14.6 Pentose Phosphate Pathway of Glucose Oxidation
In most animal tissues, the major catabolic fate of glucose 6-phosphate is glycolytic breakdown to pyruvate, much of which is then oxidized via the citric acid cycle, ultimately leading to the formation of ATP. Glucose 6-phosphate does have other catabolic fates, however, which lead to specialized products needed by the cell. Of particular importance in some tissues is the oxidation of glucose 6-phosphate to pentose phosphates by the pentose phosphate pathway (also called the phosphogluconate pathway or the hexose monophosphate pathway; Fig. 14-29). In this oxidative pathway, is the electron acceptor, yielding NADPH. Rapidly dividing cells, such as those of bone marrow, skin, and intestinal mucosa, and those of tumors, use the pentose ribose 5-phosphate to make RNA, DNA, and such coenzymes as ATP, NADH, , and coenzyme A.
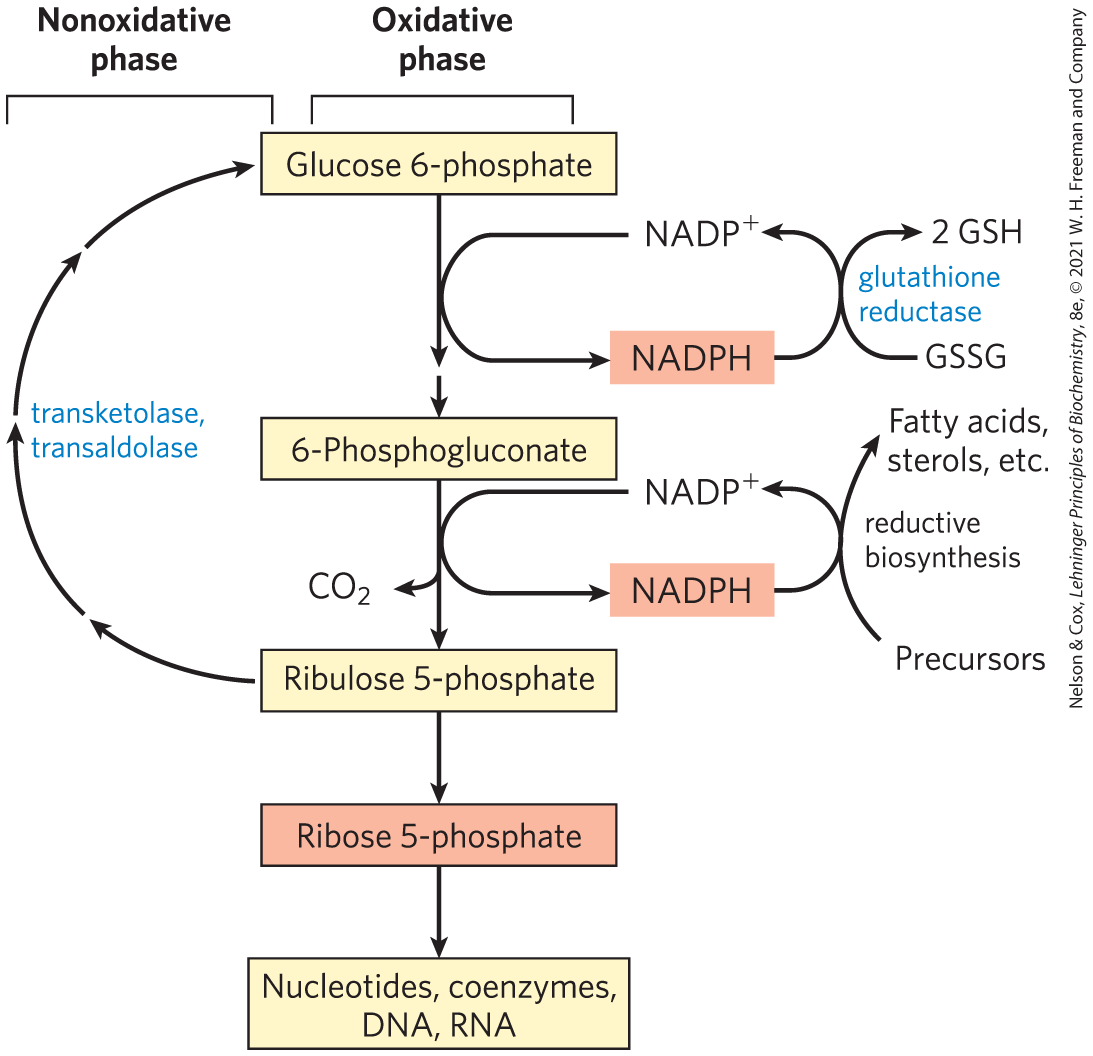
FIGURE 14-29 General scheme of the pentose phosphate pathway. NADPH formed in the oxidative phase is used to reduce glutathione, GSSG (see Box 14-4), and to support reductive biosynthesis. The other product of the oxidative phase is ribose 5-phosphate, which serves as a precursor for nucleotides, coenzymes, and nucleic acids. In cells that are not using ribose 5-phosphate for biosynthesis, the nonoxidative phase recycles six molecules of the pentose into five molecules of the hexose glucose 6-phosphate, allowing continued production of NADPH and converting glucose 6-phosphate (in six cycles) to .
In other tissues, the essential product of the pentose phosphate pathway is not the pentoses but the electron donor NADPH, needed for reductive biosynthesis or to counter the damaging effects of oxygen radicals. Tissues that carry out extensive fatty acid synthesis (liver, adipose, lactating mammary gland) or very active synthesis of cholesterol and steroid hormones (liver, adrenal glands, gonads) require the NADPH provided by this pathway. Erythrocytes and the cells of the lens and cornea are directly exposed to oxygen and thus to the damaging free radicals generated by oxygen. By maintaining a reducing environment (a high ratio of NADPH to and a high ratio of reduced glutathione to oxidized glutathione), such cells can prevent or undo oxidative damage to proteins, lipids, and other sensitive molecules. In erythrocytes, the NADPH produced by the pentose phosphate pathway is so important in preventing oxidative damage that a genetic defect in glucose 6-phosphate dehydrogenase, the first enzyme of the pathway, can have serious medical consequences (Box 14-4).
The Oxidative Phase Produces NADPH and Pentose Phosphates
The first reaction of the pentose phosphate pathway (Fig. 14-30) is the oxidation of glucose 6-phosphate by glucose 6-phosphate dehydrogenase (G6PD) to form 6-phosphoglucono-δ-lactone, an intramolecular ester. is the electron acceptor, and the overall equilibrium lies far in the direction of NADPH formation. The lactone is hydrolyzed to the free acid 6-phosphogluconate by a specific lactonase, then 6-phosphogluconate undergoes oxidation and decarboxylation by 6-phosphogluconate dehydrogenase to form the ketopentose ribulose 5-phosphate; the reaction also generates a second molecule of NADPH. Phosphopentose isomerase converts ribulose 5-phosphate to its aldose isomer, ribose 5-phosphate. In some tissues, the pentose phosphate pathway ends at this point, and its overall equation is
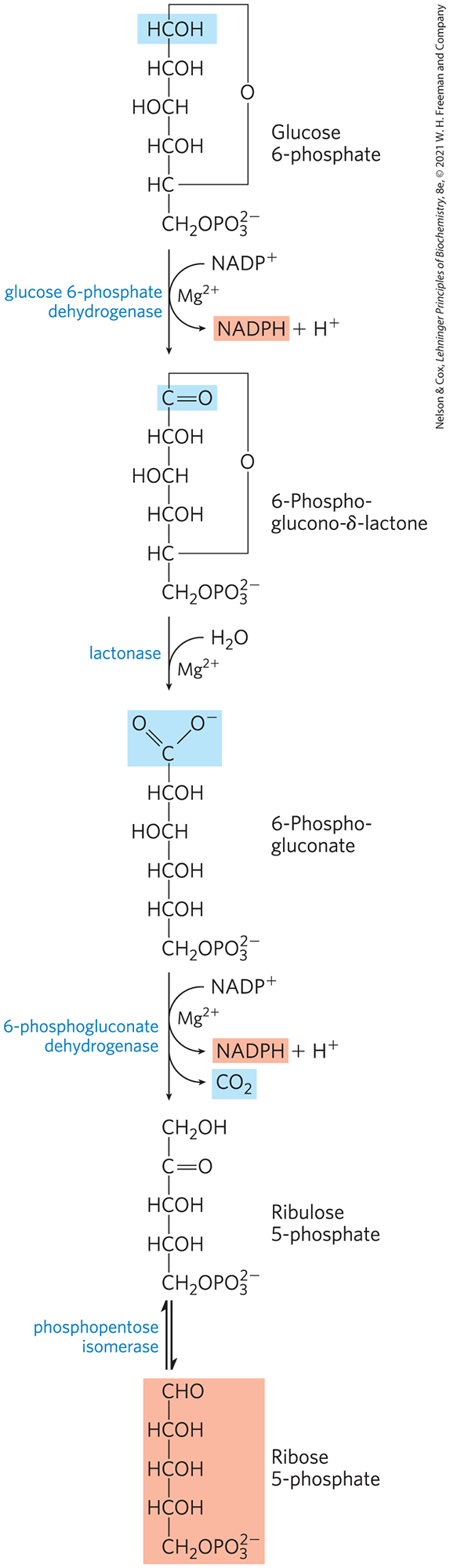
FIGURE 14-30 Oxidative reactions of the pentose phosphate pathway. The end products are ribose 5-phosphate, , and NADPH.
Glucose 6-phosphate is shown as a six-carbon vertical chain with C 1 bonded to H to the left and O H to the right within a blue box and further bonded to O that forms a ring by bonding to C 5; C 2 and C 4 bonded to H on the left and O H on the right; C 3 bonded to O H to the left and H to the right; C 5 bonded to H to the left and O to the right that further bonds to C 1; and C 6 bonded to 2 H and O P O subscript 3 superscript 2 minus. An arrow labeled glucose 6-phosphate dehydrogenase points downward. A curved arrow shows that N A D P plus is added, and a red box labeled N A D P H is produced plus H plus outside of the box. M g 2 plus is present in the inflection point of the curved arrow. This yields 6-phosphoglucono-Greek letter delta-lactone, which has a similar structure except that C 1 is double bonded to O in a blue box and bonded to O that is further bonded to C 5. An arrow labeled lactonase points down accompanied by a line showing the addition of H 2 O and by M g 2 plus. This yields 6-phosphogluconate, which has a similar structure except that C 1 is double bonded to O to the upper left and bonded to O minus to the upper right, all in a blue box, and C 5 is bonded to H to the left and O H to the right. An arrow labeled 6-phosphogluconate dehydrogenase points down accompanied by a curved arrow showing that N A D P plus is added and a red box containing N A D P H plus H plus outside of the box is lost. M g 2 plus is shown in the inflection point of this curved arrow. Another arrow shows the loss of C O 2 in a blue box. This yields ribulose 5-phosphate, which has a five-carbon vertical chain with C 1 bonded to 2 H and O H; C 2 double bonded to O; C 3 and C 4 bonded to H to the left and O H to the right; and C 5 bonded to 2 H and O P O subscript 3 superscript 2 minus. Upward- and downward-pointing arrows labeled phosphopentose isomerase indicate a reversible reaction that yields ribose 5-phosphate, which is shown in a red box as C 1 forming C H O; C 2, C 3, and C 4 all bonded to H on the left and O H on the right; and C 5 bonded to 2 H and to O P O subscript 3 superscript 2 minus.
The net result is the production of NADPH, a reductant for biosynthetic reactions, and ribose 5-phosphate, a precursor for nucleotide synthesis.
The Nonoxidative Phase Recycles Pentose Phosphates to Glucose 6-Phosphate
In tissues that require primarily NADPH, the pentose phosphates produced in the oxidative phase of the pathway are recycled into glucose 6-phosphate. In this nonoxidative phase, ribulose 5-phosphate is first epimerized to xylulose 5-phosphate:
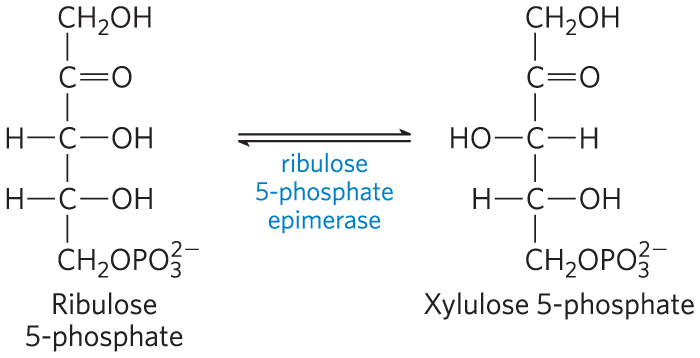
Ribulose 5-phosphate is a five-carbon vertical chain with C 1 bonded to 2 H and O H, C 2 double bonded to O to the right, C 3 and C 4 bonded to H to the left and O H to the right, and C 5 bonded to 2 H and O P O subscript 3 superscript 2 minus. Xylulose 5-phosphate is a vertical five-carbon chain that is similar except that C 3 is bonded to O H to the left and H to the right.
Then, in a series of rearrangements of the carbon skeletons (Fig. 14-31), six five-carbon sugar phosphates are converted to five six-carbon sugar phosphates, completing the cycle and allowing continued oxidation of glucose 6-phosphate with production of NADPH. Continued recycling leads ultimately to the conversion of glucose 6-phosphate to six . Two enzymes unique to the pentose phosphate pathway act in these interconversions of sugars: transketolase and transaldolase. Transketolase catalyzes the transfer of a two-carbon fragment from a ketose donor to an aldose acceptor (Fig. 14-32a). In its first appearance in the pentose phosphate pathway, transketolase transfers C-1 and C-2 of xylulose 5-phosphate to ribose 5-phosphate, forming the seven-carbon product sedoheptulose 7-phosphate (Fig. 14-32b). The remaining three-carbon fragment from xylulose is glyceraldehyde 3-phosphate.
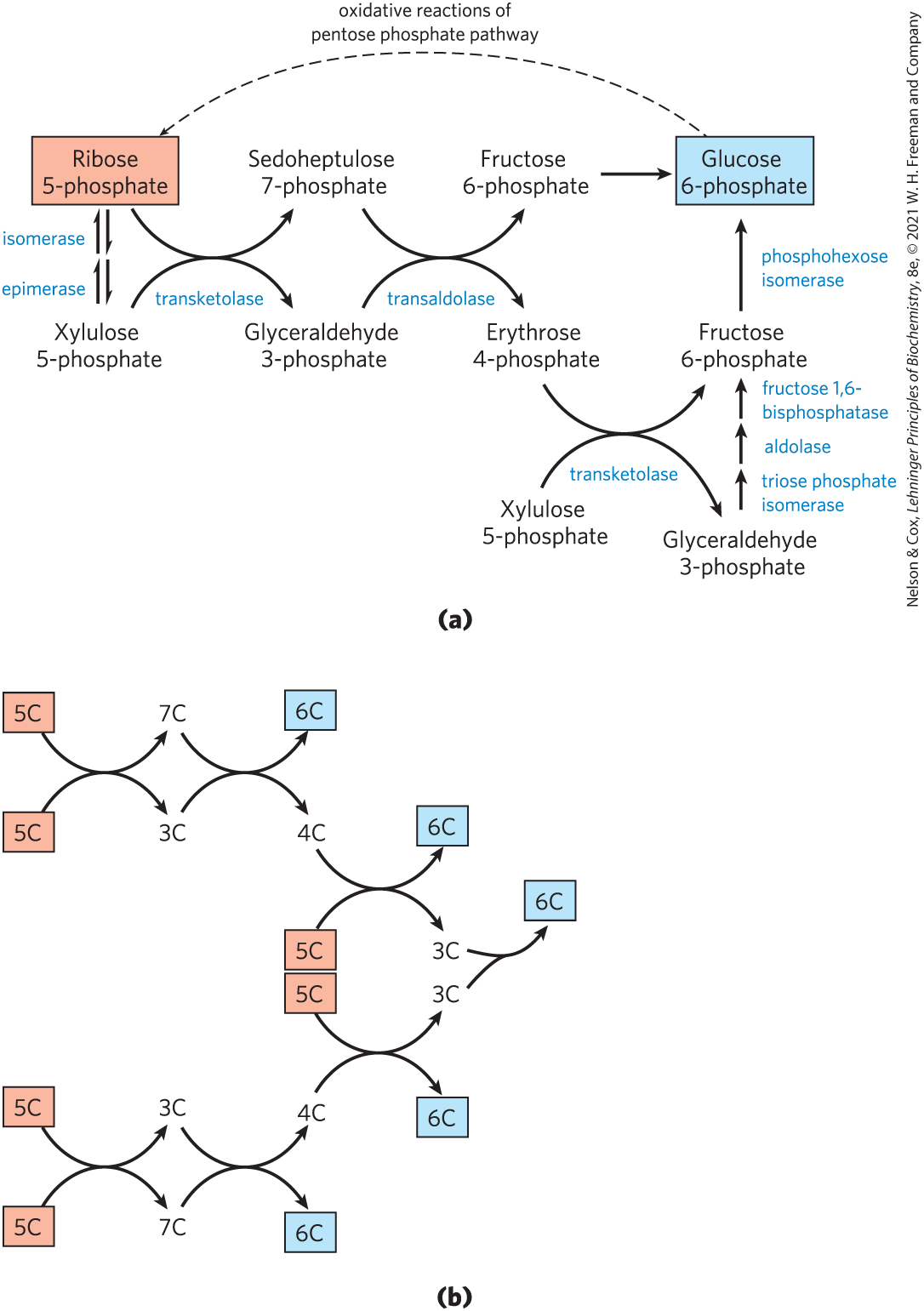
FIGURE 14-31 Nonoxidative reactions of the pentose phosphate pathway. (a) These reactions convert pentose phosphates to hexose phosphates, allowing the oxidative reactions to continue. Transketolase and transaldolase are specific to this pathway; the other enzymes also serve in the glycolytic or gluconeogenic pathways. (b) A schematic diagram showing the pathway from six pentoses (5C) to five hexoses (6C). Note that this involves two sets of the interconversions shown in (a). Every reaction shown here is reversible; unidirectional arrows are used only to make clear the direction of the reactions during continuous oxidation of glucose 6-phosphate. In the light-independent reactions of photosynthesis, the direction of these reactions is reversed.
Part a shows a red box labeled ribose 5-phosphate on the left. Upward- and downward-pointing arrows labeled isomerase are above upward- and downward-pointing arrows labeled epimerase. This yields xylulose 5-phosphate. A curved arrow from xylulose 5-phosphate to glyceraldehyde 3-phosphate meets a curved arrow from ribose 5-phosphate to sedoheptulose 7-phosphate above the word transketolase. A curved arrow from sedoheptulose 7-phosphate to fructose 6-phosphate meets a curved arrow from glyceraldehyde 3-phosphate to erythrose 4-phosphate above the word transaldolase. An arrow points from fructose 6-phosphate to a blue box labeled glucose 6-phosphate that has a dashed arrow back to ribose 5-phosphate labeled oxidative reactions of pentose phosphate pathway. A curved arrow from erythrose 4-phosphate to fructose 6-phosphate meets a curved arrow from xylulose 5-phosphate to glyceraldehyde 3-phosphate above the word transketolase. This glyceraldehyde has a series of three vertical arrows pointing up to fructose 6-phosphate. From bottom to top, these arrows are labeled triose phosphate isomerase, aldolase, and fructose 1,6-bisphosphatase. An arrow labeled phosphohexose isomerase points up from fructose 6-phosphate to glucose 6-phosphate. Part b shows curved arrows from two red squares each labeled 5 C that meet in the middle before the top arrow ends at 7 C and the bottom arrow ends at 3 C. A curved arrow from 7 C to a blue box labeled 6 C meets a curved arrow from 3 C to 4 C. A curved arrow from 4 C to a blue box labeled 6 C meets a curved arrow below from a red box labeled 5 C to 3 C. The red box labeled 5 C is above a similar red box and has a curved arrow to a similar 3 C beneath the first one, forming a circular structure. Arrows from the top and bottom 3 C meet and point to a blue box labeled 6 C. To the lower left, curved arrows from two red squares each labeled 5 C meet in the middle before the top arrow ends at 3 C and the bottom arrow ends at 7 C. A curved arrow from 3 C to 4 C meets a curved arrow from 7 C to a blue box labeled 6 C. A curved arrow from 4 C to a blue box meets the curved arrow from the red box labeled 5 C to 3 C forming the bottom half of the circular structure already described.
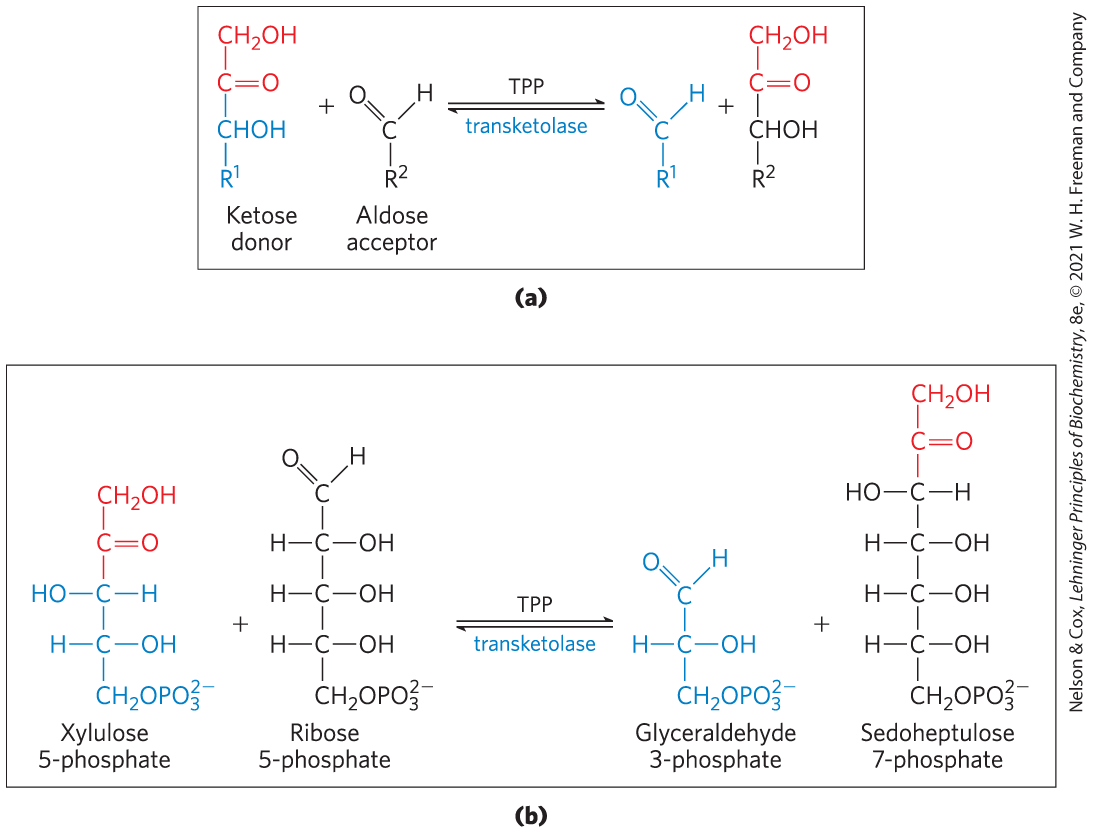
FIGURE 14-32 The first reaction catalyzed by transketolase. (a) The general reaction catalyzed by transketolase is the transfer of a two-carbon group, carried temporarily on enzyme-bound TPP, from a ketose donor to an aldose acceptor. (b) Conversion of two pentose phosphates to a triose phosphate and a seven-carbon sugar phosphate, sedoheptulose 7-phosphate.
Part a shows a ketose donor as a vertical chain with its top half in red and its bottom half in blue. The red half has C 1 bonded to 2 H and to O H and C 2 double bonded to O. The blue half has C 3 bonded to H and O H and to R superscript 1 below. The ketose donor is added to an aldose acceptor, shown as a central C double bonded to O to the upper left, bonded to H to the upper right, and bonded to R superscript 2 below. Right- and left-pointing arrows are below T P P and above transketolase. This yields a blue molecule with a central C double bonded to O to the upper left, bonded to H to the upper right, and bonded to R superscript 1 below plus a molecule with a red top half and black bottom half. The second molecule has a red top half of C 1 bonded to 2 H and O H and C 2 double bonded to O. The bottom half has C bonded to H and O H and to R superscript 2 below. Part b shows xylulose 5-phosphate with a red top half and blue bottom half. The red half has C 1 bonded to 2 H and O H and C 2 double bonded to O. The bottom half has C 3 bonded to O H to the left and H to the right, C 4 bonded to H to the left and O H to the right, and C 5 bonded to 2 H and to O P O subscript 3 superscript 2 minus. Xylulose 5-phosphate is added to ribose 5-phosphate, which is a five-carbon vertical chain with C 1 double bonded to O to the upper left and bonded to H to the upper right; C 2, C 3, and C 4 bonded to H on the left and O H on the right; and C 5 bonded to 2 H and to O P O subscript 3 superscript 2 minus. Right- and left-pointing arrows are below T P P and above transketolase. This yields a blue molecule of glyceraldehyde 3-phosphate, shown as C 1 double bonded to O to the upper left and bonded to H to the upper right, C 2 bonded to H to the left and O H to the right, and C 3 bonded to 2 H and to O P O subscript 3 superscript 2 minus. Sedoheptulose 7-phosphate is also present with the top two carbons and their substituents highlighted in red. The red part has C 1 bonded to 2 H and O H and C 2 double bonded to O. The rest of the molecule has C 3 bonded to O H to the left and H to the right; C 4, C 5, and C 6 bonded to H to the left and to O H to the right; and C 7 bonded to 2 H and to O P O subscript 3 superscript 2 minus.
Next, transaldolase catalyzes a reaction similar to the aldolase reaction of glycolysis: a three-carbon fragment is removed from sedoheptulose 7-phosphate and condensed with glyceraldehyde 3-phosphate, forming fructose 6-phosphate and the tetrose erythrose 4-phosphate (Fig. 14-33). Now transketolase acts again, forming fructose 6-phosphate and glyceraldehyde 3-phosphate from erythrose 4-phosphate and xylulose 5-phosphate (Fig. 14-34). Two molecules of glyceraldehyde 3-phosphate formed by two iterations of these reactions can be converted to a molecule of fructose 1,6-bisphosphate as in gluconeogenesis (Fig. 14-16), and finally FBPase-1 and phosphohexose isomerase convert fructose 1,6-bisphosphate to glucose 6-phosphate. Overall, six pentose phosphates have been converted to five hexose phosphates (Fig. 14-32b) — the cycle is now complete.
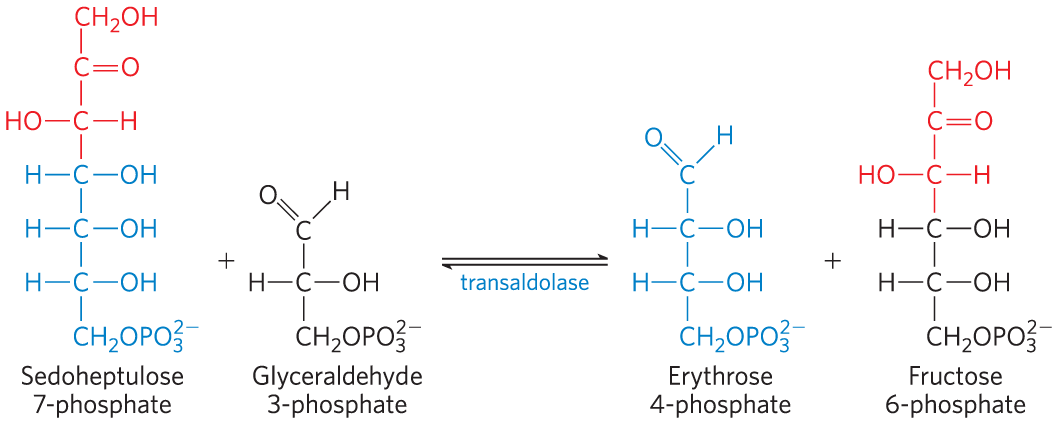
FIGURE 14-33 The reaction catalyzed by transaldolase.
Sedoheptulose 7-phosphate is a vertical seven-carbon chain shown with the top three carbons and their substituents highlighted in red and the bottom four carbons and their substituents highlighted in blue. The red part has C 1 bonded to 2 H and O H, C 2 double bonded to O, and C 3 bonded to O H to the left and H to the right. The blue part has C 4, C 5, and C 6 bonded to H to the left and to O H to the right and C 7 bonded to 2 H and to O P O subscript 3 superscript 2 minus. Glyceraldehyde 3-phosphate is added, shown as C 1 double bonded to O to the upper left and bonded to H to the upper right, C 2 bonded to H to the left and O H to the right, and C 3 bonded to 2 H and to O P O subscript 3 superscript 2 minus. Right- and left-pointing arrows are below T P P and above transaldolase. This yields a blue molecule of erythrose 4-phosphate and fructose 6-phosphate that has the top three carbons and their substituents highlighted in red. Erythrose 4-phosphate has C 1 double bonded to O to the upper left and bonded to H to the upper right, C 2 and C 3 bonded to H on the left and O H on the right, and C 4 bonded to 2 H and to O P O subscript 3 superscript 2 minus. The red part of fructose 6-phosphate has C 1 bonded to 2 H and O H, C 2 double bonded to O, and C 3 bonded to O H to the left and H to the right. The rest of the molecule is C 4 and C 5 bonded to H to the left and O H to the right and C 5 bonded to 2 H and to O P O subscript 3 superscript 2 minus.
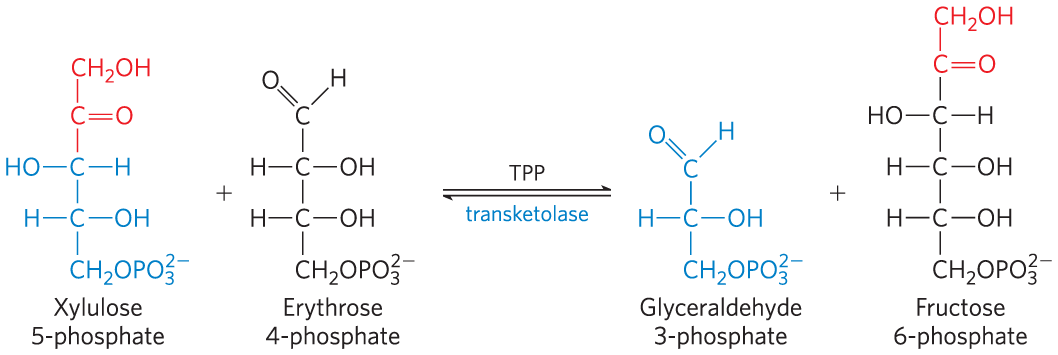
FIGURE 14-34 The second reaction catalyzed by transketolase.
Xylulose 5-phosphate is a vertical five-carbon chain shown with the top two carbons and their substituents highlighted in red and the bottom three carbons and their substituents highlighted in blue. The red part has C 1 bonded to 2 H and O H and C 2 double bonded to O. The bottom part has C 3 bonded to O H to the left and H to the right, C 4 bonded to H to the left and O H to the right, and C 5 bonded to 2 H and to O P O subscript 3 superscript 2 minus. Erythrose 4-phosphate has C 1 double bonded to O to the upper left and bonded to H to the upper right, C 2 and C 3 bonded to H on the left and O H on the right, and C 4 bonded to 2 H and to O P O subscript 3 superscript 2 minus. Right- and left-pointing arrows are below T P P and above transketolase. This yields a blue molecule of glyceraldehyde 3-phosphate and fructose 6-phosphate that has the top two carbons and their substituents highlighted in red. Glyceraldehyde 3-phosphate has C 1 double bonded to O to the upper left and bonded to H to the upper right, C 2 bonded to H to the left and O H to the right, and C 3 bonded to 2 H and to O P O subscript 3 superscript 2 minus. The red part of fructose 6-phosphate has C 1 bonded to 2 H and O H and C 2 double bonded to O. The rest of the molecule has C 3 bonded to O H to the left and H to the right, C 4 and C 5 bonded to H to the left and O H to the right and C 5 bonded to 2 H and to O P O subscript 3 superscript 2 minus.
Transketolase requires the cofactor thiamine pyrophosphate (TPP), which stabilizes a two-carbon carbanion in this reaction (Fig. 14-35a), just as it does in the pyruvate decarboxylase reaction (Fig. 14-13). Transaldolase uses a Lys side chain to form a Schiff base with the carbonyl group of its substrate, a ketose, thereby stabilizing a carbanion (Fig. 14-35b) that is central to the reaction mechanism.
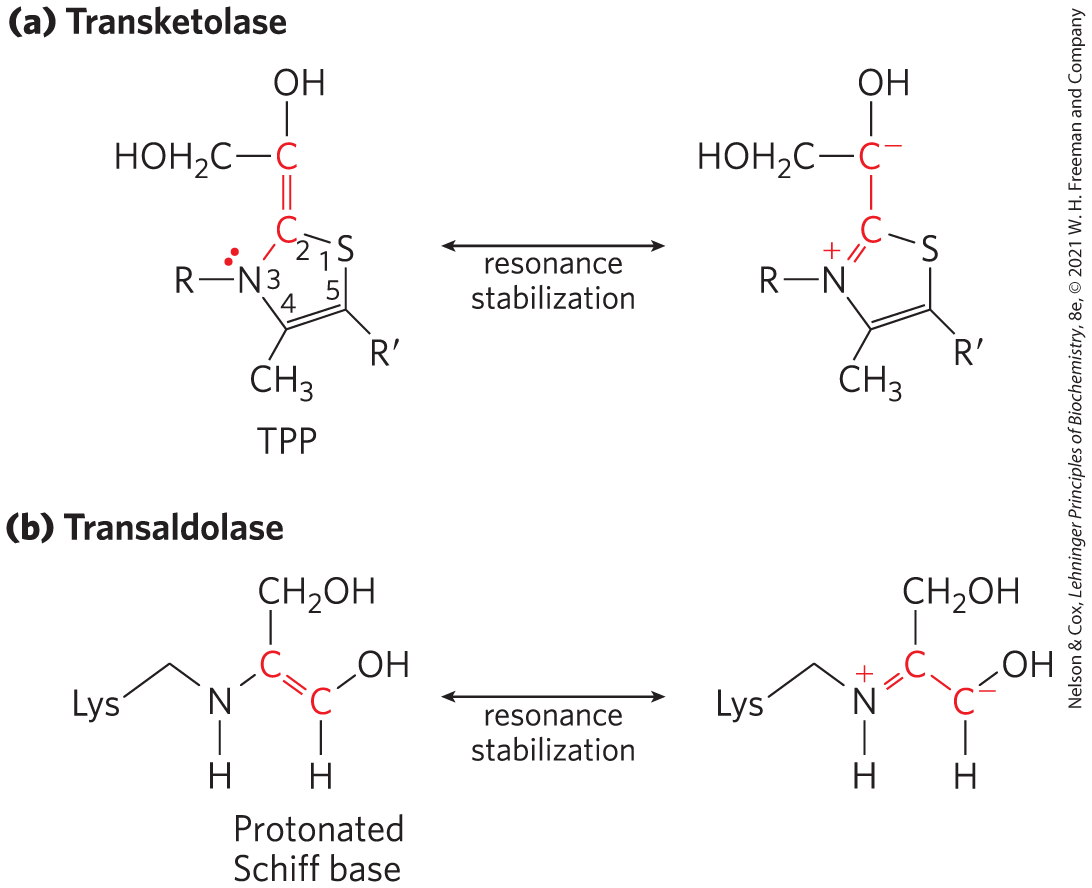
FIGURE 14-35 Carbanion intermediates stabilized by covalent interactions with transketolase and transaldolase. (a) The ring of TPP stabilizes the carbanion in the dihydroxyethyl group carried by transketolase. (b) In the transaldolase reaction, the protonated Schiff base formed between the ε-amino group of a Lys side chain and the substrate stabilizes the C-3 carbanion formed after aldol cleavage.
Part a is labeled transketolase. T P P is shown with a five-membered ring with numbered substituents. S is substituted for C at position 1; C at position 2 is highlighted in red and connected by a red double bond to red highlighted C above further bonded to O H above and to C on the left bonded to 2 H and O H; the bond between positions 2 and 3 is red; N is substituted for C at position 3 with a red pair of electrons and a bond to R; C 4 is bonded to C H 3; there is a double bond between positions 4 and 5; and position 5 is bonded to R prime. A double-headed arrow labeled resonance stabilization points to a similar molecule in which N at position 3 has a red highlighted positive charge, there is a red highlighted double bond between positions 2 and 3, and red highlighted C at position 2 has a single bond to red highlighted C above with a red highlighted negative charge. Part b is labeled transaldolase. The left-hand structure contains a protonated Schiff base. It has L y s connected by a chain with one peak to N bonded to H below and to red highlighted C to the upper right further bonded to C H 2 O H above and connected by a red highlighted double bond to red highlighted C to the lower right bonded to H below and to O H to the upper right. A double-headed arrow labeled resonance stabilization points to a similar molecule in which N has a positive charge, there is a red highlighted double bond between N and C to its right, the double bond between that C and the terminal C has become a single bond, and the right-hand terminal C has a negative charge.
The first and third steps of the oxidative pentose phosphate pathway shown in Figure 14-30 are oxidations with large, negative standard free-energy changes and are essentially irreversible in the cell. The reactions of the nonoxidative part of the pentose phosphate pathway (Fig. 14-31) are readily reversible and thus also provide a means of converting hexose phosphates to pentose phosphates. As we shall see in Chapter 20, a process that converts hexose phosphates to pentose phosphates is central to the photosynthetic assimilation of by plants. That pathway, the reductive pentose phosphate pathway, is essentially the reversal of the reactions shown in Figure 14-31 and employs many of the same enzymes.
All the enzymes of the pentose phosphate pathway are located in the cytosol, like those of glycolysis and most of those of gluconeogenesis. In fact, these three pathways are connected through several shared intermediates and enzymes. The glyceraldehyde 3-phosphate formed by the action of transketolase is readily converted to dihydroxyacetone phosphate by the glycolytic enzyme triose phosphate isomerase, and these two trioses can be joined by the aldolase as in gluconeogenesis, forming fructose 1,6-bisphosphate. Alternatively, the triose phosphates can be oxidized to pyruvate by the glycolytic reactions. The fate of the trioses is determined by the cell’s relative needs for pentose phosphates, NADPH, and ATP.
Glucose 6-Phosphate Is Partitioned between Glycolysis and the Pentose Phosphate Pathway
Whether glucose 6-phosphate enters glycolysis or the pentose phosphate pathway depends on the current needs of the cell and on the concentration of in the cytosol. Without this electron acceptor, the first reaction of the pentose phosphate pathway (catalyzed by glucose 6-phosphate dehydrogenase) cannot proceed. When a cell is rapidly converting NADPH to in biosynthetic reductions, rises, allosterically stimulating glucose 6-phosphate dehydrogenase and thereby increasing the flux of glucose 6-phosphate through the pentose phosphate pathway (Fig. 14-36). When the demand for NADPH slows, the level of drops, the pentose phosphate pathway slows, and glucose 6-phosphate is instead used to fuel glycolysis.
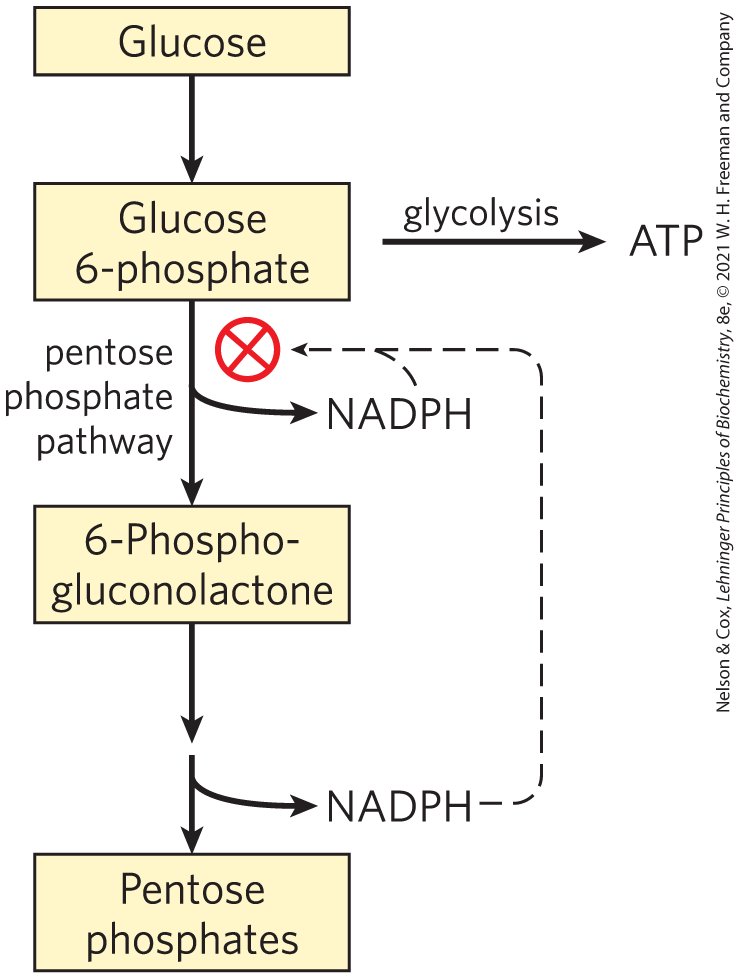
FIGURE 14-36 Role of NADPH in regulating the partitioning of glucose 6-phosphate between glycolysis and the pentose phosphate pathway. When NADPH is forming faster than it is being used for biosynthesis and glutathione reduction, [NADPH] rises and inhibits the first enzyme in the pentose phosphate pathway. As a result, more glucose 6-phosphate is available for glycolysis.
A yellow box labeled glucose has an arrow pointing down to a yellow box labeled glucose 6-phosphate, from which an arrow labeled glycolysis points right to A T P and an arrow labeled pentose phosphate pathway points down with an arrow branching to show the loss of N A D P H before ending at a yellow box labeled 6-phosphogluconolactone. A series of two arrows points down to a yellow box labeled pentose phosphates, with the second arrow branching to show the loss of N A D P H. Both N A D P H molecules have dashed arrows that join to indicate a red circle containing a red X next to the arrow labeled pentose phosphate pathway.
Thiamine Deficiency Causes Beriberi and Wernicke-Korsakoff Syndrome
Thiamine, precursor to the cofactor thiamine pyrophosphate (TPP), is one of the B vitamins, essential in humans. Lack of vitamin in the diet leads to a range of medical problems. The condition known as beriberi is characterized by an accumulation of body fluids (swelling), pain, paralysis, and ultimately, without treatment, death. Wernicke-Korsakoff syndrome, also caused by a severe deficiency of thiamine, typically includes problems with voluntary movements, reflected in abnormal eye movements and gait, and neurological defects. The syndrome is more common among heavy drinkers than in the general population because chronic, heavy alcohol consumption interferes with the intestinal absorption of thiamine. The syndrome can be exacerbated by a mutation in the gene for transketolase that results in an enzyme with a lowered affinity for TPP — an affinity one-tenth that of the normal enzyme. This defect makes individuals much more sensitive to a thiamine deficiency: even a moderate thiamine deficiency (tolerable in individuals with an unmutated transketolase) can result in a transketolase that is not saturated with TPP at its normal concentration. The result is a slowing down of the whole pentose phosphate pathway. In people with Wernicke-Korsakoff syndrome, this mutation results in a worsening of symptoms, which can include severe memory loss, mental confusion, and partial paralysis.
SUMMARY 14.6 Pentose Phosphate Pathway of Glucose Oxidation
- The oxidative pentose phosphate pathway produces NADPH and pentose phosphates. Tissues that carry out extensive fatty acid synthesis (liver, adipose, lactating mammary gland) or very active synthesis of cholesterol and steroid hormones (liver, adrenal glands, gonads) require the NADPH provided by this pathway.
- Ribose 5-phosphate is a precursor for nucleotide and nucleic acid synthesis.
- The first, oxidative phase of the pentose phosphate pathway consists of two oxidations that convert glucose 6-phosphate to ribulose 5-phosphate and reduce to NADPH.
- The second, nonoxidative phase of the pentose phosphate pathway comprises steps that convert pentose phosphates to glucose 6-phosphate, which begins the oxidative cycle again.
- Entry of glucose 6-phosphate either into glycolysis or into the pentose phosphate pathway is largely determined by the relative concentrations of and NADPH.
 In most animal tissues, the major catabolic fate of glucose 6-phosphate is glycolytic breakdown to pyruvate, much of which is then oxidized via the citric acid cycle, ultimately leading to the formation of ATP. Glucose 6-phosphate does have other catabolic fates, however, which lead to specialized products needed by the cell. Of particular importance in some tissues is the oxidation of glucose 6-phosphate to pentose phosphates by the
In most animal tissues, the major catabolic fate of glucose 6-phosphate is glycolytic breakdown to pyruvate, much of which is then oxidized via the citric acid cycle, ultimately leading to the formation of ATP. Glucose 6-phosphate does have other catabolic fates, however, which lead to specialized products needed by the cell. Of particular importance in some tissues is the oxidation of glucose 6-phosphate to pentose phosphates by the  Thiamine, precursor to the cofactor thiamine pyrophosphate (TPP), is one of the B vitamins, essential in humans. Lack of vitamin in the diet leads to a range of medical problems. The condition known as beriberi is characterized by an accumulation of body fluids (swelling), pain, paralysis, and ultimately, without treatment, death. Wernicke-Korsakoff syndrome, also caused by a severe deficiency of thiamine, typically includes problems with voluntary movements, reflected in abnormal eye movements and gait, and neurological defects. The syndrome is more common among heavy drinkers than in the general population because chronic, heavy alcohol consumption interferes with the intestinal absorption of thiamine. The syndrome can be exacerbated by a mutation in the gene for transketolase that results in an enzyme with a lowered affinity for TPP — an affinity one-tenth that of the normal enzyme. This defect makes individuals much more sensitive to a thiamine deficiency: even a moderate thiamine deficiency (tolerable in individuals with an unmutated transketolase) can result in a transketolase that is not saturated with TPP at its normal concentration. The result is a slowing down of the whole pentose phosphate pathway. In people with Wernicke-Korsakoff syndrome, this mutation results in a worsening of symptoms, which can include severe memory loss, mental confusion, and partial paralysis.
Thiamine, precursor to the cofactor thiamine pyrophosphate (TPP), is one of the B vitamins, essential in humans. Lack of vitamin in the diet leads to a range of medical problems. The condition known as beriberi is characterized by an accumulation of body fluids (swelling), pain, paralysis, and ultimately, without treatment, death. Wernicke-Korsakoff syndrome, also caused by a severe deficiency of thiamine, typically includes problems with voluntary movements, reflected in abnormal eye movements and gait, and neurological defects. The syndrome is more common among heavy drinkers than in the general population because chronic, heavy alcohol consumption interferes with the intestinal absorption of thiamine. The syndrome can be exacerbated by a mutation in the gene for transketolase that results in an enzyme with a lowered affinity for TPP — an affinity one-tenth that of the normal enzyme. This defect makes individuals much more sensitive to a thiamine deficiency: even a moderate thiamine deficiency (tolerable in individuals with an unmutated transketolase) can result in a transketolase that is not saturated with TPP at its normal concentration. The result is a slowing down of the whole pentose phosphate pathway. In people with Wernicke-Korsakoff syndrome, this mutation results in a worsening of symptoms, which can include severe memory loss, mental confusion, and partial paralysis. 
 The oxidative pentose phosphate pathway produces NADPH and pentose phosphates. Tissues that carry out extensive fatty acid synthesis (liver, adipose, lactating mammary gland) or very active synthesis of cholesterol and steroid hormones (liver, adrenal glands, gonads) require the NADPH provided by this pathway.
The oxidative pentose phosphate pathway produces NADPH and pentose phosphates. Tissues that carry out extensive fatty acid synthesis (liver, adipose, lactating mammary gland) or very active synthesis of cholesterol and steroid hormones (liver, adrenal glands, gonads) require the NADPH provided by this pathway.