8.4 Other Functions of Nucleotides
In addition to their roles as the subunits of nucleic acids, nucleotides have cellular functions as energy carriers, components of enzyme cofactors, and chemical messengers.
Nucleotides Carry Chemical Energy in Cells
The phosphate group covalently linked at the hydroxyl of a ribonucleotide may have one or two additional phosphates attached. The resulting molecules are referred to as nucleoside mono-, di-, and triphosphates (Fig. 8-39). Starting from the ribose, the three phosphates are generally labeled α, β, and γ. Hydrolysis of nucleoside triphosphates provides the chemical energy to drive many cellular reactions. Adenosine -triphosphate, ATP, is by far the most widely used nucleoside triphosphate for this purpose, but UTP, GTP, and CTP are also used in some reactions. Nucleoside triphosphates also serve as the activated precursors of DNA and RNA synthesis, as described in Chapters 25 and 26 (see also Fig. 8-34).
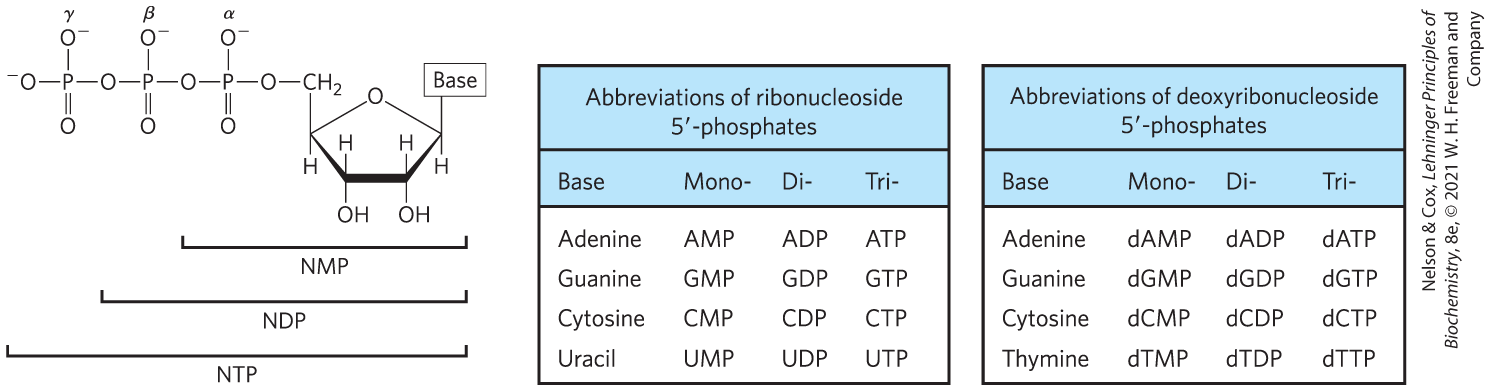
FIGURE 8-39 Nucleoside phosphates. General structure of the nucleoside -mono-, di-, and triphosphates (NMPs, NDPs, and NTPs) and their standard abbreviations. In the deoxyribonucleoside phosphates (dNMPs, dNDPs, and dNTPs), the pentose is -deoxy-d-ribose.
The energy released by hydrolysis of ATP and the other nucleoside triphosphates is accounted for by the structure of the triphosphate group. The bond between the ribose and the α phosphate is an ester linkage. The α, β and β, γ linkages are phosphoanhydrides (Fig. 8-40). Hydrolysis of the ester linkage yields about under standard conditions, whereas hydrolysis of each anhydride bond yields about . ATP hydrolysis often plays an important thermodynamic role in biosynthesis. When coupled to a reaction with a positive free-energy change, ATP hydrolysis shifts the equilibrium of the overall process to favor product formation (recall the relationship between the equilibrium constant and free-energy change described by Eqn 6-3 on p. 182).
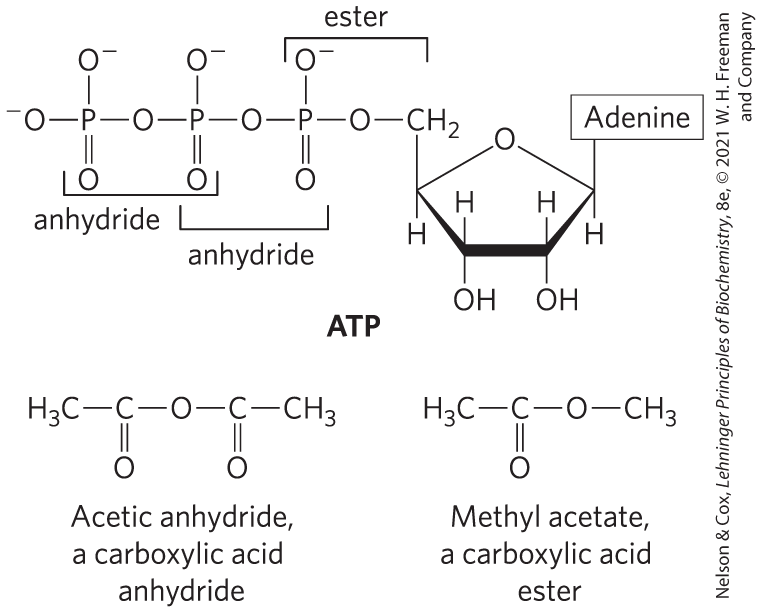
FIGURE 8-40 The phosphate ester and phosphoanhydride bonds of ATP. Hydrolysis of an anhydride bond yields more energy than hydrolysis of the ester. A carboxylic acid anhydride and a carboxylic acid ester are shown for comparison.
Adenine Nucleotides Are Components of Many Enzyme Cofactors
Enzyme cofactors serving a wide range of chemical functions include adenosine as part of their structure (Fig. 8-41). They are unrelated structurally except for the presence of adenosine. In none of these cofactors does the adenosine portion participate directly in the primary function, but removal of adenosine generally results in a drastic reduction of cofactor activities. For example, removal of the adenine nucleotide (-phosphoadenosine diphosphate) from acetoacetyl-CoA, the coenzyme A derivative of acetoacetate, reduces its reactivity as a substrate for β-ketoacyl-CoA transferase (an enzyme of lipid metabolism) by a factor of . Although this requirement for adenosine has not been investigated in detail, it must involve the binding energy between enzyme and substrate (or cofactor) that is used both in catalysis and in stabilizing the initial enzyme-substrate complex (Chapter 6). In the case of β-ketoacyl-CoA transferase, the nucleotide moiety of coenzyme A seems to be a binding “handle” that helps to pull the substrate (acetoacetyl-CoA) into the active site. Similar roles may be found for the nucleoside portion of other nucleotide cofactors.
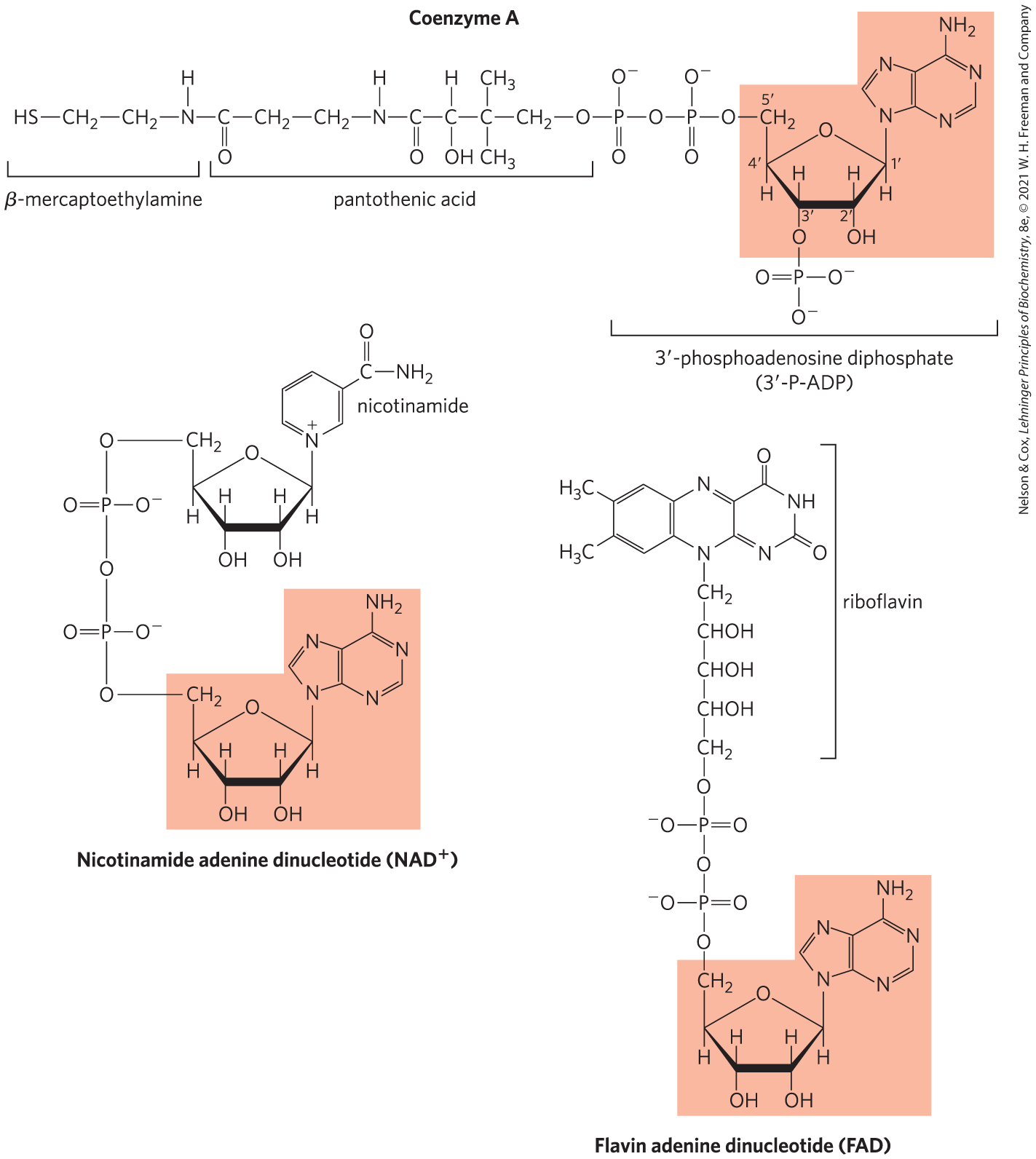
FIGURE 8-41 Some coenzymes containing adenosine. The adenosine portion is shaded in light red. Coenzyme A (CoA) functions in acyl group transfer reactions; the acyl group (such as the acetyl or acetoacetyl group) is attached to the CoA through a thioester linkage to the β-mercaptoethylamine moiety. functions in hydride transfers, and FAD, the active form of vitamin (riboflavin), functions in electron transfers. Another coenzyme incorporating adenosine is -deoxyadenosylcobalamin, the active form of vitamin (see Box 17-2), which participates in intramolecular group transfers between adjacent carbons.
Why is adenosine, rather than some other large molecule, used in these structures? The answer here may involve a form of evolutionary economy. Adenosine is certainly not unique in the amount of potential binding energy it can contribute. The importance of adenosine probably lies not so much in some special chemical characteristic as in the evolutionary advantage of using one compound for multiple roles. Once ATP became the universal source of chemical energy, systems developed to synthesize ATP in greater abundance than the other nucleotides; because it is abundant, it becomes the logical choice for incorporation into a wide variety of structures. The economy extends to protein structure. A single protein domain that binds adenosine can be used in different enzymes. Such a domain, called a nucleotide-binding fold, is found in many enzymes that bind ATP and nucleotide cofactors.
Some Nucleotides Are Regulatory Molecules
Cells respond to their environment by taking cues from hormones or other external chemical signals. The interaction of these extracellular chemical signals (“first messengers”) with receptors on the cell surface often leads to the production of second messengers inside the cell, which in turn leads to adaptive changes in the cell interior (Chapter 12). Often, the second messenger is a nucleotide (Fig. 8-42). One of the most common is adenosine ,-cyclic monophosphate (cyclic AMP, or cAMP), formed from ATP in a reaction catalyzed by adenylyl cyclase, an enzyme associated with the inner face of the plasma membrane. Cyclic AMP serves regulatory functions in virtually every cell outside the plant kingdom. Guanosine ,-cyclic monophosphate (cGMP) also has regulatory functions in many cells.
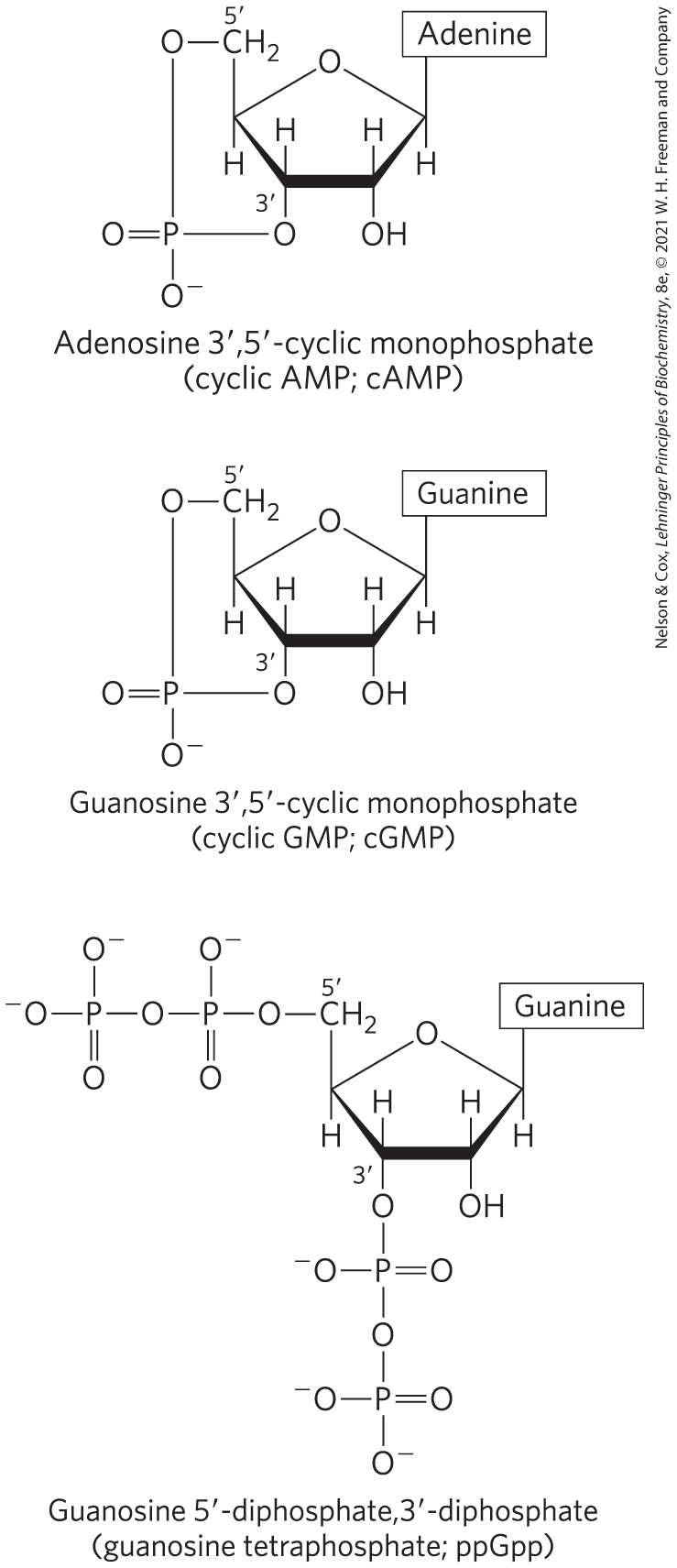
FIGURE 8-42 Three regulatory nucleotides.
Another regulatory nucleotide, ppGpp (Fig. 8-42), is produced in bacteria in response to a slowdown in protein synthesis during amino acid starvation. This nucleotide inhibits the synthesis of the rRNA and tRNA molecules (see Fig. 28-22) needed for protein synthesis, preventing the unnecessary production of nucleic acids.
Adenine Nucleotides Also Serve as Signals
ATP and ADP also serve as signaling molecules in many unicellular and multicellular organisms, including humans. In mammals, certain neurons release ATP at synapses. The ATP binds receptors on the postsynaptic cell, triggering changes in membrane potential or the release of an intracellular second messenger that initiates diverse physiological processes, including taste, inflammation, and smooth muscle contraction. One important class of ATP receptors that mediate the sensation of pain is an obvious target for drug development. Extracellular ADP is a signaling molecule that acts through receptors in sensitive cell types. By preventing ADP from binding the receptors of platelets, the drug clopidogrel (Plavix) inhibits undesirable blood clotting in patients with cardiac disease. Signaling pathways are discussed in more detail in Chapter 12.
SUMMARY 8.4 Other Functions of Nucleotides
- ATP is the central carrier of chemical energy in cells.
- The presence of an adenosine moiety in a variety of enzyme cofactors may be related to binding-energy requirements.
- Cyclic AMP, formed from ATP in a reaction catalyzed by adenylyl cyclase, is a common second messenger produced in response to hormones and other chemical signals.
- ATP and ADP serve as neurotransmitters in a variety of signaling pathways.
 Hydrolysis of nucleoside triphosphates provides the chemical energy to drive many cellular reactions. Adenosine -triphosphate, ATP, is by far the most widely used nucleoside triphosphate for this purpose, but UTP, GTP, and CTP are also used in some reactions. Nucleoside triphosphates also serve as the activated precursors of DNA and RNA synthesis, as described in
Hydrolysis of nucleoside triphosphates provides the chemical energy to drive many cellular reactions. Adenosine -triphosphate, ATP, is by far the most widely used nucleoside triphosphate for this purpose, but UTP, GTP, and CTP are also used in some reactions. Nucleoside triphosphates also serve as the activated precursors of DNA and RNA synthesis, as described in  ATP and ADP also serve as signaling molecules in many unicellular and multicellular organisms, including humans. In mammals, certain neurons release ATP at synapses. The ATP binds receptors on the postsynaptic cell, triggering changes in membrane potential or the release of an intracellular second messenger that initiates diverse physiological processes, including taste, inflammation, and smooth muscle contraction. One important class of ATP receptors that mediate the sensation of pain is an obvious target for drug development. Extracellular ADP is a signaling molecule that acts through receptors in sensitive cell types. By preventing ADP from binding the receptors of platelets, the drug clopidogrel (Plavix) inhibits undesirable blood clotting in patients with cardiac disease. Signaling pathways are discussed in more detail in
ATP and ADP also serve as signaling molecules in many unicellular and multicellular organisms, including humans. In mammals, certain neurons release ATP at synapses. The ATP binds receptors on the postsynaptic cell, triggering changes in membrane potential or the release of an intracellular second messenger that initiates diverse physiological processes, including taste, inflammation, and smooth muscle contraction. One important class of ATP receptors that mediate the sensation of pain is an obvious target for drug development. Extracellular ADP is a signaling molecule that acts through receptors in sensitive cell types. By preventing ADP from binding the receptors of platelets, the drug clopidogrel (Plavix) inhibits undesirable blood clotting in patients with cardiac disease. Signaling pathways are discussed in more detail in 
 ATP is the central carrier of chemical energy in cells.
ATP is the central carrier of chemical energy in cells.