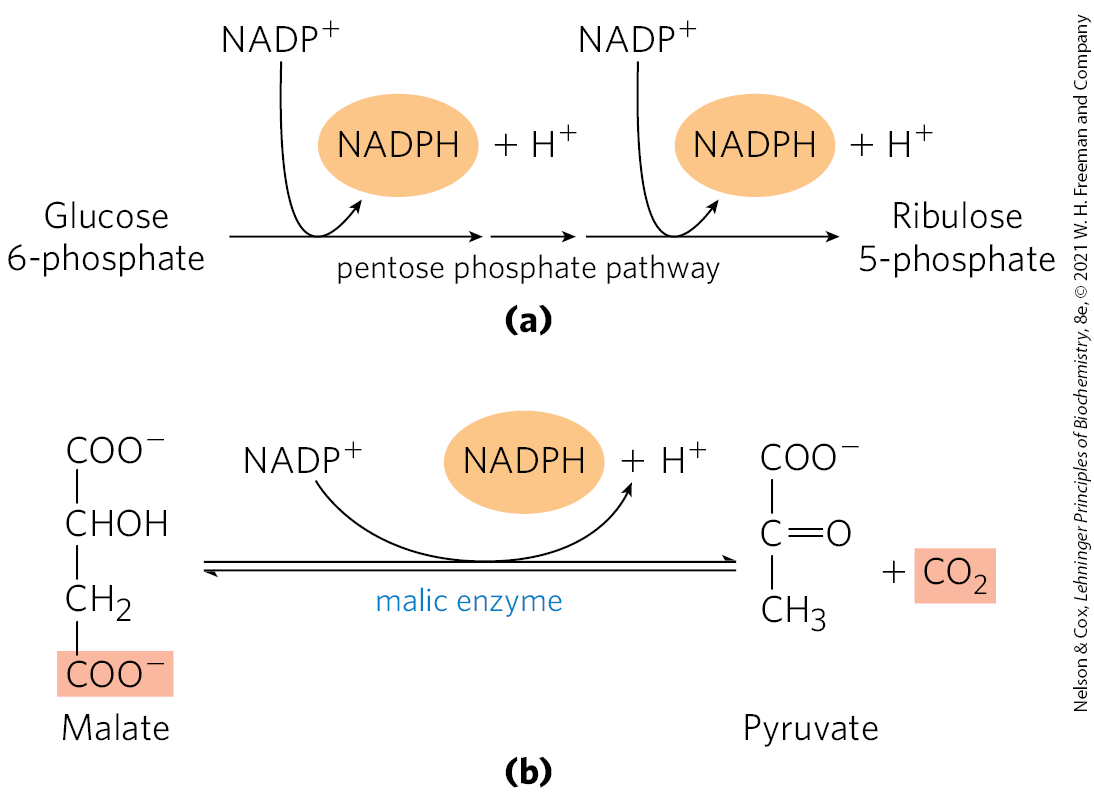
FIGURE 21-8 Production of NADPH. Two routes to NADPH, catalyzed by (a) the pentose phosphate pathway and (b) malic enzyme.
In the photosynthetic cells of plants, fatty acid synthesis occurs not in the cytosol but in the chloroplast stroma (Fig. 21-9). This makes sense, given that NADPH is produced in chloroplasts by the light-dependent reactions of photosynthesis:
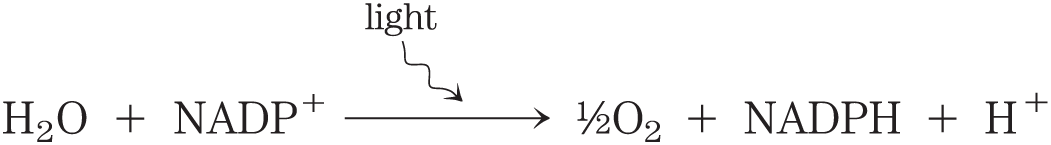
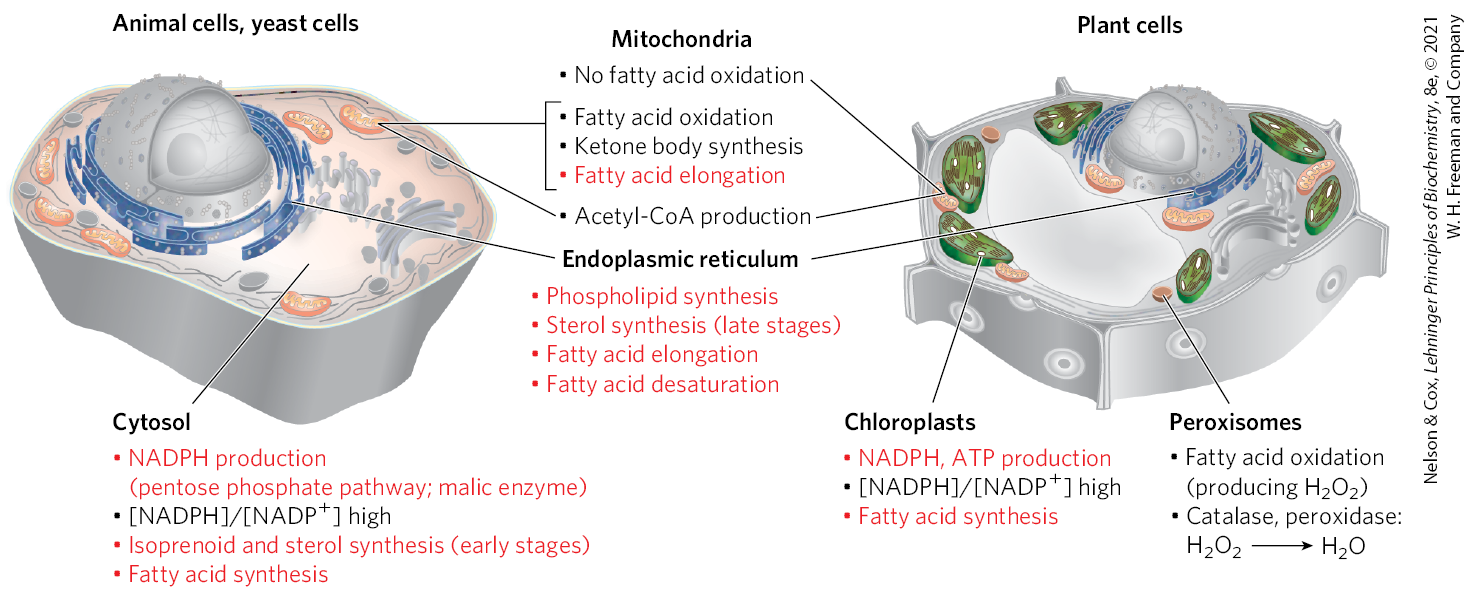
FIGURE 21-9 Subcellular localization of lipid metabolism. Yeast and animal cells differ from higher plant cells in the compartmentation of lipid metabolism. Fatty acid synthesis takes place in the compartment in which NADPH is available for reductive synthesis (i.e., where the ratio is high); this is the cytosol in animals and yeast, and the chloroplast in plants. Processes in red type are addressed in this chapter.
Acetate Is Shuttled out of Mitochondria as Citrate
In nonphotosynthetic eukaryotes, nearly all the acetyl-CoA used in fatty acid synthesis is formed in mitochondria from pyruvate oxidation and from catabolism of the carbon skeletons of amino acids. Acetyl-CoA arising from the oxidation of fatty acids is not a significant source of acetyl-CoA for fatty acid biosynthesis in animals, because the two pathways are reciprocally regulated, as described below.
The inner mitochondrial membrane is impermeable to acetyl-CoA, so an indirect shuttle transfers acetyl group equivalents across the membrane (Fig. 21-10). Intramitochondrial acetyl-CoA first reacts with oxaloacetate to form citrate, in the citric acid cycle reaction catalyzed by citrate synthase (see Fig. 16-7). Citrate then passes through the inner membrane on the citrate transporter. In the cytosol, citrate cleavage by citrate lyase regenerates acetyl-CoA and oxaloacetate in an ATP-dependent reaction. Oxaloacetate cannot return to the mitochondrial matrix directly, as there is no oxaloacetate transporter. Instead, cytosolic malate dehydrogenase reduces the oxaloacetate to malate, which can return to the mitochondrial matrix on the malate–α-ketoglutarate transporter, in exchange for citrate. In the matrix, malate is reoxidized to oxaloacetate to complete the shuttle. However, most of the malate produced in the cytosol is used to generate cytosolic NADPH through the activity of malic enzyme (Fig. 21-8b). The pyruvate produced is transported into the mitochondria by the pyruvate transporter (Fig. 21-10), then converted back into oxaloacetate by pyruvate carboxylase in the matrix. In the resulting cycle, two ATP molecules are consumed (by citrate lyase and pyruvate carboxylase) for every molecule of acetyl-CoA delivered to fatty acid synthesis.
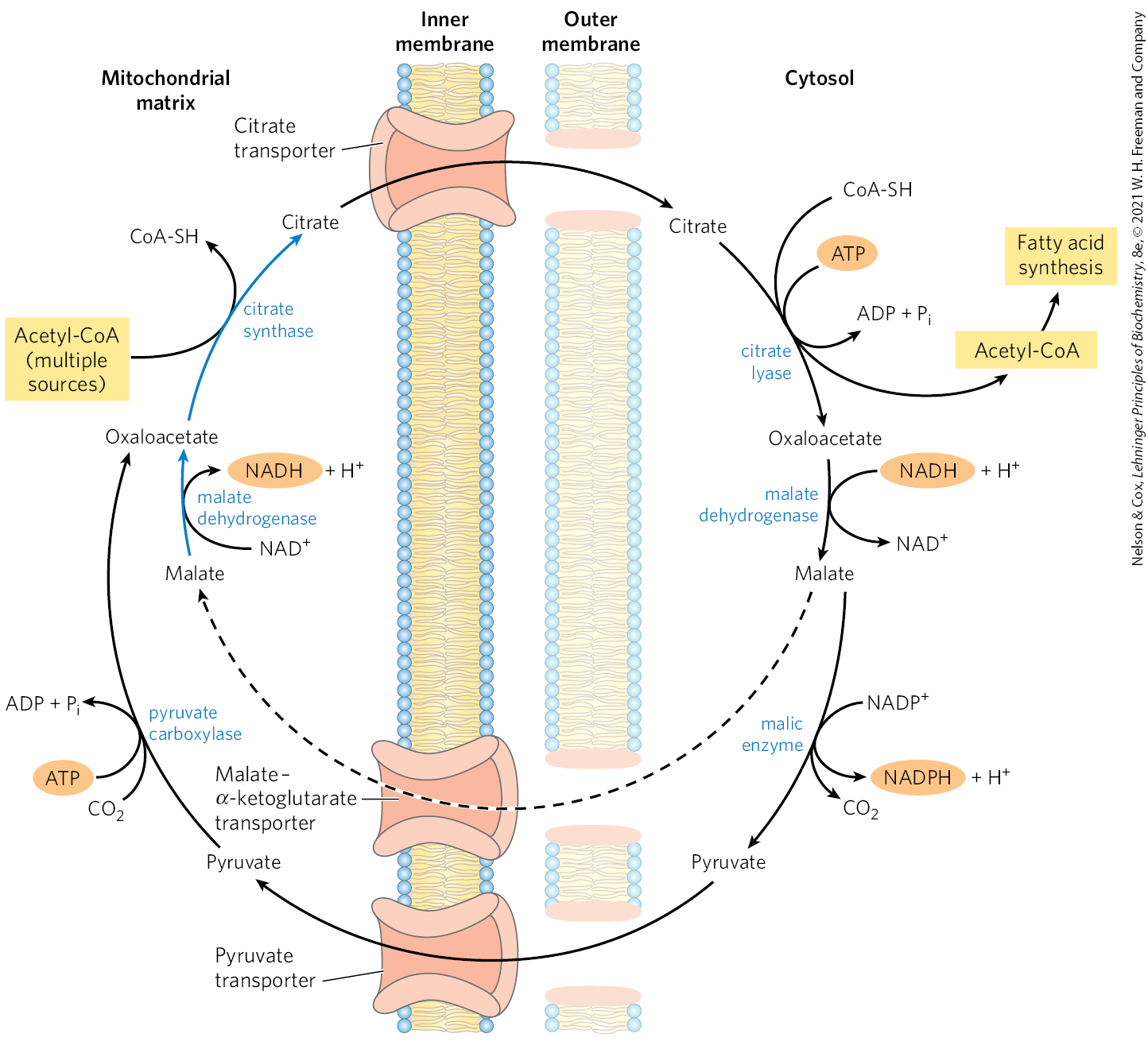
FIGURE 21-10 Shuttle for transfer of acetyl groups from mitochondria to the cytosol. The outer mitochondrial membrane is freely permeable to all these compounds. Pyruvate derived from amino acid catabolism in the mitochondrial matrix, or from glucose by glycolysis in the cytosol, is converted to acetyl-CoA in the matrix. Acetyl groups pass out of the mitochondrion as citrate; in the cytosol they are delivered as acetyl-CoA for fatty acid synthesis. Oxaloacetate is reduced to malate, which can be returned to the mitochondrial matrix. The steps converting malate to oxaloacetate and oxaloacetate plus acetyl-CoA to citrate (indicated by blue arrows) are part of the citric acid cycle. The major fate of cytosolic malate, however, is oxidation by malic enzyme to generate cytosolic NADPH; the pyruvate produced returns to the mitochondrial matrix.
Malate thus has two fates in metabolism. In the mitochondrial matrix, malate is part of the citric acid cycle. In the cytosol, malate degradation becomes a significant source of NADPH. After citrate cleavage to generate acetyl-CoA, conversion of the four remaining carbons to pyruvate and by malic enzyme generates about half the NADPH required for fatty acid synthesis. The pentose phosphate pathway contributes the rest of the needed NADPH.
Fatty Acid Biosynthesis Is Tightly Regulated
When a cell or an organism has more than enough metabolic fuel to meet its energy needs, the excess is generally converted to fatty acids and stored as lipids such as triacylglycerols. The reaction catalyzed by acetyl-CoA carboxylase is the rate-limiting step in the biosynthesis of fatty acids, and this enzyme is an important site of regulation. In vertebrates, palmitoyl-CoA, the principal product of fatty acid synthesis, is a feedback inhibitor of the enzyme; citrate is an allosteric activator (Fig. 21-11a), increasing . Citrate plays a central role in diverting cellular metabolism from the consumption (oxidation) of metabolic fuel to the storage of fuel as fatty acids. When the concentrations of mitochondrial acetyl-CoA and ATP increase, citrate is transported out of mitochondria; it then becomes both the precursor of cytosolic acetyl-CoA and an allosteric signal for the activation of acetyl-CoA carboxylase. At the same time, citrate inhibits the activity of phosphofructokinase-1 (see Fig. 14-23), reducing the flow of carbon through glycolysis.

FIGURE 21-11 Regulation of fatty acid synthesis. (a) In the cells of vertebrates, both allosteric regulation and hormone-dependent covalent modification influence the flow of precursors into malonyl-CoA. In plants, acetyl-CoA carboxylase is activated by the changes in and pH that accompany illumination (not shown here). (b) Filaments of acetyl-CoA carboxylase from chicken hepatocytes (the active, dephosphorylated form), as seen with the electron microscope.
Acetyl-CoA carboxylase is also regulated by covalent modification. Phosphorylation, triggered by the hormones glucagon and epinephrine or by high [AMP], inactivates the enzyme and reduces its sensitivity to activation by citrate, thereby slowing fatty acid synthesis. Phosphorylation occurs on at least three Ser residues and is catalyzed primarily by the AMP-activated protein kinase (AMPK). In its active (dephosphorylated) form, acetyl-CoA carboxylase polymerizes into long filaments (Fig. 21-11b); phosphorylation is accompanied by dissociation into monomeric subunits and loss of activity.
The acetyl-CoA carboxylase of plants and bacteria is not regulated by citrate or by a phosphorylation-dephosphorylation cycle. Instead, the plant enzyme is activated by an increase in stromal pH and , which occurs on illumination of the plant (see Fig. 20-35). Bacteria do not use triacylglycerols as energy stores. In Escherichia coli, the primary role of fatty acid synthesis is to provide precursors for membrane lipids; the regulation of this process is complex, employing guanine nucleotides (such as ppGpp; see Fig. 8-42) that coordinate cell growth with membrane formation.
In addition to the moment-by-moment regulation of enzymatic activity, these pathways are regulated at the level of gene expression. For example, when animals ingest an excess of certain polyunsaturated fatty acids, the expression of genes encoding many lipogenic enzymes in the liver is suppressed. This gene regulation is mediated by a family of nuclear receptor proteins called PPARs, which we encountered in Section 17.2.
If fatty acid synthesis and β oxidation were to proceed simultaneously, the two processes would constitute a futile cycle, wasting energy. We noted earlier (see Fig. 17-13) that β oxidation is blocked by malonyl-CoA, which inhibits carnitine acyltransferase I. Thus, during fatty acid synthesis, production of the first intermediate, malonyl-CoA, shuts down β oxidation at the level of a transport system in the inner mitochondrial membrane. This control mechanism illustrates another advantage of segregating synthetic and degradative pathways in different cellular compartments.
Long-Chain Saturated Fatty Acids Are Synthesized from Palmitate
Palmitate, the principal product of the fatty acid synthase system in animal cells, is the precursor of other long-chain fatty acids (Fig. 21-12). It may be lengthened to form stearate (18:0) or even longer saturated fatty acids by further additions of acetyl groups, through the action of fatty acid elongation systems present in the smooth endoplasmic reticulum (smooth ER) and in mitochondria. The more active elongation system of the ER extends the 16-carbon chain of palmitoyl-CoA by two carbons, forming stearoyl-CoA. Although different enzyme systems are used, and coenzyme A rather than ACP is the acyl carrier in the reaction, the mechanism of elongation in the ER is otherwise identical to that in palmitate synthesis: donation of two carbons by malonyl-CoA, followed by reduction, dehydration, and reduction to the saturated 18-carbon product, stearoyl-CoA.
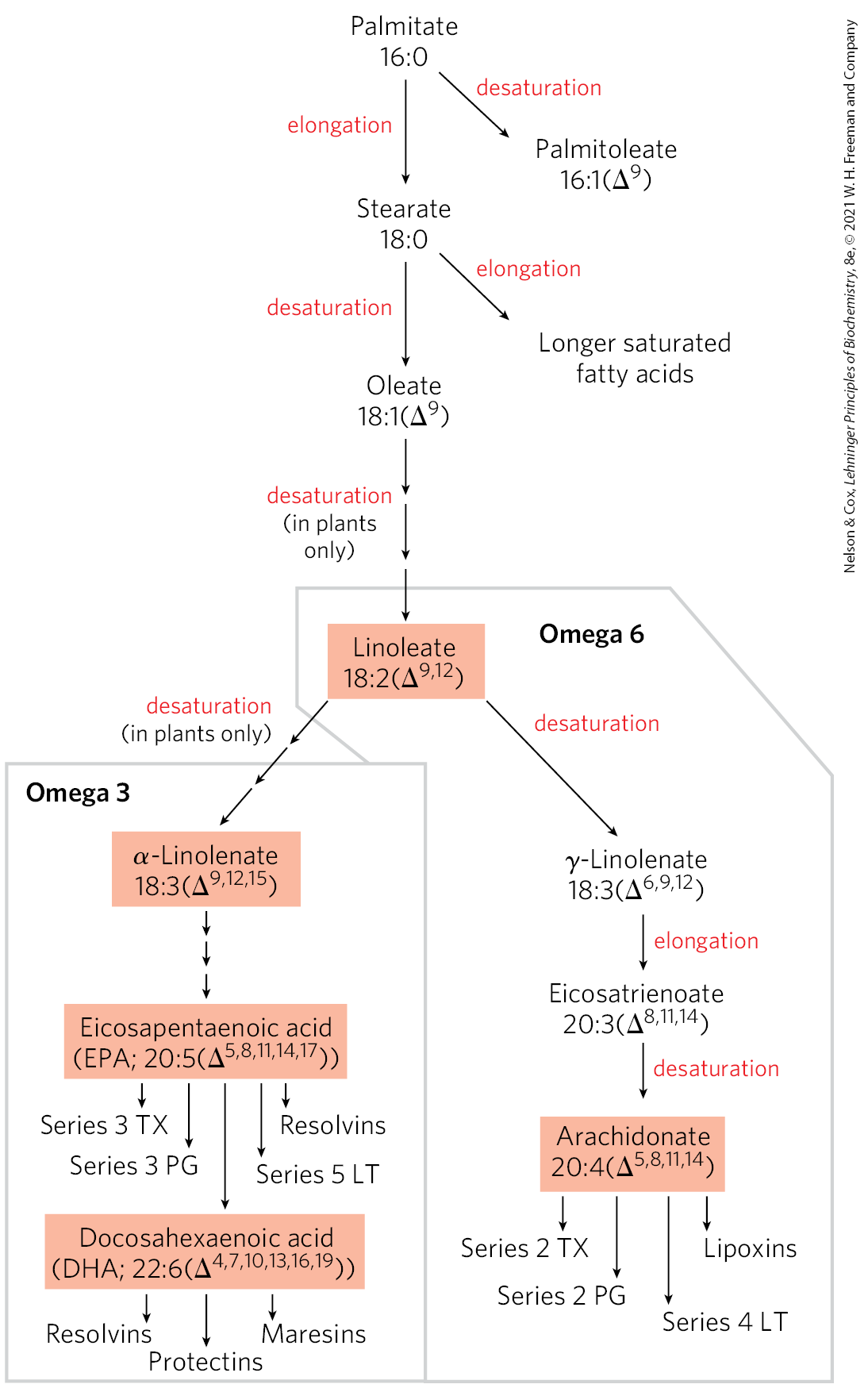
FIGURE 21-12 Routes of synthesis of unsaturated fatty acids and their derivatives. Palmitate is the precursor of stearate and longer-chain saturated fatty acids, as well as the monounsaturated acids palmitoleate and oleate. Mammals cannot convert oleate to linoleate or α-linolenate (shaded), which are therefore required in the diet as essential fatty acids. Conversion of linoleate to other polyunsaturated fatty acids and eicosanoids is outlined. Unsaturated fatty acids are symbolized by indicating the number of carbons and the number and position of double bonds, as in Table 10-1. Linoleate and α-linolenate are important omega-6 and omega-3 fatty acids, respectively; they are also precursors for a wide range of unsaturated fatty acids that act as signaling molecules. Two-letter abbreviations specify the eicosanoid prostaglandins (PG), thromboxanes (TX), and leukotrienes (LT). Particular classes of unsaturated fatty acids are further delineated by the number of double bonds, which defines subclasses referred to as series. For example, series 2 TX are thromboxanes with two double bonds in the hydrocarbon chain.
Two key products of elongation pathways are linoleate, an omega-6 fatty acid (see Chapter 10 for the alternative nomenclature), and α-linolenate, an omega-3 fatty acid. These are precursors for two extensive families of derivative unsaturated fatty acids, the omega-6 and omega-3 families. Humans cannot synthesize linoleate and α-linolenate and must obtain them in the diet. The ratio of omega-6 to omega-3 fatty acids in the diet, if too high, can lead to cardiovascular disease. The importance of this ratio may reflect the multitude of signaling molecules in the omega-6 and omega-3 families (Fig. 21-12), with their equally complex physiological effects. Several of these derivative unsaturated fatty acids are considered below.
Desaturation of Fatty Acids Requires a Mixed-Function Oxidase
Palmitate and stearate serve as precursors of the two most common monounsaturated fatty acids of animal tissues: palmitoleate, , and oleate, ; both of these fatty acids have a single cis double bond between C-9 and C-10 (see Table 10-1). The double bond is introduced into the fatty acid chain by an oxidative reaction catalyzed by fatty acyl–CoA desaturase (Fig. 21-13), a mixed-function oxidase (Box 21-1). Two different substrates, the fatty acid and NADPH, simultaneously undergo two-electron oxidations. The path of electron flow includes a cytochrome (cytochrome ) and a flavoprotein (cytochrome reductase), both of which, like fatty acyl–CoA desaturase, are in the smooth ER. In plants, oleate is produced by a stearoyl-ACP desaturase (SCD) that uses reduced ferredoxin as the electron donor in the chloroplast stroma.
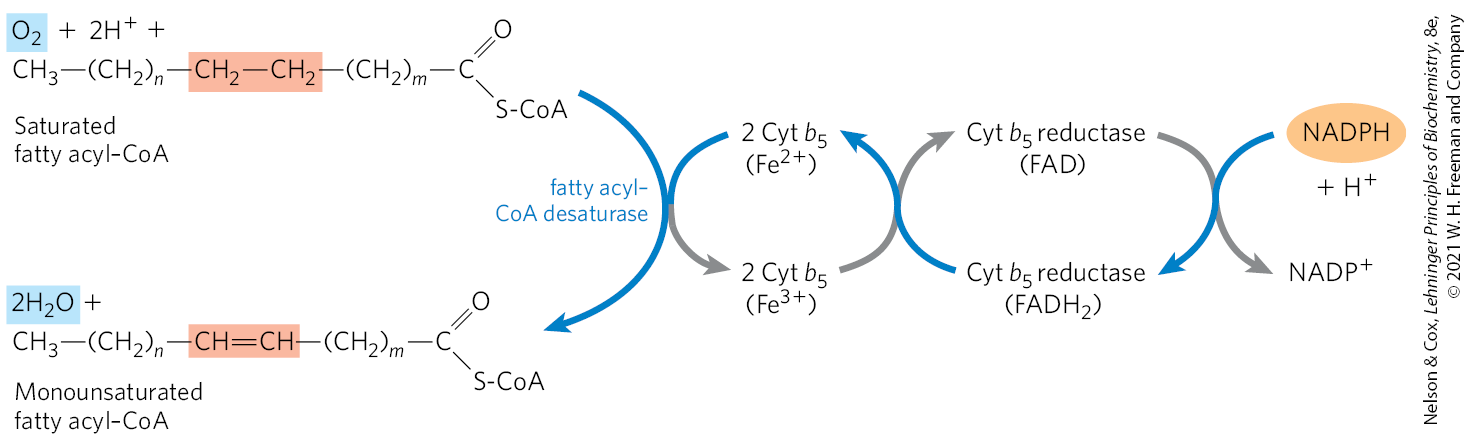
FIGURE 21-13 Electron transfer in the desaturation of fatty acids in vertebrates. Blue arrows show the path of electrons as two substrates — a fatty acyl–CoA and NADPH — undergo oxidation by molecular oxygen. These reactions take place on the lumenal face of the smooth ER. A similar pathway, but with different electron carriers, occurs in plants.
The SCD of animals (as studied in mice) has an important role in the development of obesity and the insulin resistance that often accompanies obesity and precedes development of type 2 diabetes mellitus. Mice have four isozymes, SCD1 through SCD4, of which SCD1 is the best understood. Its synthesis is induced by dietary saturated fatty acids, and also by the action of SREBP and LXR, two protein regulators of lipid metabolism that activate transcription of lipid-synthesizing enzymes (described in Section 21.4). Mice with mutant forms of SCD1 are resistant to diet-induced obesity and do not develop diabetes under conditions that cause both obesity and diabetes in mice with normal SCD1.
Mammalian hepatocytes can readily introduce double bonds at the position of fatty acids but cannot introduce additional double bonds between C-10 and the methyl-terminal end. Thus, as noted above, mammals cannot synthesize the omega-6 family precursor linoleate, , or the omega-3 family precursor α-linolenate, . Plants, however, can synthesize both; the desaturases that introduce double bonds at the and positions are located in the ER and in chloroplasts. The ER enzymes act not on free fatty acids but on a phospholipid, phosphatidylcholine, that contains at least one oleate linked to the glycerol (Fig. 21-14). Both plants and bacteria must synthesize polyunsaturated fatty acids to ensure membrane fluidity at reduced temperatures.
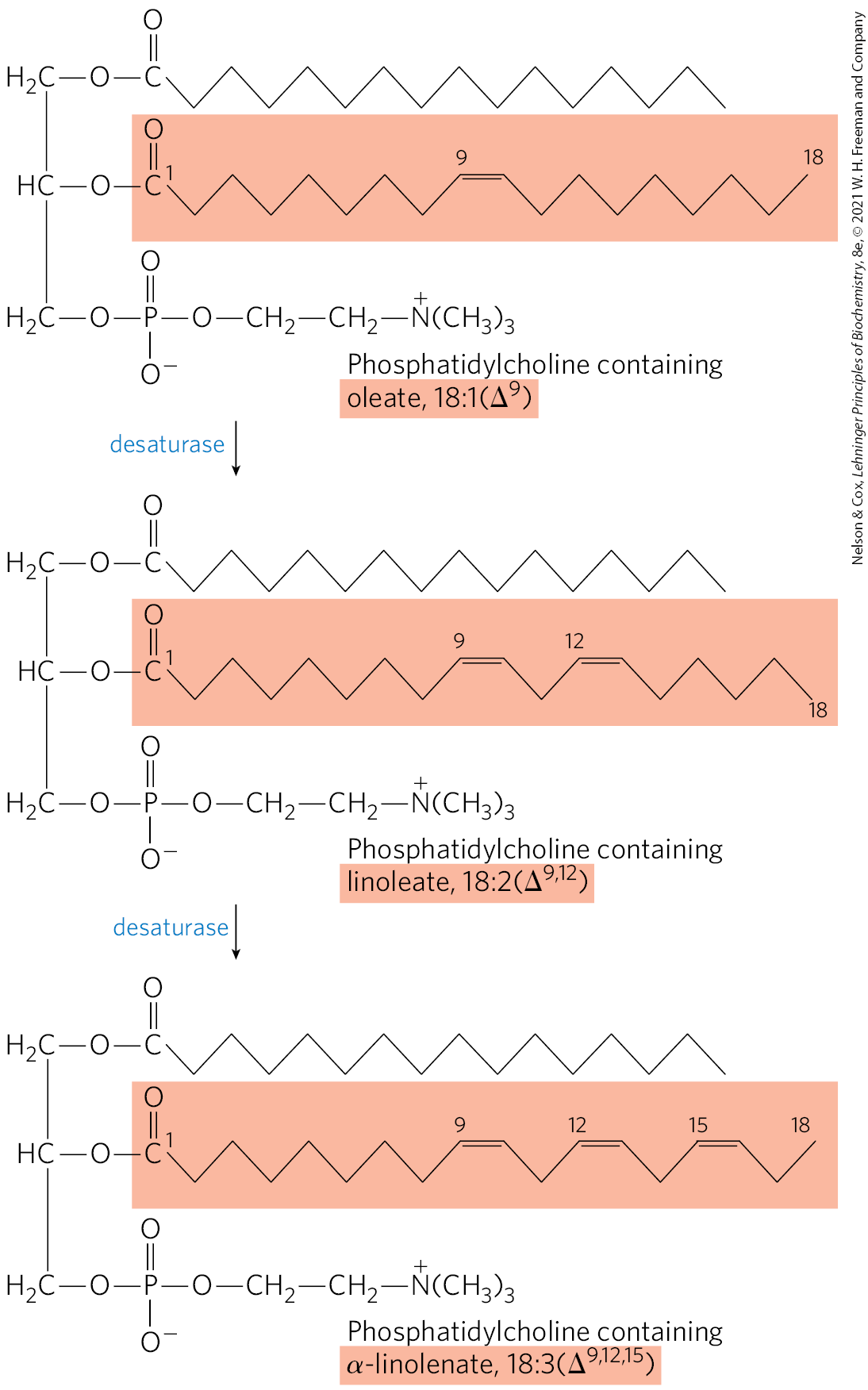
FIGURE 21-14 Action of plant desaturases. Desaturases in plants oxidize phosphatidylcholine-bound oleate to polyunsaturated fatty acids. Some of the products are released from the phosphatidylcholine by hydrolysis.
The top molecule has a vertical three-carbon chain with the top carbon bonded to 2 H and to O to the right bonded to C double bonded to O above further bonded to a zigzag chain of 15 additional carbons to the right. The middle carbon is bonded to H and to O to the right bonded to C in a light red box that is numbered 1, double bonded to O above, and further bonded to a zigzag chain of 17 additional carbons with the final carbon numbered as 18. There is a cis double bond between C 9, which is numbered, and C 10 to the right. The entire eighteen-carbon sequence from C double bonded to O to C 18 is enclosed in a light red box. The bottom C is bonded to 2 H and to O on the right further bonded to P double bonded to O above, bonded to O minus below, and bonded to O to the right further bonded to C H 2 bonded to C H 2 bonded to N plus (C H 3) 3. This molecule is labeled phosphatidylcholine containing oleate, 18:1 (delta superscript 9 end superscript), with oleate and its structural abbreviation shown in a light red box. An arrow labeled desaturase points down to a similar molecule in which the eighteen-carbon chain now has cis double bonds between C 9 and C 10 and between C 11 and C 13. This molecule is labeled phosphatidylcholine containing linoleate, 18:2 (delta superscript 9, 12 end superscript), with linoleate and its structural abbreviation shown in a light red box. An arrow labeled desaturase points down to a similar molecule in which the eighteen-carbon chain now has cis double bonds between C 9 and C 10, C 11 and C 13, and C 15 and C 16. This molecule is labeled phosphatidylcholine containing alpha-linoleate, 18:3 (delta superscript 9, 12, 15 end superscript), with alpha-linoleate and its structural abbreviation shown in a light red box.
Because they are necessary precursors for the synthesis of other products, linoleate and α-linolenate are essential fatty acids for mammals; they must be obtained from dietary plant material. Once ingested, linoleate may be converted to certain other polyunsaturated acids, particularly γ-linolenate, eicosatrienoate, and arachidonate (eicosatetraenoate), all of which can be made only from linoleate (Fig. 21-12). Similarly, α-linolenate is converted to two important derivatives, eicosapentaenoic acid (EPA) and docosa-hexaenoic acid (DHA). Arachidonate, , EPA, , and DHA, , are essential precursors of distinct classes of eicosanoids, lipids with important regulatory functions. The 20- and 22-carbon fatty acids are synthesized from linoleate and -linolenate by fatty acid elongation reactions analogous to those described on page 753.
Eicosanoids Are Formed from 20- and 22-Carbon Polyunsaturated Fatty Acids
Eicosanoids are a family of very potent biological signaling molecules that act as short-range messengers, affecting tissues near the cells that produce them.
In response to hormonal or other stimuli, phospholipase , present in most types of mammalian cells, attacks membrane phospholipids, releasing arachidonate from the middle carbon of glycerol. Enzymes of the smooth ER then convert arachidonate to prostaglandins, beginning with the formation of prostaglandin , the immediate precursor of many other prostaglandins and thromboxanes (Fig. 21-15a). The two reactions that lead to are catalyzed by a bifunctional enzyme, cyclooxygenase (COX), also called prostaglandin synthase. In the first step, the cyclooxygenase activity introduces molecular oxygen to convert arachidonate to . The second step, catalyzed by the peroxidase activity of COX, converts to .
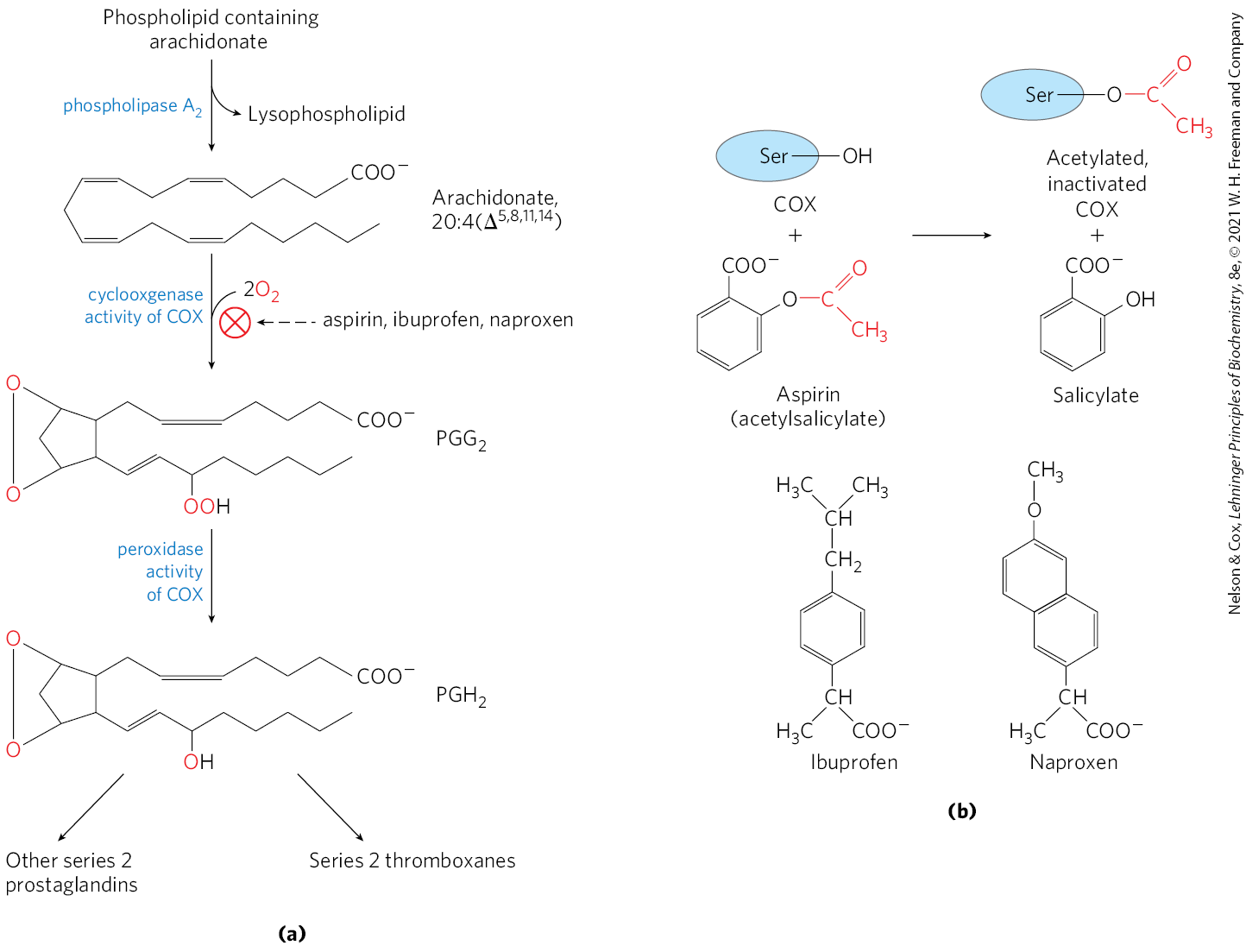
FIGURE 21-15 The “cyclic” pathway from arachidonate to prostaglandins and thromboxanes. (a) After arachidonate is released from phospholipids by the action of phospholipase , the cyclooxygenase and peroxidase activities of COX (also called prostaglandin synthase) catalyze the production of , the precursor of other prostaglandins and of thromboxanes. (b) Aspirin inhibits the first reaction by acetylating an essential Ser residue on the enzyme. Ibuprofen and naproxen inhibit the same step, probably by mimicking the structure of the substrate or an intermediate in the reaction.
Part a begins with text at the upper left reading, phospholipid containing arachidonate. An arrow labeled phospholipidase A subscript 2 points down to arachidonate, 20:4 (delta superscript 5, 8, 11, 14), accompanied by a curved arrow showing the loss of lysophospholipid. Arachidonate has C O O minus at the upper right that begins a chain of 20 carbons with a curve at one end to form a looped structure that is open on the right side. C O O minus at the top right has a zigzag chain of three carbons to its left before reaching a cis double bond between the fifth and sixth carbons, which forms the top of a structure that resembles a hexagon with sides that don’t come together to form left and right vertices. From C 6, there is a bond to C H 2 and then a bond C 8, which is cis double bonded to C 9 at the upper left corner. C 9 has a bond to C H 2 at the lower left forming the left end, from which a bond extends to C 11 at the lower right that forms a double bond with C 12 beneath the double bond between C 8 and C 9. This gives the appearance of a partial ring that is open on its right side. C 12 is bonded to C H 2 to the upper right, which is bonded to C 14. C 14 is double bonded to C 15, forming the bottom side of the second partial ring-like structure. From C 15, a zigzag chain of five additional carbons completes the bottom half of the structure. An arrow labeled cyclooxygenase activity of C O X points down accompanied by a curved arrow showing the addition of 2 red highlighted O 2. A red “X” in a red circle next to the downward arrow is indicated by a dashed horizontal arrow from aspirin, ibuprofen, naproxen. This points down to a similar structure below labeled P G G 2. It has C O O minus at the upper right bonded to a zigzag chain of four carbons with the fourth carbon in this chain, C 5, forming a cis double bond to C 6. C 6 is bonded to C 7 to the upper left, forming the top vertex of a partial double six-membered ring missing its right side, with a double bond at its lower right side, and with a left side shared with a five-membered ring that has a left side vertex and upper and lower left vertices each bonded to red highlighted O that are joined together by a vertical bond. The lower right vertex of the partial six-membered ring has a bond down to a vertex from which a bond extends down to red highlighted O bonded to red highlighted O bonded to nonhighlighted H. From here, a zigzag chain of five carbons completes the bottom half of the molecule. An arrow labeled peroxidase activity of C O X points down to P G H 2, which is similar except that the bond to O bonded to O H at the bottom center is now a bond to red highlighted O bonded to nonhighlighted H. Arrows point down to the lower left and right. The left-hand arrow points to other series 2 prostaglandins and the right-hand arrow points to series 2 thromboxanes. Part b begins with a blue oval labeled S e r with a bond to O H to the right. Text below reads, C O X. This is added to aspirin (acetylsalicylate), which is a benzene ring with the top vertex bonded to CO O minus and the upper right vertex bonded to O further connected by a red bond to red highlighted C connected by a red double bond to red highlighted O to the upper right and by a red bond to red highlighted C H 3 to the lower right. An arrow points right to show that this yields a blue oval labeled S e r bonded to O to the right further connected by a red bond to red highlighted C connected by a red highlighted double bond to O to the upper right and by a red bond to red highlighted C H 3 to the lower right. Text below reads, acetylated, inactivated C O X. Salicylate is also present and is a benzene ring with the top vertex bonded to C O O minus and the upper right vertex bonded to O H. Below, ibuprofen is shown as a benzene ring with its top vertex bonded to C H 2 bonded to C H 2 bonded to C H 3 to the upper right and left and with its bottom vertex bonded to C H bonded to C H 3 to the lower left and bonded to C O O minus to the lower right. Naproxen is shown as a benzene ring with its top vertex bonded to O bonded to C H 3 above and with its lower right side shared with the upper let side of a second benzene ring with its bottom vertex bonded to C H bonded to C H 3 to the lower left and to C O O minus to the lower right.
Key Convention
Prostaglandins with different functional groups on the ring are given different letter designations: A, B, C, D, E, F, G, H, and R. The subscript number following the letter, as in and , indicates the number of double bonds. Prostaglandins with two double bonds, all of which are derived from arachidonate, are referred to as series 2 prostaglandins; those with three double bonds, derived from EPA, as series 3 (Fig. 21-12). Similar naming patterns are used for other classes of eicosanoids described below.
Series 2 prostaglandins have important roles in the immediate response to stress or injury, including inflammation, pain, swelling, and dilation of blood vessels. Series 3 prostaglandins, in general, act more slowly and usually moderate the responses associated with series 2 prostaglandins.
Mammals have two isozymes of prostaglandin synthase, COX-1 and COX-2. These have different functions but closely similar amino acid sequences (60% to 65% sequence identity) and similar reaction mechanisms at both of their catalytic centers. COX-1 is responsible for synthesis of the prostaglandins that regulate the secretion of gastric mucin, and COX-2 is responsible for synthesis of the prostaglandins that mediate inflammation, pain, and fever.
Pain can be relieved by inhibiting COX-2. The first drug widely marketed for this purpose was aspirin (acetylsalicylate; Fig. 21-15b). The name “aspirin” (from a for acetyl and spir for Spirsaüre, the German word for the salicylates prepared from the plant Spiraea ulmaria) appeared in 1899 when the drug was introduced by the Bayer company. Aspirin irreversibly inactivates the cyclooxygenase activity of both COX isozymes, by acetylating a Ser residue and blocking each enzyme’s active site. The synthesis of prostaglandins and thromboxanes is thereby inhibited. Additional widely used nonsteroidal anti-inflammatory drugs (NSAIDs; Fig. 21-15b), ibuprofen and naproxen, inhibit the same pair of enzymes. However, the inhibition of COX-1 can result in undesired side effects, including stomach irritation and more serious conditions. In the 1990s, NSAID compounds that had a greater specificity for COX-2 were developed as advanced therapies for severe pain. Three of these drugs were approved for use worldwide: rofecoxib (Vioxx), valdecoxib (Bextra), and celecoxib (Celebrex). Though initially considered a success, Vioxx and Bextra were withdrawn as field reports and clinical studies connected the drugs with an increased risk of heart attack and stroke. Celebrex is still on the market but is being used with increased caution. The detailed reasons for the problems with these drugs are still not clear but serve as a cautionary note. We are increasingly aware of the complexity of the web of these signaling interactions, and predicting the consequences of targeting specific components with pharmaceutical agents remains an imperfect process.
Thromboxane synthase, present in blood platelets (thrombocytes), converts to thromboxane , from which other series 2 thromboxanes are derived (Fig. 21-15a). The series 2 thromboxanes induce constriction of blood vessels and platelet aggregation, early steps in blood clotting. Low doses of aspirin, taken regularly, reduce the probability of heart attacks and strokes by reducing thromboxane production.
Thromboxanes, like prostaglandins, contain a ring of five or six atoms; the pathway from arachidonate to the series 2 prostaglandins and thromboxanes is sometimes called the “cyclic” pathway, to distinguish it from the “linear” pathway that leads from arachidonate to the leukotrienes, which are linear compounds (Fig. 21-16). Leukotriene synthesis begins with the action of several lipoxygenases that catalyze the incorporation of molecular oxygen into arachidonate. These enzymes, found in leukocytes and in heart, brain, lung, and spleen, are mixed-function oxidases of the cytochrome P-450 family (see Box 21-1). The various leukotrienes differ in the position of the peroxide group introduced by the lipoxygenases. The linear pathway from arachidonate, unlike the cyclic pathway, is not inhibited by aspirin or other NSAIDs.
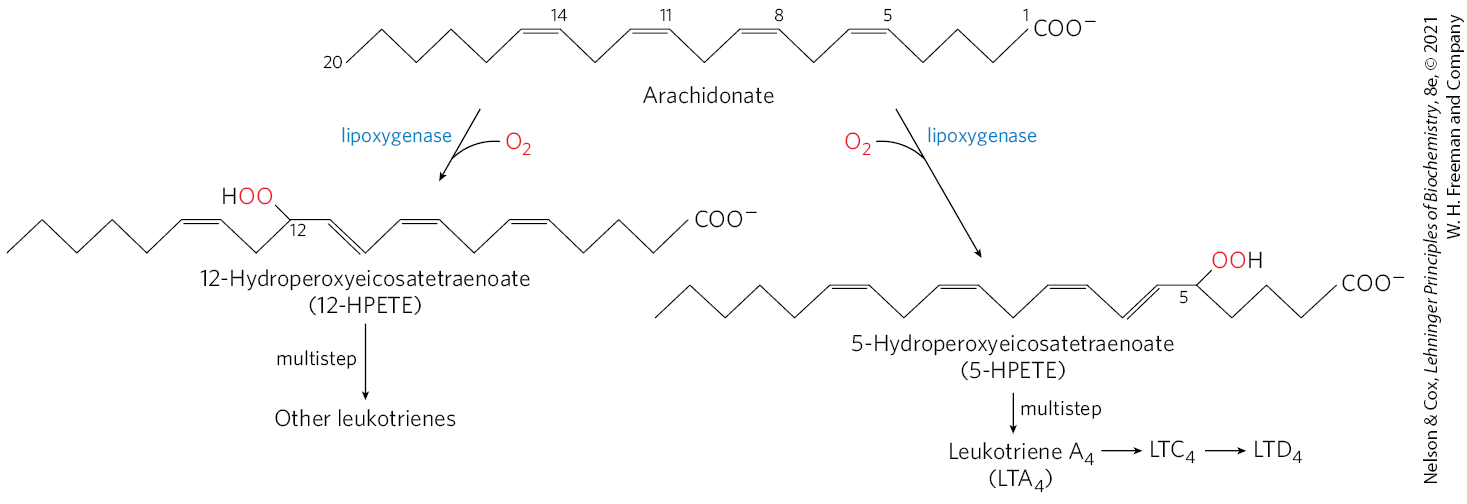
FIGURE 21-16 The “linear” pathway from arachidonate to leukotrienes.
Arachidonate is shown at the top as a horizontal zigzag chain with carbons 1, 5, 8, 11, 14, and 20 numbered. C 1 at the right forms C O O minus, C 5 forms a cis double bond with C 6, C 8 forms a cis double bond with C 9, C 11 forms a cis double bond with C 12, C 14 forms a cis double bond with C 15, and C 20 is the terminal carbon on the left. Arrows point down to the lower left and right. The left-hand arrow is labeled lipoxygenase and a curved arrows shows the addition of red highlighted O 2. This yields 12-hydroperoxyeicosatetraenoate (12-H P T E). This has a similar structure to the reactant except that there is a double bond between C 10 and C 11, a single bond between C 11 and C 12, and a bond from C 12 to red highlighted O bonded to red highlighted O bonded to nonhighlighted H. An arrow labeled multistep points down to text reading, other leukotrienes. The right-hand arrow is labeled lipoxygenase and a curved arrows shows the addition of red highlighted O 2. This yields 5-hydroperoxyeicosatetraenoate (5-H P T E). This has a similar structure to the reactant except that C 5 is bonded to red highlighted O bonded to red highlighted O bonded to nonhighlighted H, there is no double bond between C 5 and C 6, and there is a double bond between C 6 and C 7. An arrow labeled multistep points down to leukotriene A subscript 4 end subscript (L T A subscript 4 end subscript), from which an arrow points right to L T C subscript 4 end subscript, from which an arrow points right to L T D subscript 4 and subscript.
Pathogenic organisms, as well as irritants such as air pollution and tobacco smoke, trigger an inflammatory response in the affected tissue, which consists of two phases: initiation and resolution. Eicosanoids of the omega-6 family are critical to initiation — playing key roles in recruiting leukocytes, making blood vessels more permeable, and stimulating chemotaxis and migration of immune system cells. As the source of tissue damage is brought under control, the inflammation must be resolved and the tissue brought back to its normal state. Resolution of inflammation is called catabasis, and it is promoted by several classes of signaling molecules; prominent among these are several leukotrienes and prostaglandins. Many eicosanoids of the omega-3 family (including series 3 prostaglandins and thromboxanes) are anti-inflammatory, although the classification is not absolute; individual eicosanoids can be inflammatory in one tissue and anti-inflammatory in another.
Catabasis is also promoted by a set of eicosanoids termed specialized pro-resolving mediators (SPMs). The first family of SPMs to be discovered was the lipoxins, followed more recently by resolvins, protectins, and maresins. All SPMs are derived from essential fatty acids (Fig. 21-12). They affect different target cells and tissues in different ways. The sum of their action is to promote removal of debris, microbes, and dead cells, to restore blood vessel integrity, and to regenerate tissue. Particular SPMs also reduce pain and fever, and play roles in resolving the tissue inflammation leading to diabetes, obesity, and asthma. Further research on SPMs thus has potential for the development of new pharmaceutical targets.
Plants also derive important signaling molecules from fatty acids. As in animals, a key step in the initiation of signaling is activation of a specific phospholipase. In plants, the fatty acid substrate released by phospholipase action is α-linolenate. A lipoxygenase then catalyzes the first step in a pathway that converts α-linolenate to jasmonate, a substance known to have signaling roles in defense against insects, resistance to fungal pathogens, and maturation of pollen. Jasmonate also affects seed germination, root growth, and fruit and seed development.
SUMMARY 21.1 Biosynthesis of Fatty Acids and Eicosanoids
- Malonyl-CoA, a key precursor of fatty acids, is synthesized by the action of acetyl-CoA carboxylase.
- Beginning with malonyl-CoA and acetyl-CoA, fatty acids are synthesized in a repeating cycle of four steps.
- Long-chain saturated fatty acids are synthesized from acetyl-CoA by a cytosolic system of six enzymatic activities plus acyl carrier protein (ACP). There are two types of fatty acid synthase. FAS I, found in vertebrates and fungi, consists of multifunctional polypeptides. FAS II is a dissociated system found in bacteria and plants. Both contain two types of OSH groups (one furnished by the phosphopantetheine of ACP, the other by a Cys residue of β-ketoacyl-ACP synthase) that function as carriers of the fatty acyl intermediates.
- Malonyl-ACP, formed from acetyl-CoA (shuttled out of mitochondria) and , condenses with an acetyl bound to the Cys OSH to yield acetoacetyl-ACP, with release of . This is followed by reduction to the d-β-hydroxy derivative, dehydration to the trans--unsaturated acyl-ACP, and reduction to butyryl-ACP. NADPH is the electron donor for both reductions. Fatty acid synthesis is regulated at the level of malonyl-CoA formation.
- Six more molecules of malonyl-ACP react successively at the carboxyl end of the growing fatty acid chain to form palmitoyl-ACP — the end product of the fatty acid synthase reaction. Free palmitate is released by hydrolysis.
- Fatty acid synthesis occurs in the cytosol of animal cells, and in chloroplasts in plants.
- Acetate is exported from the mitochondria as citrate.
- Fatty acid synthesis is tightly regulated, principally by regulation of acetyl-CoA carboxylase.
- Palmitate may be elongated to the 18-carbon stearate.
- Palmitate and stearate can be desaturated to yield palmitoleate and oleate, respectively, by the action of mixed-function oxidases.
- Mammals cannot make linoleate and must obtain it from plant sources; they convert exogenous linoleate to arachidonate, the parent compound of eicosanoids (prostaglandins, thromboxanes, leukotrienes, and specialized pro-resolving mediators), a family of very potent signaling molecules. The synthesis of prostaglandins and thromboxanes is inhibited by NSAIDs that act on the cyclooxygenase activity of prostaglandin synthase.
 Even when compared to other major classes of metabolites, the division between fatty acid biosynthesis and breakdown is particularly striking. The two processes occur by different pathways, catalyzed by different sets of enzymes, and, in eukaryotes, occur in different cellular compartments. Fatty acid breakdown occurs in the mitochondria, whereas biosynthesis occurs in the cytosol. Moreover, biosynthesis requires the participation of a three-carbon intermediate, malonyl-CoA, that does not appear in the path of fatty acid breakdown.
Even when compared to other major classes of metabolites, the division between fatty acid biosynthesis and breakdown is particularly striking. The two processes occur by different pathways, catalyzed by different sets of enzymes, and, in eukaryotes, occur in different cellular compartments. Fatty acid breakdown occurs in the mitochondria, whereas biosynthesis occurs in the cytosol. Moreover, biosynthesis requires the participation of a three-carbon intermediate, malonyl-CoA, that does not appear in the path of fatty acid breakdown. Condensation of an activated acyl group (an acetyl group from acetyl-CoA is the first acyl group) and two carbons derived from malonyl-CoA, with elimination of from the malonyl group, extends the acyl chain by two carbons. The mechanism of the first step of this reaction is given to illustrate the role of decarboxylation in facilitating condensation. The β-keto product of the condensation is then reduced in three more steps nearly identical to the reactions of β oxidation, but in the reverse sequence:
Condensation of an activated acyl group (an acetyl group from acetyl-CoA is the first acyl group) and two carbons derived from malonyl-CoA, with elimination of from the malonyl group, extends the acyl chain by two carbons. The mechanism of the first step of this reaction is given to illustrate the role of decarboxylation in facilitating condensation. The β-keto product of the condensation is then reduced in three more steps nearly identical to the reactions of β oxidation, but in the reverse sequence:  the β-keto group is reduced to an alcohol,
the β-keto group is reduced to an alcohol,  elimination of creates a double bond, and
elimination of creates a double bond, and  the double bond is reduced to form the corresponding saturated fatty acyl group.
the double bond is reduced to form the corresponding saturated fatty acyl group. in
in  are described in the text.
are described in the text. The use of activated malonyl groups rather than acetyl groups makes the condensation reactions thermodynamically favorable. The methylene carbon (C-2) of the malonyl group, sandwiched between carbonyl and carboxyl carbons, forms a good nucleophile. In the condensation step, decarboxylation of the malonyl group facilitates nucleophilic attack of the methylene carbon on the thioester linking the acetyl group to β-ketoacyl-ACP synthase, displacing the enzyme’s group. Coupling the condensation to the decarboxylation of the malonyl group renders the overall process highly exergonic. A similar carboxylation-decarboxylation sequence facilitates the formation of phosphoenolpyruvate from pyruvate in gluconeogenesis (see
The use of activated malonyl groups rather than acetyl groups makes the condensation reactions thermodynamically favorable. The methylene carbon (C-2) of the malonyl group, sandwiched between carbonyl and carboxyl carbons, forms a good nucleophile. In the condensation step, decarboxylation of the malonyl group facilitates nucleophilic attack of the methylene carbon on the thioester linking the acetyl group to β-ketoacyl-ACP synthase, displacing the enzyme’s group. Coupling the condensation to the decarboxylation of the malonyl group renders the overall process highly exergonic. A similar carboxylation-decarboxylation sequence facilitates the formation of phosphoenolpyruvate from pyruvate in gluconeogenesis (see  When a cell or an organism has more than enough metabolic fuel to meet its energy needs, the excess is generally converted to fatty acids and stored as lipids such as triacylglycerols.
When a cell or an organism has more than enough metabolic fuel to meet its energy needs, the excess is generally converted to fatty acids and stored as lipids such as triacylglycerols.  The reaction catalyzed by acetyl-CoA carboxylase is the rate-limiting step in the biosynthesis of fatty acids, and this enzyme is an important site of regulation. In vertebrates, palmitoyl-CoA, the principal product of fatty acid synthesis, is a feedback inhibitor of the enzyme; citrate is an allosteric activator
The reaction catalyzed by acetyl-CoA carboxylase is the rate-limiting step in the biosynthesis of fatty acids, and this enzyme is an important site of regulation. In vertebrates, palmitoyl-CoA, the principal product of fatty acid synthesis, is a feedback inhibitor of the enzyme; citrate is an allosteric activator  The SCD of animals (as studied in mice) has an important role in the development of obesity and the insulin resistance that often accompanies obesity and precedes development of type 2 diabetes mellitus. Mice have four isozymes, SCD1 through SCD4, of which SCD1 is the best understood. Its synthesis is induced by dietary saturated fatty acids, and also by the action of SREBP and LXR, two protein regulators of lipid metabolism that activate transcription of lipid-synthesizing enzymes (described in
The SCD of animals (as studied in mice) has an important role in the development of obesity and the insulin resistance that often accompanies obesity and precedes development of type 2 diabetes mellitus. Mice have four isozymes, SCD1 through SCD4, of which SCD1 is the best understood. Its synthesis is induced by dietary saturated fatty acids, and also by the action of SREBP and LXR, two protein regulators of lipid metabolism that activate transcription of lipid-synthesizing enzymes (described in 
 Eicosanoids are a family of very potent biological signaling molecules that act as short-range messengers, affecting tissues near the cells that produce them.
Eicosanoids are a family of very potent biological signaling molecules that act as short-range messengers, affecting tissues near the cells that produce them.
 Malonyl-CoA, a key precursor of fatty acids, is synthesized by the action of acetyl-CoA carboxylase.
Malonyl-CoA, a key precursor of fatty acids, is synthesized by the action of acetyl-CoA carboxylase.