10.1 Storage Lipids
The fats and oils almost universally used as stored forms of energy in living organisms are derivatives of fatty acids. The fatty acids are hydrocarbon derivatives, at about the same low oxidation state (that is, as highly reduced) as the hydrocarbons in fossil fuels. The cellular oxidation of fatty acids (to and ), like the controlled, rapid burning of fossil fuels in internal combustion engines, is highly exergonic.
We introduce here the structures and nomenclature of the fatty acids most commonly found in living organisms. We describe two types of fatty acid–containing compounds, triacylglycerols and waxes, to illustrate the diversity of structures and physical properties in this family of compounds.
Fatty Acids Are Hydrocarbon Derivatives
Fatty acids are carboxylic acids with hydrocarbon chains ranging from 4 to 36 carbons long ( to ). In some fatty acids, this chain is unbranched and fully saturated (contains no double bonds); in others, the chain contains one or more double bonds (Table 10-1). A few contain three-carbon rings, hydroxyl groups, or methyl-group branches.
| Solubility at (mg/g solvent) | ||||||
|---|---|---|---|---|---|---|
| Carbon skeleton | Structurea | Systematic nameb | Common name (derivation) | Melting point () | Water | Benzene |
12:0 |
n-Dodecanoic acid |
Lauric acid (Latin laurus, “laurel plant”) |
44.2 |
0.063 |
2,600 |
|
14:0 |
n-Tetradecanoic acid |
Myristic acid (Latin Myristica, nutmeg genus) |
53.9 |
0.024 |
874 |
|
16:0 |
n-Hexadecanoic acid |
Palmitic acid (Latin palma “palm tree”) |
63.1 |
0.0083 |
348 |
|
18:0 |
n-Octadecanoic acid |
Stearic acid (Greek stear, “hard fat”) |
69.6 |
0.0034 |
124 |
|
20:0 |
n-Eicosanoic acid |
Arachidic acid (Latin Arachis, legume genus) |
76.5 |
|||
24:0 |
n-Tetracosanoic acid |
Lignoceric acid (Latin lignum, “wood” + cera, “wax”) |
86.0 |
|||
cis-9-Hexadecenoic acid |
Palmitoleic acid |
1 to –0.5 |
||||
cis-9-Octadecenoic acid |
Oleic acid (Latin oleum, “oil”) |
13.4 |
||||
cis-,cis-9,12- Octadecadienoic acid |
Linoleic acid (Greek linon, “flax”) |
1–5 |
||||
cis-,cis-,cis-9,12, 15-Octadecatrienoic acid |
α-Linolenic acid |
–11 |
||||
cis-,cis-,cis-,cis-5,8,11, 14-Icosatetraenoic acid |
Arachidonic acid |
–49.5 |
||||
aAll acids are shown in their nonionized form. At pH 7, all free fatty acids have an ionized carboxylate. Note that numbering of carbon atoms begins at the carboxyl carbon. bThe prefix n- indicates the “normal” unbranched structure. For instance, “dodecanoic” simply indicates 12 carbon atoms, which could be arranged in a variety of branched forms; “n-dodecanic” specifies the linear unbranched form. For unsaturated fatty acids, the configuration of each double bond is indicated; in biological fatty acids the configuration is almost always cis. |
||||||
Key Convention
A simplified nomenclature for unbranched fatty acids specifies the chain length and number of double bonds, separated by a colon. For example, the 16-carbon saturated palmitic acid is abbreviated 16:0, and the 18-carbon oleic (octadecenoic) acid, with one double bond (shown below), is 18:1. Each line segment of the zigzag in the structure represents a single bond between adjacent carbons. The carboxyl carbon is assigned the number 1 (C-1), and the α carbon next to it is C-2. The positions of any double bonds, designated Δ (delta), are specified relative to C-1 by a superscript number indicating the lower-numbered carbon in the double bond. By this convention, oleic acid, with a double bond between C-9 and C-10, is designated 18:1(); a 20-carbon fatty acid with one double bond between C-9 and C-10 and another between C-12 and C-13 is designated 20:2().
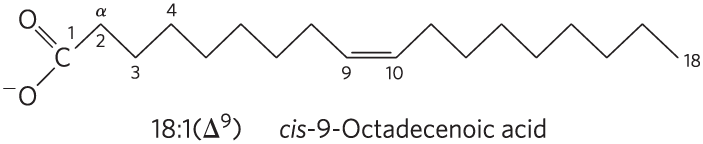
The most commonly occurring fatty acids have even numbers of carbon atoms in an unbranched chain of 12 to 24 carbons (Table 10-1). As we shall see in Chapter 21, the even number of carbons results from the mode of synthesis of these compounds, which involves successive condensations of two-carbon acetyl units.
There is also a common pattern in the location of double bonds; in most monounsaturated fatty acids the double bond is between C-9 and C-10 (), and the other double bonds of polyunsaturated fatty acids are generally and . (Arachidonic acid is an exception to this generalization; see Table 10-1.) The double bonds of polyunsaturated fatty acids are almost never conjugated (alternating single and double bonds, as in ), but are separated by a methylene group: . In nearly all naturally occurring unsaturated fatty acids, the double bonds are in the cis configuration. Notable exceptions include the trans fatty acids produced by fermentation in the rumen of dairy animals. Humans can ingest these trans fats from dairy products and meat.
Key Convention
The family of polyunsaturated fatty acids (PUFAs) with a double bond between the third carbon and the fourth carbon from the methyl end of the chain are of special importance in human nutrition. Because the physiological role of PUFAs is related more to the position of the first double bond near the methyl end of the chain than to that near the carboxyl end, an alternative nomenclature is sometimes used for these fatty acids. The carbon of the methyl group — that is, the carbon most distant from the carboxyl group — is called the ω (omega; the last letter in the Greek alphabet) carbon and is given the number 1 (C-1); the carboxyl carbon in this convention has the highest number. The positions of the double bonds are indicated relative to the ω carbon. In this convention, PUFAs with a double bond between C-3 and C-4 are called omega-3 (ω-3) fatty acids, and those with a double bond between C-6 and C-7 are omega-6 (ω-6) fatty acids. Shown below is eicosapentaenoic acid, which can be designated as 20:5() by the standard nomenclature but is also referred to as an omega-3 fatty acid, emphasizing the biologically important double bond in the omega-3 position.
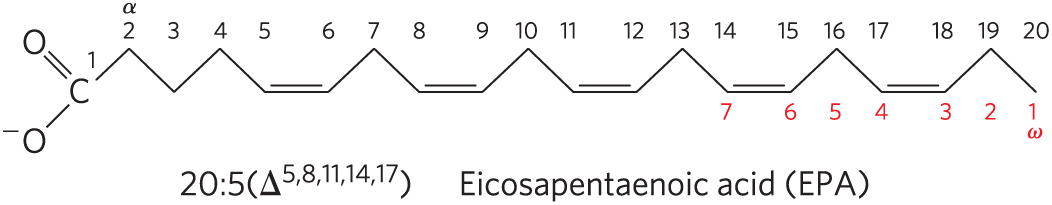
Humans require the omega-3 PUFA α-linolenic acid (ALA; 18:3(), in the standard convention), but do not have the enzymatic capacity to synthesize it and must therefore obtain ALA from the diet. Humans also use ALA to synthesize two other omega-3 PUFAs important in cellular function: eicosapentaenoic acid (EPA; 20:5(), shown in the Key Convention above) and docosahexaenoic acid (DHA; 22:6()). An imbalance of omega-6 and omega-3 PUFAs in the diet is associated with an increased risk of cardiovascular disease. The optimal dietary ratio of omega-6 to omega-3 PUFAs is between 1:1 and 4:1, but the ratio in the diets of most North Americans is closer to 10:1 to 30:1. The “Mediterranean diet,” which has been associated with lowered cardiovascular risk, is richer in omega-3 PUFAs, obtained in leafy vegetables (salads) and fish oils. Fish oils especially rich in EPA and DHA are a common dietary supplement even though their exact role in preventing cardiovascular disease is controversial.
The physical properties of the fatty acids, and of compounds that contain them, are largely determined by the length and degree of unsaturation of the hydrocarbon chain. The nonpolar hydrocarbon chain accounts for the poor solubility of fatty acids in water. Lauric acid (12:0, 200), for example, has a solubility in water of 0.063 mg/g — much less than that of glucose ( 180), which is 1,100 mg/g. The longer the fatty acyl chain and the fewer the double bonds, the lower the solubility in water. The carboxylic acid group is polar (and ionized at neutral pH) and accounts for the slight solubility of short-chain fatty acids in water.
Melting points are also strongly influenced by the length and degree of unsaturation of the hydrocarbon chain. At room temperature (), the saturated fatty acids from 12:0 to 24:0 have a waxy consistency, whereas unsaturated fatty acids of these lengths are oily liquids. This difference in melting points is due to different degrees of packing of the fatty acid molecules (Fig. 10-1). In the fully saturated compounds, free rotation around each carbon–carbon bond gives the hydrocarbon chain great flexibility; the most stable conformation is the fully extended form, in which the steric hindrance of neighboring atoms is minimized. These molecules can pack together tightly in nearly crystalline arrays, with atoms along their lengths in van der Waals contact with the atoms of neighboring molecules. In unsaturated fatty acids, a cis double bond forces a kink in the hydrocarbon chain. Fatty acids with one or several such kinks cannot pack together as tightly as fully saturated fatty acids, and their interactions with each other are therefore weaker. Because less thermal energy is needed to disorder these poorly ordered arrays of unsaturated fatty acids, they have markedly lower melting points than saturated fatty acids of the same chain length (Table 10-1).
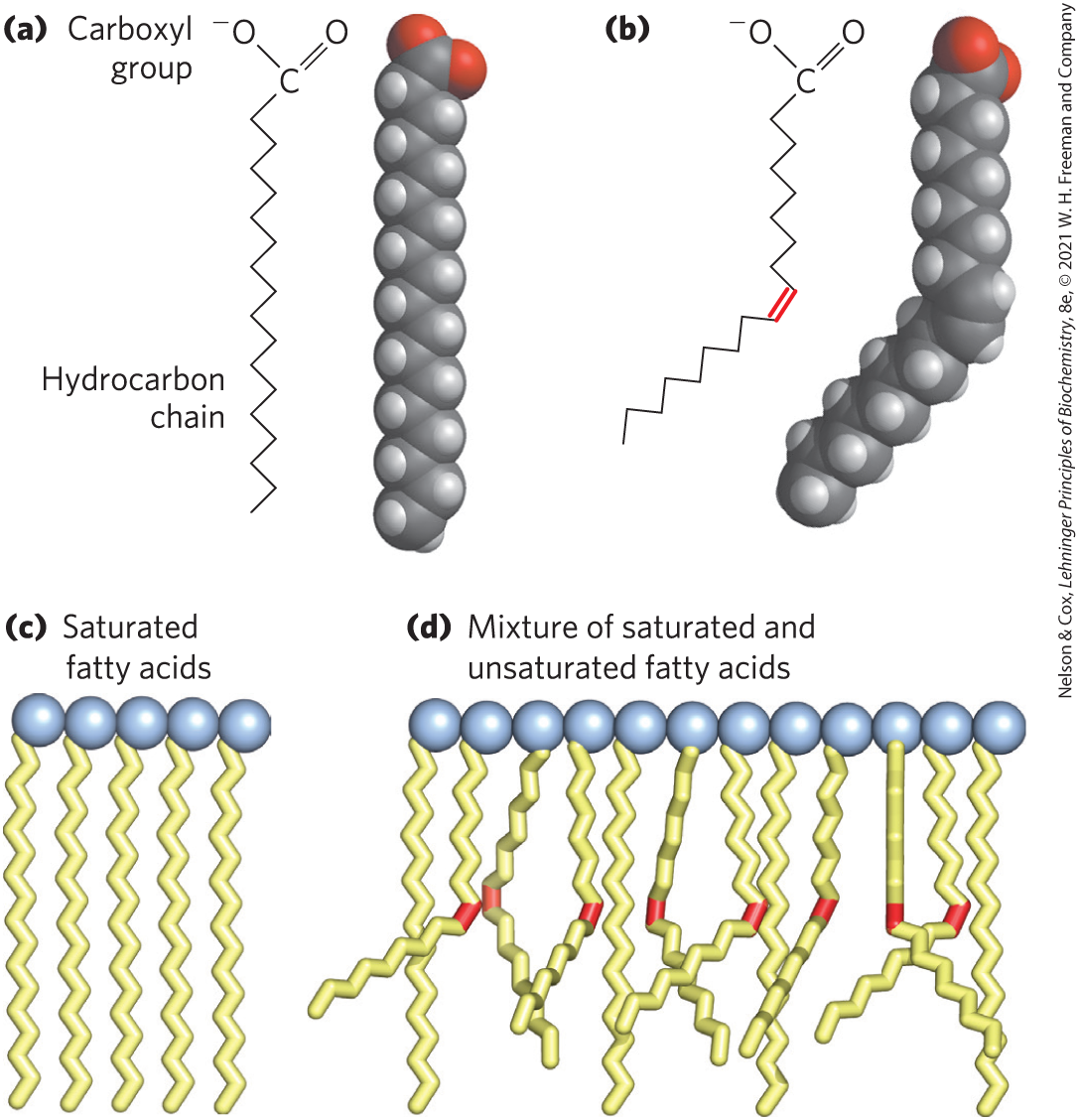
FIGURE 10-1 The packing of fatty acids into stable aggregates. The extent of packing depends on the degree of saturation. (a) Two representations of the fully saturated acid stearic acid, 18:0 (stearate at pH 7), in its usual extended conformation. (b) The cis double bond (red) in oleic acid, 18:1() (oleate), restricts rotation and introduces a rigid bend in the hydrocarbon tail. All other bonds in the chain are free to rotate. (c) Fully saturated fatty acids in the extended form pack into nearly crystalline arrays, stabilized by extensive hydrophobic interaction. (d) The presence of one or more fatty acids with cis double bonds (red) interferes with this tight packing and results in less stable aggregates.
In vertebrates, free fatty acids (unesterified fatty acids, with a free carboxylate group) can circulate in the blood bound noncovalently to carrier proteins such as serum albumin. However, fatty acids are present in blood plasma mostly as carboxylic acid derivatives such as esters or amides. Lacking the charged carboxylate group, these fatty acid derivatives are generally even less soluble in water than are the free fatty acids and are transported through the blood primarily as lipoprotein particles (see Chapter 21).
Triacylglycerols Are Fatty Acid Esters of Glycerol
The simplest lipids constructed from fatty acids are the triacylglycerols, also referred to as triglycerides, fats, or neutral fats. Triacylglycerols are composed of three fatty acids, each in ester linkage with a single glycerol (Fig. 10-2). Those containing the same kind of fatty acid in all three positions are called simple triacylglycerols and are named after the fatty acid they contain. Simple triacylglycerols of 16:0, 18:0, and 18:1, for example, are tripalmitin, tristearin, and triolein, respectively. Most naturally occurring triacylglycerols are mixed; they contain two or three different fatty acids. To name these compounds unambiguously, the name and position of each fatty acid must be specified.
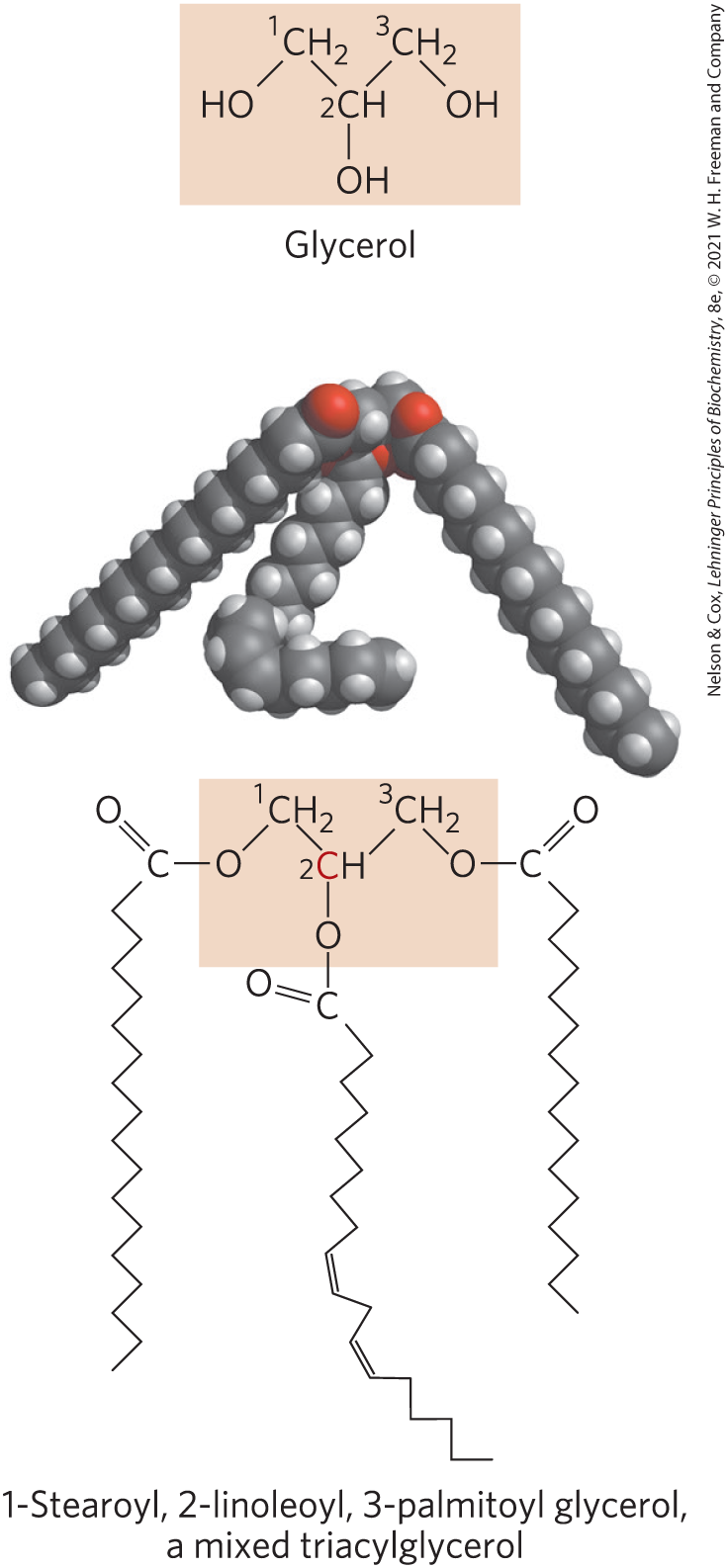
FIGURE 10-2 Glycerol and a triacylglycerol. The mixed triacylglycerol shown here has three different fatty acids attached to the glycerol backbone. When glycerol has different fatty acids at C-1 and C-3, C-2 is a chiral center.
Because the polar hydroxyls of glycerol and the polar carboxylates of the fatty acids are bound in ester linkages, triacylglycerols are nonpolar, hydrophobic molecules, essentially insoluble in water. Lipids have lower specific gravities than water, which explains why mixtures of oil and water (oil-and-vinegar salad dressing, for example) have two phases: oil, with the lower specific gravity, floats on the aqueous phase.
Triacylglycerols Provide Stored Energy and Insulation
In most eukaryotic cells, triacylglycerols form a separate phase of microscopic, oily droplets in the aqueous cytosol, serving as depots of metabolic fuel. In vertebrates, specialized cells called adipocytes, or fat cells, store large amounts of triacylglycerols as fat droplets that nearly fill the cell (Fig. 10-3a). Triacylglycerols are also stored as oils in the seeds of many types of plants, providing energy and biosynthetic precursors during seed germination (Fig. 10-3b). Adipocytes and germinating seeds contain lipases, enzymes that catalyze the hydrolysis of stored triacylglycerols, releasing fatty acids for export to sites where they are required as fuel.
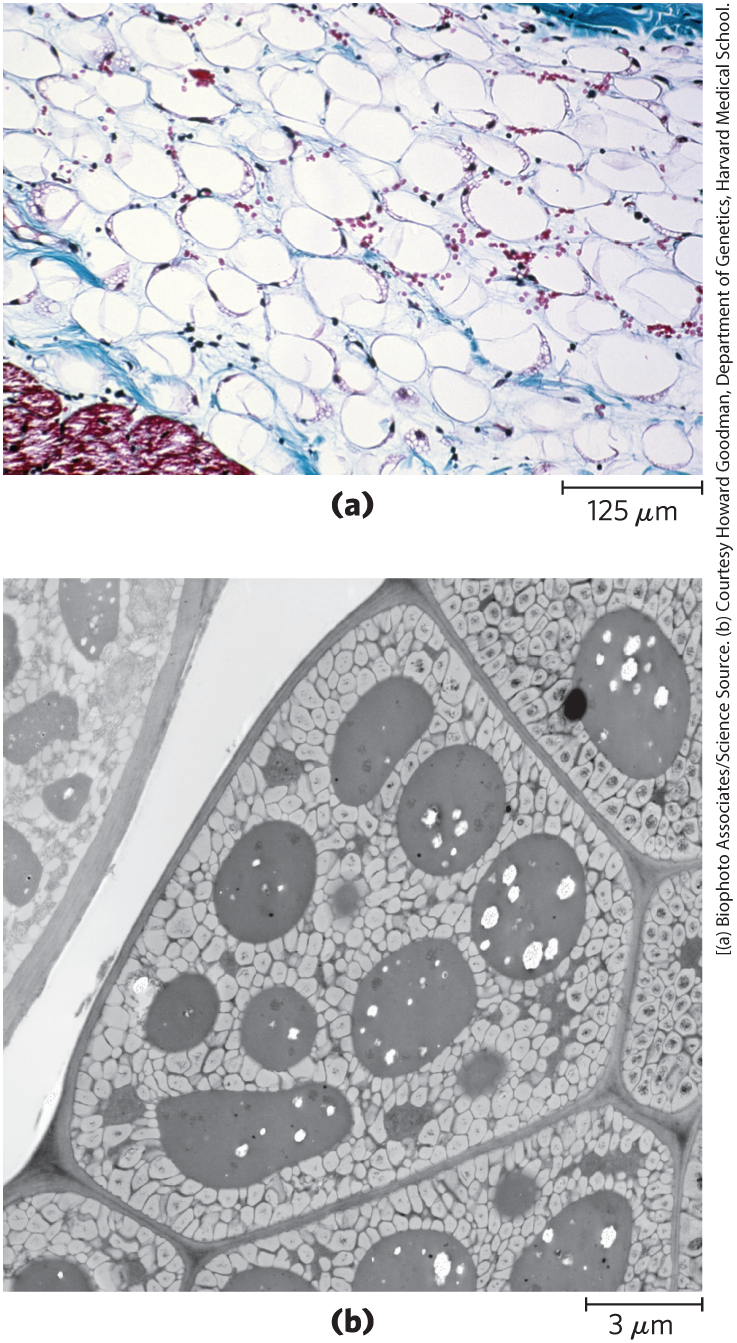
FIGURE 10-3 Fat stores in cells. (a) Cross section of human white adipose tissue. Each cell contains a fat droplet (white) so large that it squeezes the nucleus (stained red) against the plasma membrane. (b) Cross section of a cotyledon cell from a seed of the plant Arabidopsis. The large dark structures are protein bodies, which are surrounded by stored oils in the light-colored oil bodies.
There are two significant advantages to using triacylglycerols as stored fuels, rather than polysaccharides such as glycogen and starch. First, the carbon atoms of fatty acids are more reduced than those of sugars, so oxidation of triacylglycerols yields more than twice as much energy, gram for gram, as the oxidation of carbohydrates. Second, because triacylglycerols are hydrophobic and therefore unhydrated, the organism that carries stored fuel in the form of fat does not have to carry the extra weight of water of hydration that is associated with stored polysaccharides (2 g per gram of polysaccharide). Humans have fat tissue (composed primarily of adipocytes) under the skin, in the abdominal cavity, and in the mammary glands. Moderately obese people with 15 to 20 kg of triacylglycerols deposited in their adipocytes could meet their energy needs for months by drawing on their fat stores. In contrast, the human body can store less than a day’s energy supply in the form of glycogen. Carbohydrates such as glucose do offer certain advantages as quick sources of metabolic energy; one of those advantages is their ready solubility in water.
In some animals, triacylglycerols stored under the skin serve not only as energy stores but also as insulation against low temperatures. Seals, walruses, penguins, and other warm-blooded polar animals are amply padded with triacylglycerols. In hibernating animals (bears, for example), the huge fat reserves accumulated before hibernation serve the dual purposes of insulation and energy storage (see Box 17-1).
Partial Hydrogenation of Cooking Oils Improves Their Stability but Creates Fatty Acids with Harmful Health Effects
Most natural fats, such as those in vegetable oils, dairy products, and animal fat, are complex mixtures of simple and mixed triacylglycerols. These contain a variety of fatty acids differing in chain length and degree of saturation (Fig. 10-4). Vegetable oils such as corn (maize) oil and olive oil are composed largely of triacylglycerols with unsaturated fatty acids and thus are liquids at room temperature. Triacylglycerols containing only saturated fatty acids, such as tristearin, the major component of beef fat, are white, greasy solids at room temperature.
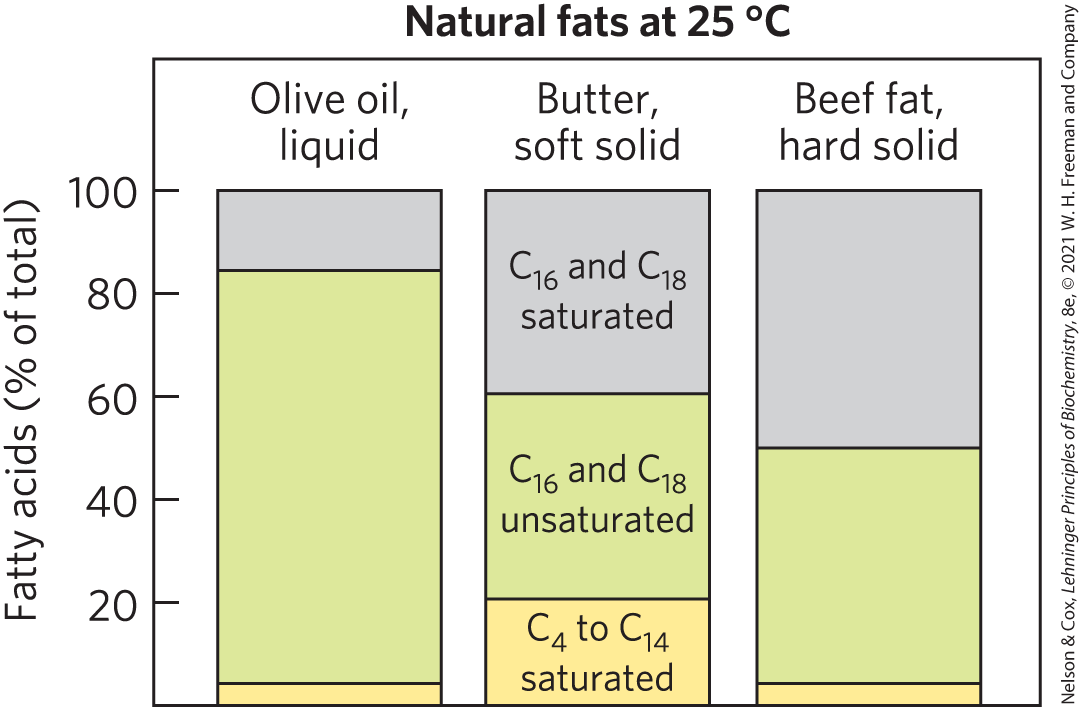
FIGURE 10-4 Fatty acid composition of three food fats. Olive oil, butter, and beef fat consist of mixtures of triacylglycerols differing in their fatty acid composition. The melting points of these fats — and hence their physical state at room temperature () — are a direct function of their fatty acid composition. Olive oil has a high proportion of long-chain ( and ) unsaturated fatty acids, which accounts for its liquid state at . The higher proportion of long-chain ( and ) saturated fatty acids in butter increases its melting point, so butter is a soft solid at room temperature. Beef fat, with an even higher proportion of long-chain saturated fatty acids, is a hard solid.
When lipid-rich foods are exposed too long to the oxygen in air, they may spoil and become rancid. The unpleasant taste and smell associated with rancidity result from the oxidative cleavage of double bonds in unsaturated fatty acids, which produces aldehydes and carboxylic acids of shorter chain length and therefore higher volatility; these compounds pass readily through the air to your nose. Throughout the twentieth century, partial hydrogenation of commercial vegetable oils was used to improve shelf life and to increase the stability of cooking oils at the high temperatures used in deep-frying. This process converts many of the cis double bonds in the fatty acids to single bonds and increases the melting temperature of the oils so that they are more nearly solid at room temperature (margarine is produced from vegetable oil in this way). Partial hydrogenation, however, has another, undesirable, effect: some cis double bonds are converted to trans double bonds. There is now strong evidence that dietary intake of trans fatty acids (often referred to simply as “trans fats”) leads to a higher incidence of cardiovascular disease, and that avoiding these fats in the diet substantially reduces the risk of coronary heart disease. Dietary trans fatty acids raise the level of triacylglycerols and of LDL (“bad”) cholesterol in the blood, and lower the level of HDL (“good”) cholesterol, and these changes alone are enough to increase the risk of coronary heart disease. But trans fatty acids may have further adverse effects. They seem, for example, to increase the body’s inflammatory response, which is another risk factor for heart disease. (See Chapter 21 for descriptions of LDL and HDL — low-density and high-density lipoprotein — cholesterol and their health effects.) Regulatory agencies around the world now limit or ban the use of trans fatty acids in prepared and packaged foods.
Waxes Serve as Energy Stores and Water Repellents
Biological waxes are esters of long-chain ( to ) saturated and unsaturated fatty acids with long-chain ( to ) alcohols (Fig. 10-5). Their melting points (60 to ) are generally higher than those of triacylglycerols. In plankton, the free-floating microorganisms at the bottom of the food chain for marine animals, waxes are the chief storage form of metabolic fuel.
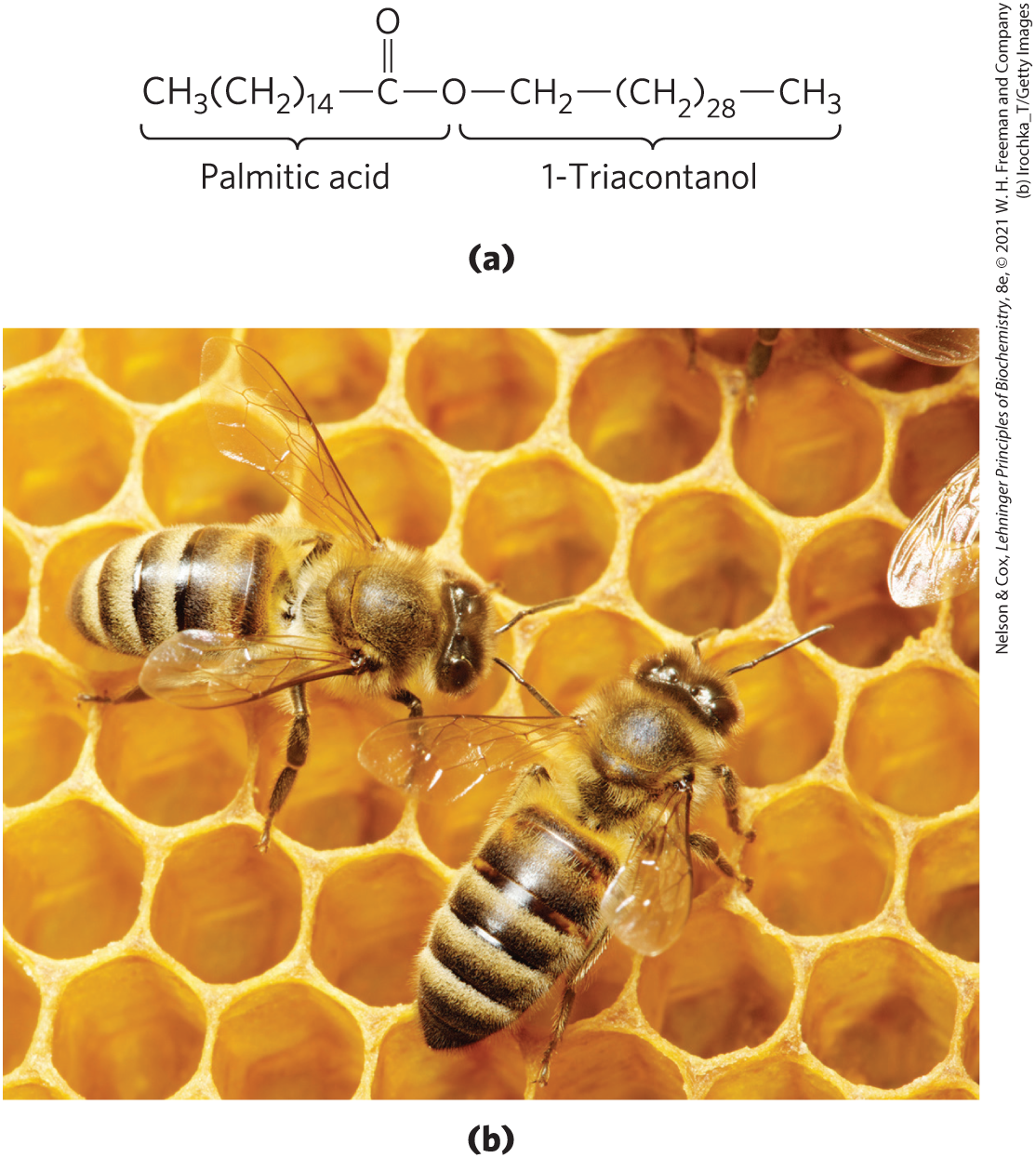
FIGURE 10-5 Biological wax. (a) Triacontanoylpalmitate, the major component of beeswax, is an ester of palmitic acid with the alcohol triacontanol. (b) The beeswax of a honeycomb is firm at and completely impervious to water. The term “wax” originates in the Old English weax, meaning “the material of the honeycomb.”
Waxes also serve a diversity of other functions related to their water-repellent properties and their firm consistency. Certain skin glands of vertebrates secrete waxes to protect hair and skin and keep it pliable, lubricated, and waterproof. Birds, particularly waterfowl, secrete waxes from preen glands to keep their feathers water-repellent. The shiny leaves of holly, rhododendrons, poison ivy, and many tropical plants are coated with a thick layer of waxes, which prevents excessive evaporation of water and protects against parasites.
Biological waxes find a variety of applications in the pharmaceutical, cosmetic, and other industries. Lanolin (from lamb’s wool), beeswax (Fig. 10-5), carnauba wax (from a Brazilian palm tree), and wax extracted from the seeds of the jojoba bush are widely used in the manufacture of lotions, ointments, and polishes.
SUMMARY 10.1 Storage Lipids
- Lipids are water-insoluble cellular components of diverse structure that can be extracted from tissues by nonpolar solvents. Almost all fatty acids, the hydrocarbon components of many lipids, have an even number of carbon atoms (usually 12 to 24); they are either saturated or unsaturated, with double bonds almost always in the cis configuration.
- Triacylglycerols contain three fatty acid molecules esterified to the three hydroxyl groups of glycerol. Simple triacylglycerols contain only one type of fatty acid; mixed triacylglycerols contain two or three types.
- Triacylglycerols are present in many foods and are primarily used for storing energy and providing insulation in animals. Lipases release fatty acids from storage so they can be used as fuel.
- Since many natural fats can easily turn rancid, partial hydrogenation has been used to extend their shelf life. This process can produce trans fatty acids, which increase risk for coronary heart disease. As a result, their use in prepared and processed foods has become highly regulated.
- Waxes are esters of long-chain fatty acids and long-chain alcohols with diverse uses in biology and industry.
 The fats and oils almost universally used as stored forms of energy in living organisms are derivatives of
The fats and oils almost universally used as stored forms of energy in living organisms are derivatives of 
 Humans require the omega-3 PUFA α-linolenic acid (ALA; 18:3(), in the standard convention), but do not have the enzymatic capacity to synthesize it and must therefore obtain ALA from the diet. Humans also use ALA to synthesize two other omega-3 PUFAs important in cellular function: eicosapentaenoic acid (EPA; 20:5(), shown in the Key Convention above) and docosahexaenoic acid (DHA; 22:6()). An imbalance of omega-6 and omega-3 PUFAs in the diet is associated with an increased risk of cardiovascular disease. The optimal dietary ratio of omega-6 to omega-3 PUFAs is between 1:1 and 4:1, but the ratio in the diets of most North Americans is closer to 10:1 to 30:1. The “Mediterranean diet,” which has been associated with lowered cardiovascular risk, is richer in omega-3 PUFAs, obtained in leafy vegetables (salads) and fish oils. Fish oils especially rich in EPA and DHA are a common dietary supplement even though their exact role in preventing cardiovascular disease is controversial.
Humans require the omega-3 PUFA α-linolenic acid (ALA; 18:3(), in the standard convention), but do not have the enzymatic capacity to synthesize it and must therefore obtain ALA from the diet. Humans also use ALA to synthesize two other omega-3 PUFAs important in cellular function: eicosapentaenoic acid (EPA; 20:5(), shown in the Key Convention above) and docosahexaenoic acid (DHA; 22:6()). An imbalance of omega-6 and omega-3 PUFAs in the diet is associated with an increased risk of cardiovascular disease. The optimal dietary ratio of omega-6 to omega-3 PUFAs is between 1:1 and 4:1, but the ratio in the diets of most North Americans is closer to 10:1 to 30:1. The “Mediterranean diet,” which has been associated with lowered cardiovascular risk, is richer in omega-3 PUFAs, obtained in leafy vegetables (salads) and fish oils. Fish oils especially rich in EPA and DHA are a common dietary supplement even though their exact role in preventing cardiovascular disease is controversial. 
 Lipids are water-insoluble cellular components of diverse structure that can be extracted from tissues by nonpolar solvents. Almost all fatty acids, the hydrocarbon components of many lipids, have an even number of carbon atoms (usually 12 to 24); they are either saturated or unsaturated, with double bonds almost always in the cis configuration.
Lipids are water-insoluble cellular components of diverse structure that can be extracted from tissues by nonpolar solvents. Almost all fatty acids, the hydrocarbon components of many lipids, have an even number of carbon atoms (usually 12 to 24); they are either saturated or unsaturated, with double bonds almost always in the cis configuration.