6.5 Regulatory Enzymes
In cellular metabolism, groups of enzymes work together in sequential pathways to carry out a given metabolic process, such as the multireaction breakdown of glucose to lactate or the multireaction synthesis of an amino acid from simpler precursors. Each separate reaction is catalyzed by a different enzyme. In such enzyme systems, the reaction product of one enzyme becomes the substrate of the next. This functional compartmentalization of cellular chemistry does more than accelerate individual reactions; it provides opportunities for the exquisitely precise regulation of all cellular processes.
Most of the enzymes in each metabolic pathway follow the kinetic patterns we have already described. Each pathway, however, includes one or more enzymes that have a greater effect on the rate of the overall sequence. The catalytic activity of these regulatory enzymes increases or decreases in response to certain signals. Adjustments in the rate of reactions catalyzed by regulatory enzymes, and therefore in the rate of entire metabolic sequences, allow the cell to meet changing needs for energy and for biomolecules required in growth and repair.
The activities of regulatory enzymes are modulated in a variety of ways. Allosteric enzymes function through reversible, noncovalent binding of regulatory compounds called allosteric modulators or allosteric effectors, which are generally small metabolites or cofactors. Other enzymes are regulated by reversible covalent modification. Both classes of regulatory enzymes tend to be multisubunit proteins, and in some cases the regulatory site(s) and the active site are on separate subunits. Metabolic systems have at least two other mechanisms of enzyme regulation. Some enzymes are stimulated or inhibited when they are bound by separate regulatory proteins. Others are activated when peptide segments are removed by proteolytic cleavage; unlike effector-mediated regulation, regulation by proteolytic cleavage is irreversible. Important examples of both mechanisms are found in physiological processes such as digestion, blood clotting, hormone action, and vision.
Cell growth and survival depend on efficient use of resources, and this efficiency is made possible by regulatory enzymes. No single rule governs which of the various types of regulation occur in different systems. Several types of regulation may occur in a single regulatory enzyme. The remainder of this chapter is devoted to a discussion of these major mechanisms of enzyme regulation.
Allosteric Enzymes Undergo Conformational Changes in Response to Modulator Binding
As we saw in Chapter 5, allosteric proteins are those having “other shapes” or conformations induced by the binding of modulators. The same concept applies to certain regulatory enzymes, as conformational changes induced by one or more modulators interconvert more-active and less-active forms of the enzyme. The modulators for allosteric enzymes may be inhibitory or stimulatory. The modulator can be the substrate itself; regulation in which substrate and modulator are identical is referred to as homotropic. The effect is similar to that of binding to hemoglobin (Chapter 5): binding of the ligand — or substrate, in the case of enzymes — causes conformational changes that affect the subsequent activity of other sites on the protein. In most cases, the conformational change converts a relatively inactive conformation (often referred to as a T state — a convention based on the early hemoglobin literature) to a more active conformation (an R state). When the modulator is a molecule other than the substrate, the enzyme is said to be heterotropic. Note that heterotropic modulators should not be confused with uncompetitive and mixed inhibitors. Although the latter bind at a second site on the enzyme, they do not necessarily mediate conformational changes between active and inactive forms, and the kinetic effects are distinct.
The properties of allosteric enzymes are significantly different from those of simple nonregulatory enzymes. Some of the differences are structural. In addition to active sites, allosteric enzymes often have one or more regulatory, or allosteric, sites for binding to each heterotropic modulator (Fig. 6-35). Just as an enzyme’s active site is specific for its substrate, each regulatory site is specific for its modulator. Enzymes with several modulators generally have different specific binding sites for each. In homotropic enzymes, the active site and regulatory site are the same.
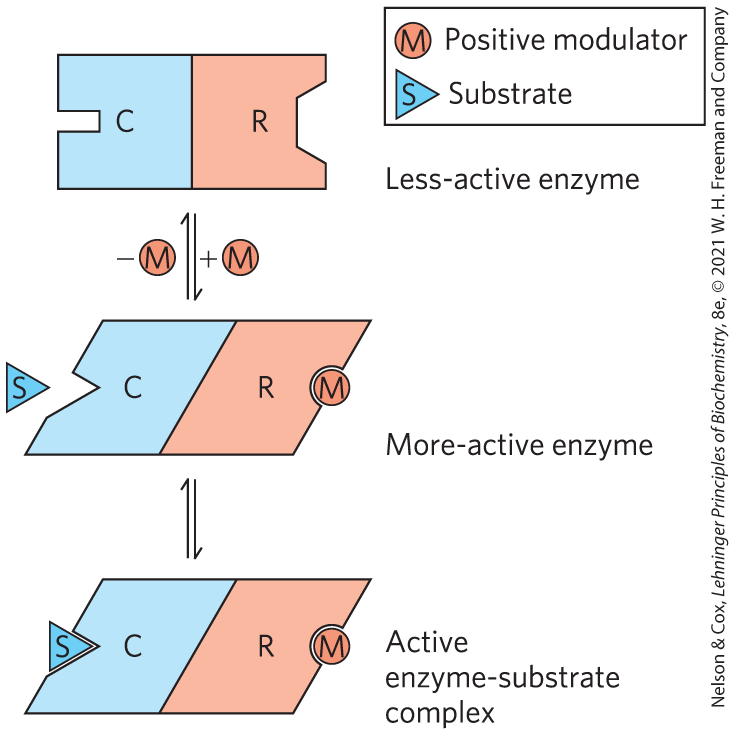
FIGURE 6-35 Subunit interactions in an allosteric enzyme, and interactions with inhibitors and activators. In many allosteric enzymes, the substrate-binding site and the modulator-binding site(s) are on different subunits: the catalytic (C) and regulatory (R) subunits, respectively. Binding of the positive (stimulatory) modulator (M) to its specific site on the regulatory subunit is communicated to the catalytic subunit through a conformational change. This change renders the catalytic subunit active and capable of binding the substrate (S) with higher affinity. Upon dissociation of the modulator from the regulatory subunit, the enzyme reverts to its inactive or less-active form.
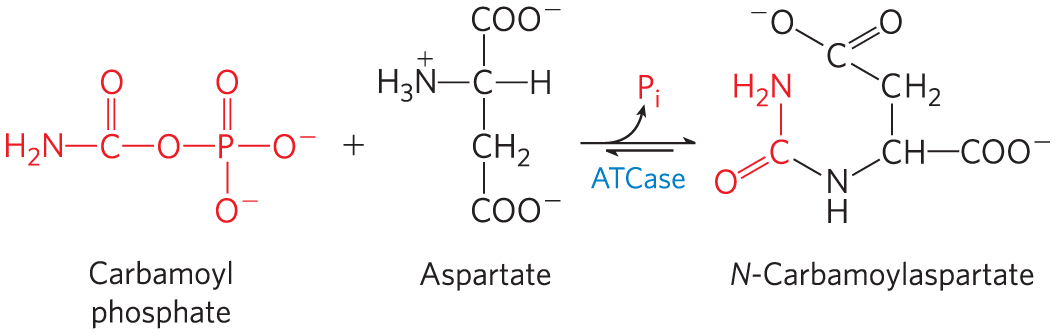
Allosteric enzymes are typically larger and more complex than nonallosteric enzymes, with two or more subunits. A classic example is aspartate transcarbamoylase (often abbreviated ATCase), which catalyzes an early step in the biosynthesis of pyrimidine nucleotides, the reaction of carbamoyl phosphate and aspartate to form carbamoyl aspartate:
ATCase has 12 polypeptide chains organized into 6 catalytic subunits (organized as 2 trimeric complexes) and 6 regulatory subunits (organized as 3 dimeric complexes). Figure 6-36 shows the quaternary structure of this enzyme, deduced from x-ray analysis. The enzyme exhibits allosteric behavior as detailed below, as the catalytic subunits function cooperatively. The regulatory subunits have binding sites for ATP and CTP, which function as positive and negative regulators, respectively. CTP is one of the end products of the pathway, and negative regulation by CTP serves to limit ATCase action under conditions when CTP is abundant. On the other hand, high concentrations of ATP indicate that cellular metabolism is robust, that the cell is growing, and that additional pyrimidine nucleotides may be needed to support RNA transcription and DNA replication.
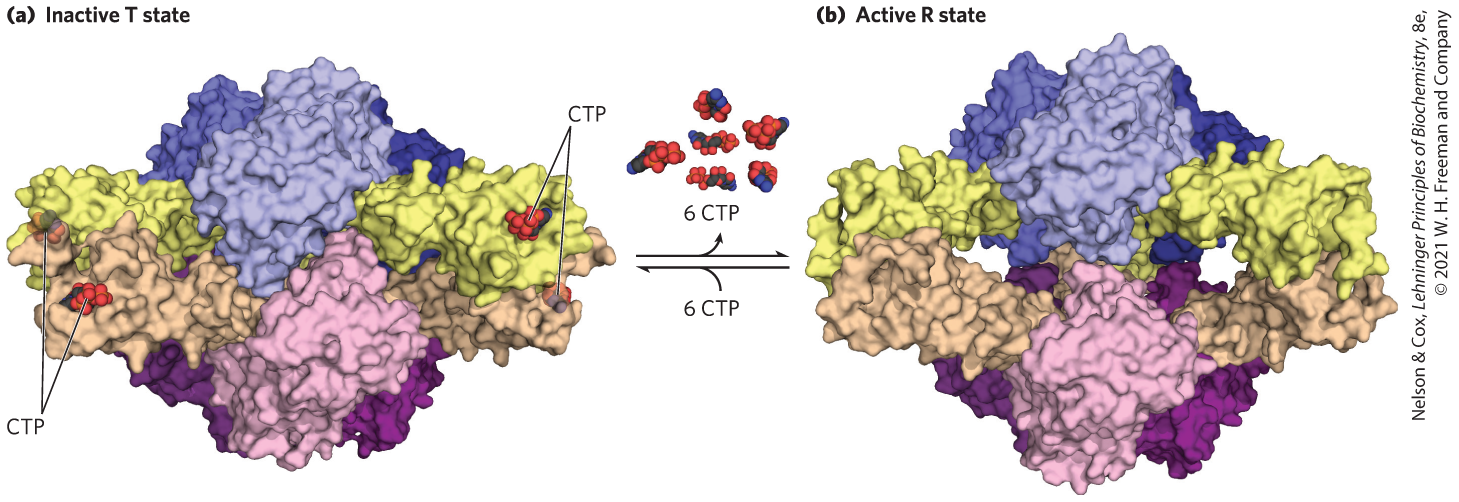
FIGURE 6-36 The regulatory enzyme aspartate transcarbamoylase. (a) The inactive T state and (b) the active R state of the enzyme are shown. This allosteric regulatory enzyme has two stacked catalytic clusters, each with three catalytic polypeptide chains (in shades of blue and purple), and three regulatory clusters, each with two regulatory polypeptide chains (in beige and yellow). The regulatory clusters form the points of a triangle (not evident in this side view) surrounding the catalytic subunits. Binding sites for allosteric modulators (including CTP) are on the regulatory subunits. Modulator binding produces large changes in enzyme conformation and activity. The role of this enzyme in nucleotide synthesis, and details of its regulation, are discussed in Chapter 22. [Data from (a) PDB ID 1RAB, R. P. Kosman et al., Proteins 15:147, 1993; (b) PDB ID 1F1B, L. Jin et al., Biochemistry 39:8058, 2000.]
The Kinetic Properties of Allosteric Enzymes Diverge from Michaelis-Menten Behavior
Allosteric enzymes show relationships between and [S] that differ from Michaelis-Menten kinetics. They do exhibit saturation with the substrate when [S] is sufficiently high, but for allosteric enzymes, plots of versus [S] (Fig. 6-37) usually produce a sigmoid saturation curve, rather than the hyperbolic curve typical of nonregulatory enzymes. On the sigmoid saturation curve we can find a value of [S] at which is half-maximal, but we cannot refer to it with the designation , because the enzyme does not follow the hyperbolic Michaelis-Menten relationship. Instead, the symbol or is often used to represent the substrate concentration giving half-maximal velocity of the reaction catalyzed by an allosteric enzyme (Fig. 6-37).
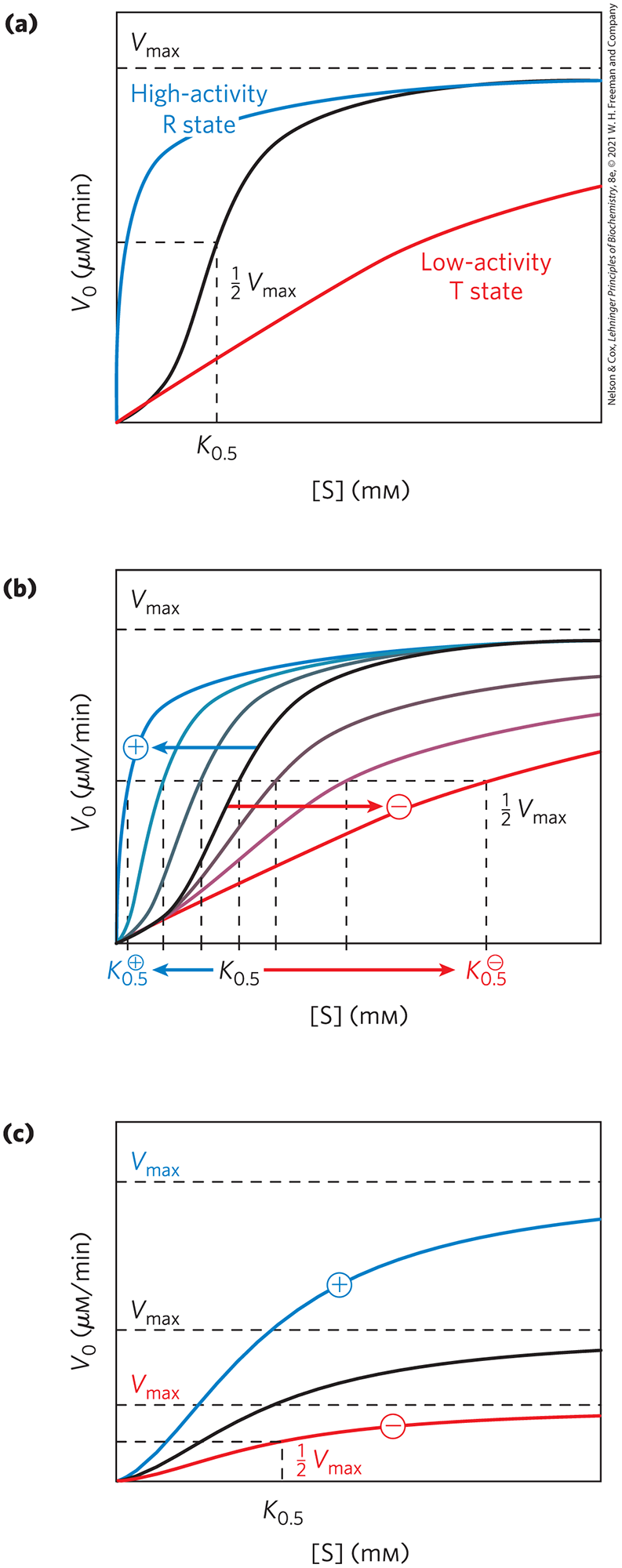
FIGURE 6-37 Substrate-activity curves for representative allosteric enzymes. Three examples of complex responses of allosteric enzymes to their modulators. (a) The sigmoid curve of a homotropic enzyme, in which the substrate also serves as a positive (stimulatory) modulator, or activator. Notice the resemblance to the oxygen-saturation curve of hemoglobin (see Fig. 5-12). The sigmoid curve is a hybrid curve in which the enzyme is present primarily in the relatively inactive T state at low substrate concentration, and primarily in the more active R state at high substrate concentration. The curves for the pure T and R states are plotted separately in color. ATCase exhibits a kinetic pattern similar to this. (b) The effects of several different concentrations of a positive modulator (+) or a negative modulator (−) on an allosteric enzyme in which is altered without a change in . The central curve shows the substrate-activity relationship without a modulator. For ATCase, CTP is a negative modulator and ATP is a positive modulator. (c) A less common type of modulation, in which is altered and is nearly constant.
Sigmoid kinetic behavior reflects cooperative interactions between multiple protein subunits. In other words, changes in the structure of one subunit are translated into structural changes in adjacent subunits, an effect mediated by noncovalent interactions at the interface between subunits. Sigmoid kinetic behavior for enzymes is well explained by the concerted and sequential models for subunit interactions we previously encountered when considering binding to hemoglobin (see Fig. 5-14).
ATCase effectively illustrates both homotropic and heterotropic allosteric kinetic behavior. Binding of the substrates, aspartate and carbamoyl phosphate, to the enzyme gradually brings about a transition from the relatively inactive T state to the more active R state. This accounts for the sigmoid rather than hyperbolic change in with increasing [S]. One characteristic of sigmoid kinetics is that small changes in the concentration of a modulator can be associated with large changes in activity. As exemplified in Figure 6-37a, a relatively small increase in [S] in the steep part of the curve causes a comparatively large increase in .
The heterotropic allosteric regulation of ATCase is brought about by its interactions with ATP and CTP. For heterotropic allosteric enzymes, an activator may cause the curve to become more nearly hyperbolic, with a decrease in but no change in , resulting in an increased reaction velocity at a fixed substrate concentration. For ATCase, the interaction with ATP brings this about, and the enzyme exhibits a versus [S] curve that is characteristic of the active R state at sufficiently high ATP concentrations ( is higher for any value of [S]; Fig. 6-37b). A negative modulator (an inhibitor) may produce a more sigmoid substrate-saturation curve, with an increase in , as illustrated by the effects of CTP on ATCase kinetics (see curves for a negative modulator in Fig. 6-37b). Other heterotropic allosteric enzymes respond to an activator by an increase in with little change in (Fig. 6-37c). Heterotropic allosteric enzymes therefore show different kinds of responses in their substrate-activity curves, because some have inhibitory modulators, some have activating modulators, and some (like ATCase) have both.
Some Enzymes Are Regulated by Reversible Covalent Modification
In another important class of regulatory enzymes, activity is modulated by covalent modification of one or more of the amino acid residues in the enzyme molecule. Over 500 different types of covalent modification have been found in proteins. Common modifying groups include phosphoryl, acetyl, adenylyl, uridylyl, methyl, amide, carboxyl, myristoyl, palmitoyl, prenyl, hydroxyl, sulfate, and adenosine diphosphate ribosyl groups (Fig. 6-38). There are even entire proteins that function as specialized modifying groups. These include ubiquitin and SUMO (small ubiquitin-like modifier), which are attached and detached from other proteins to regulate their activity in some way. All of these groups, small and large, are linked to and removed from a regulated enzyme by separate enzymes. When an amino acid residue in an enzyme is modified, a novel amino acid with altered properties has effectively been introduced into the enzyme. Introduction of a charge can alter the local properties of the enzyme and induce a change in conformation. Introduction of a hydrophobic group can trigger association with a membrane. The changes are often substantial and can be critical to the function of the altered enzyme.
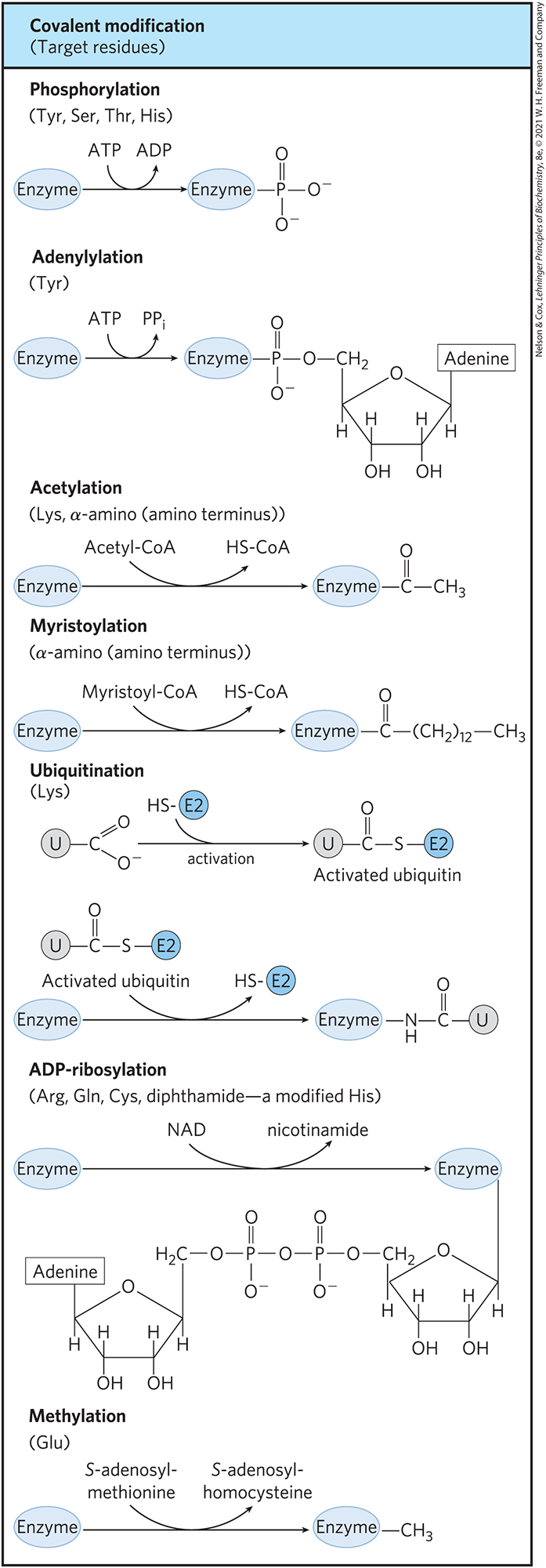
FIGURE 6-38 Some enzyme modification reactions.
Although there are too many examples of covalent modification of proteins to cover in detail, a few examples are instructive. One enzyme that is regulated by methylation is the methyl-accepting chemotaxis protein of bacteria. This protein is part of a system that permits a bacterium to swim toward an attractant (such as a sugar) in solution and away from repellent chemicals. The methylating agent is S-adenosylmethionine (adoMet) (see Fig. 18-18). Acetylation is another common modification, with approximately 80% of the soluble proteins in eukaryotes, including many enzymes, acetylated at their amino terminus. Ubiquitin is added to proteins as a tag that destines them for proteolytic degradation (see Fig. 27-47). Ubiquitination can also have a regulatory function. SUMO is found attached to many eukaryotic nuclear proteins and has roles in the regulation of transcription, chromatin structure, and DNA repair.
ADP-ribosylation is an especially interesting reaction; the ADP-ribose is derived from nicotinamide adenine dinucleotide (NAD) (see Fig. 8-41). This type of modification occurs for the bacterial enzyme dinitrogenase reductase, resulting in regulation of the important process of biological nitrogen fixation. Diphtheria toxin and cholera toxin are enzymes that catalyze the ADP-ribosylation (and inactivation) of key cellular enzymes or other proteins.
By far the most common type of regulatory modification is phosphorylation. About one-third of all proteins in a eukaryotic cell are phosphorylated, and one or (often) many phosphorylation events are part of virtually every regulatory process. Some proteins have only one phosphorylated residue, others have several, and a few have dozens of sites for phosphorylation. This mode of covalent modification is central to a large number of regulatory pathways. We discuss it in some detail here, and again in Chapter 12.
We will encounter all of these types of enzyme modification again in later chapters.
Phosphoryl Groups Affect the Structure and Catalytic Activity of Enzymes
The attachment of phosphoryl groups to specific amino acid residues of a protein is catalyzed by protein kinases. More than 500 genes encoding these critical enzymes are found in the human genome. In the reactions they catalyze, the γ-phosphoryl group derived from a nucleoside triphosphate (usually ATP) is transferred to a particular Ser, Thr, or Tyr residue (occasionally His as well) on the target protein. This introduces a bulky, charged group into a region of the target protein that was only moderately polar prior to modification. The oxygen atoms of a phosphoryl group can hydrogen-bond with one or several groups in a protein, commonly the amide groups of the peptide backbone at the start of an α helix or the charged guanidinium group of an Arg residue. The two negative charges on a phosphorylated side chain can also repel neighboring negatively charged (Asp or Glu) residues. When the modified side chain is located in a region of an enzyme critical to its three-dimensional structure, phosphorylation can have dramatic effects on enzyme conformation and thus on substrate binding and catalysis. Removal of phosphoryl groups from these same target proteins is catalyzed by phosphoprotein phosphatases, also called simply protein phosphatases.
An important example of enzyme regulation by phosphorylation is the case of glycogen phosphorylase of muscle and liver (Chapter 15), which catalyzes the reaction
The glucose 1-phosphate so formed can be used for ATP synthesis in muscle or converted to free glucose in the liver. Note that glycogen phosphorylase, though it adds a phosphate to a substrate, is not itself a kinase, because it does not utilize ATP or any other nucleotide triphosphate as a phosphoryl donor in its catalyzed reaction. It is, however, the substrate for a protein kinase that phosphorylates it. In the discussion below, the phosphoryl groups we are concerned with are those involved in regulation of the enzyme, as distinguished from its catalytic function.
Glycogen phosphorylase occurs in two forms: the more active phosphorylase a and the less active phosphorylase b (Fig. 6-39). Phosphorylase a has two subunits, each with a specific Ser residue that is phosphorylated at its hydroxyl group. Phosphorylase b is covalently transformed into active phosphorylase a by another enzyme, phosphorylase kinase, which catalyzes the transfer of phosphoryl groups from ATP to the hydroxyl groups of the two specific Ser residues in phosphorylase b:
To serve as an effective regulatory mechanism, phosphorylation must be reversible. In general, phosphoryl groups are added and removed by different enzymes, and the processes can therefore be separately regulated. The phosphoryl groups of phosphorylase a are hydrolytically removed by a separate enzyme called phosphoprotein phosphatase 1 (PP1):
These phosphoserine residues are required for maximal activity of the enzyme.
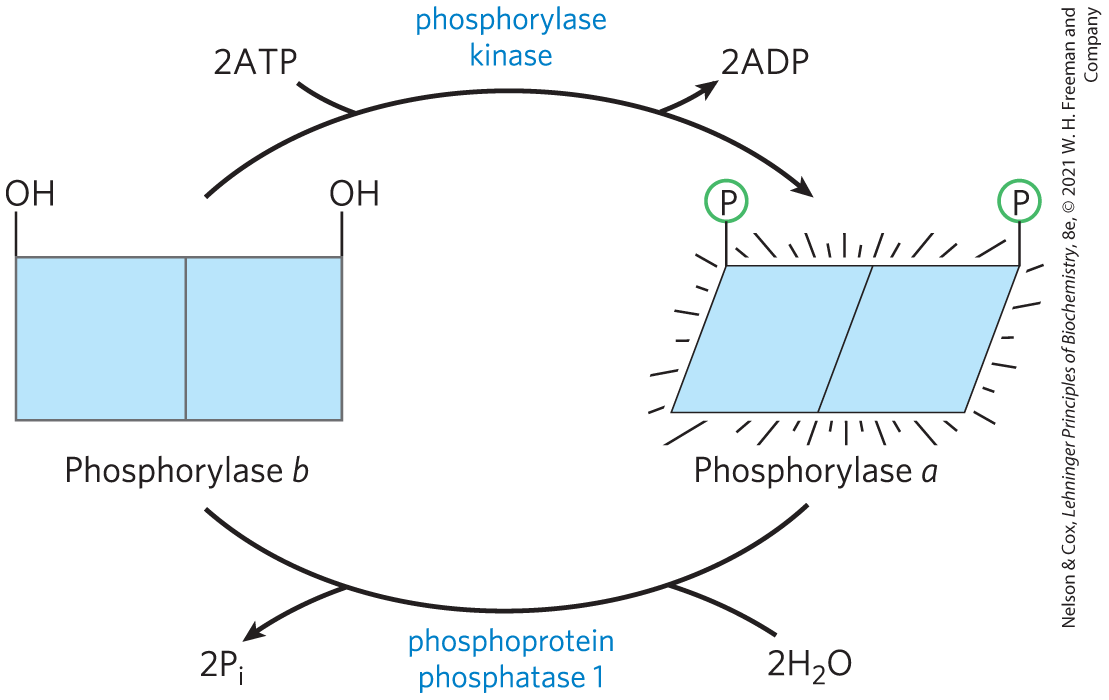
FIGURE 6-39 Regulation of muscle glycogen phosphorylase activity by phosphorylation. In the more-active form of the enzyme, phosphorylase a, specific Ser residues, one on each subunit, are phosphorylated. Phosphorylase a is converted to the less active phosphorylase b by enzymatic loss of these phosphoryl groups, promoted by phosphoprotein phosphatase 1 (PP1). Phosphorylase b can be reconverted (reactivated) to phosphorylase a by the action of phosphorylase kinase.
In this reaction, phosphorylase a is converted to phosphorylase b by the cleavage of two phosphoserine covalent bonds, one on each subunit of glycogen phosphorylase.
The regulation of glycogen phosphorylase by phosphorylation illustrates the effects on both structure and catalytic activity of adding a phosphoryl group (Fig. 6-40). In the unphosphorylated state (phosphorylase b), each subunit of this enzyme is folded so as to bring the 20 residues at its amino terminus, including some basic residues, into a region containing several acidic amino acids; this produces an electrostatic interaction that stabilizes the conformation. Phosphorylation of interferes with this interaction, forcing the amino-terminal domain out of the acidic environment and into a conformation that allows interaction between the –Ser and several Arg side chains (phosphorylase a). In this conformation, the enzyme is much more active.
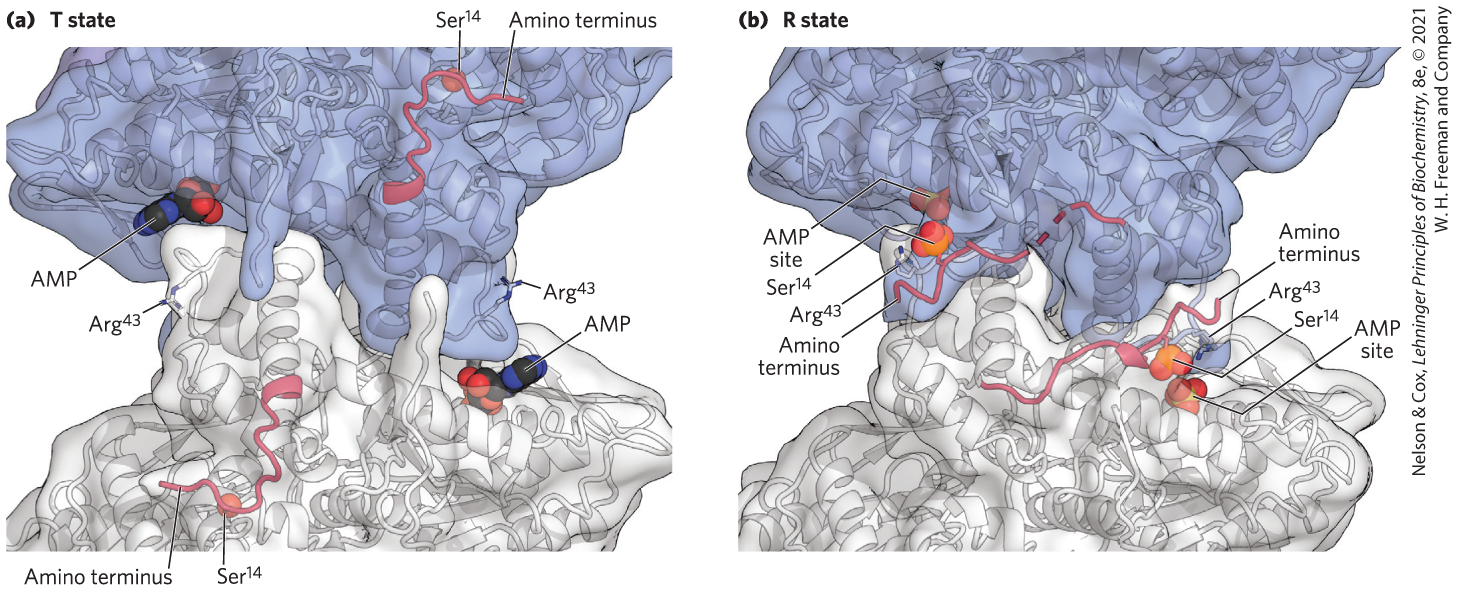
FIGURE 6-40 The conformation change brought about by phosphorylation in glycogen phosphorylase from rabbit muscle. Twenty amino acid residues at the amino terminus of each subunit are shown in red, including the residue phosphorylated in phosphorylase a . This peptide segment interacts with different parts of the protein depending upon the phosphorylation state of , stabilizing the different conformations that characterize phosphorylase a and b. [Data from (a) PDB ID 8GPB, (b) PDB ID 1GPA, D. Barford et al., J. Mol. Biol. 218:233, 1991.]
As we shall see in Chapter 15, phosphorylation is just one of the regulatory mechanisms by which the activity of glycogen phosphorylase is controlled. Allosteric regulation and additional hormonal responses also contribute to the overall precision of control needed to meet moment-to-moment cellular demands for glucose and ATP.
Multiple Phosphorylations Allow Exquisite Regulatory Control
The Ser, Thr, or Tyr residues that are typically phosphorylated in regulated proteins occur within common structural motifs, called consensus sequences, that are recognized by specific protein kinases (Table 6-10). Some kinases are basophilic, preferentially phosphorylating a residue that has basic neighbors; others have different substrate preferences, such as for a residue near a Pro residue. However, amino acid sequence is not the only important factor in determining whether a given residue will be phosphorylated. Protein folding brings together residues that are distant in the primary sequence; the resulting three-dimensional structure can determine whether a protein kinase has access to a given residue and can recognize it as a substrate.
| Protein kinase | Consensus sequence and phosphorylated residue |
|---|---|
| Protein kinase A | -x-R-[RK]-x-[ST]-B- |
| Protein kinase G | -x-R-[RK]-x-[ST]-X- |
| Protein kinase C | -[RK](2)-x-[ST]-B-[RK](2)- |
| Protein kinase B | R-x-x-R-x-[ST]-x-ψa- |
| /calmodulin kinase I | -B-x-R-x(2)-[ST]-x(3)-B- |
| /calmodulin kinase II | -B-x-[RK]-x(2)-[ST]-x(2)- |
| Myosin light chain kinase (smooth muscle) | -K(2)-R-x(2)-S-x-B(2)- |
| Phosphorylase b kinase | -K-R-K-Q-I-S-V-R- |
| Extracellular signal-regulated kinase (ERK) | -P-x-[ST]-P(2)- |
| Cyclin-dependent protein kinase (cdc2) | -x-[ST]-P-x-[KR]- |
| Casein kinase I | -[SpTp]-x(2)-[ST]-Bb |
| Casein kinase II | -x-[ST]-x(2)-[ED]-x- |
| β-Adrenergic receptor kinase | -[DE](n)-[ST]-x(3) |
| Rhodopsin kinase | -x(2)-[ST]-E(n)-vABL-[YLV]-Y--[PF]- |
| Epidermal growth factor (EGF) receptor kinase | -E(4)-Y-F-E-L-V- |
Information from L. A. Pinna and M. H. Ruzzene, Biochim. Biophys. Acta 1314:191, 1996; B. E. Kemp and R. B. Pearson, Trends Biochem. Sci. 15:342, 1990; P. J. Kennelly and E. G. Krebs, J. Biol. Chem. 266:15,555, 1991; T. P. Cujec, P. F. Madeiros, P. Hammond, C. Rise, and B. L. Kreider, Chem. Biol. 9:253, 2002. Note: Shown here are deduced consensus sequences (in roman type) and actual sequences from known substrates (italic). The Ser (S), Thr (T), or Tyr (Y) residue that undergoes phosphorylation is in bold; all amino acid residues are shown as their one-letter abbreviations (see Table 3-1). An x represents any amino acid; B, any hydrophobic amino acid; Sp and Tp are Ser and Thr residues that must already be phosphorylated for the kinase to recognize the site. aψ denotes any amino acid with a bulky hydrophobic side chain. bThe best target site has two amino acid residues separating the phosphorylated and target Ser/Thr residues; target sites with one or three intervening residues function at a reduced level. |
|
Regulation by phosphorylation is often complicated. Some proteins have consensus sequences recognized by several different protein kinases, each of which can phosphorylate the protein and alter its enzymatic activity. In some cases, phosphorylation is hierarchical: a certain residue can be phosphorylated only if a neighboring residue has already been phosphorylated. For example, glycogen synthase, the enzyme that catalyzes the condensation of glucose monomers to form glycogen (Chapter 15), is inactivated by phosphorylation of specific Ser residues and is also modulated by at least four other protein kinases that phosphorylate four other sites in the enzyme (Fig. 6-41). The enzyme does not become a substrate for glycogen synthase kinase 3 until one site has been phosphorylated by casein kinase II. Some phosphorylations inhibit glycogen synthase more than others, and some combinations of phosphorylations are cumulative. These multiple regulatory phosphorylations provide the potential for extremely subtle modulation of enzyme activity.
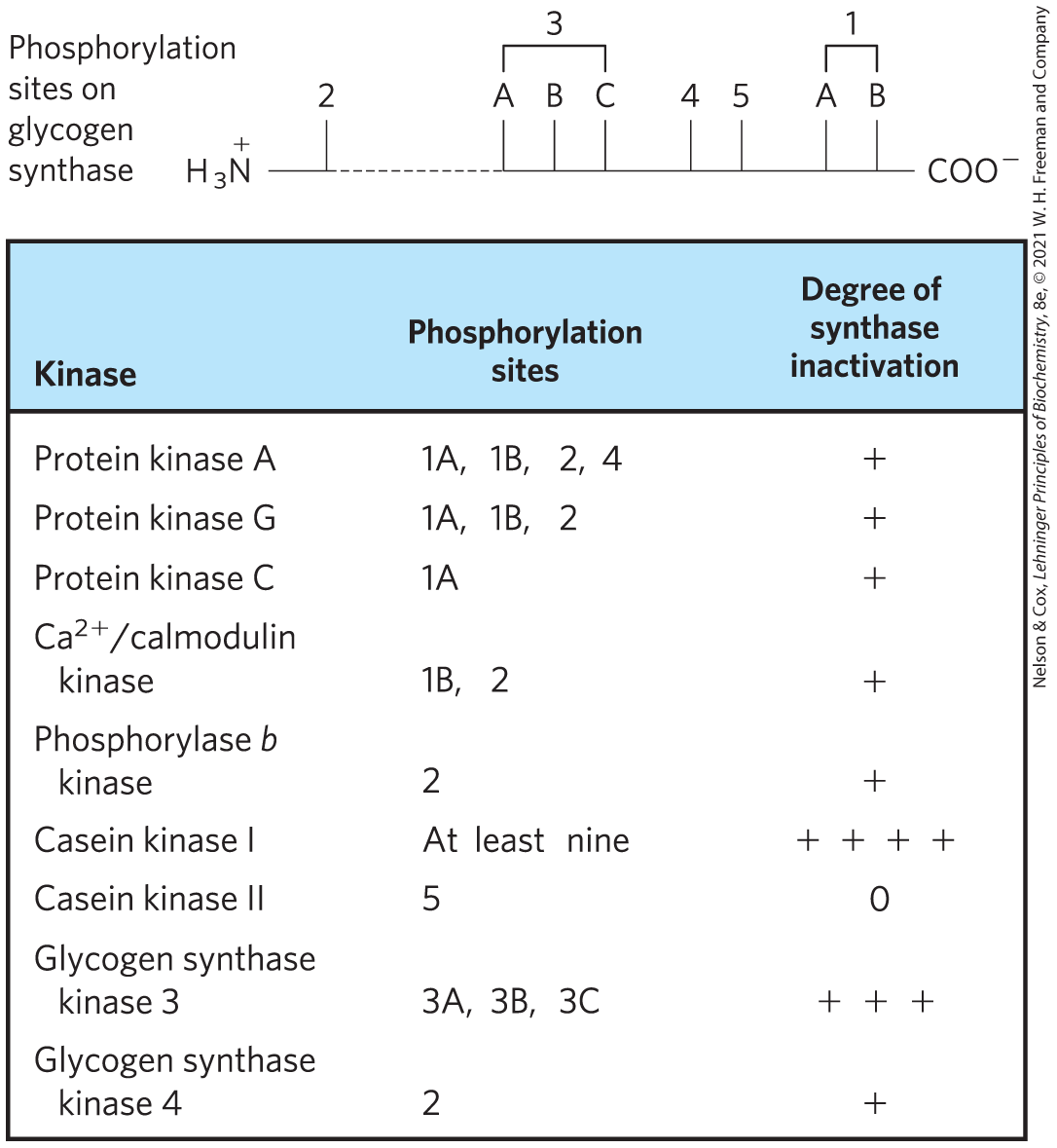
FIGURE 6-41 Multiple regulatory phosphorylations. The enzyme glycogen synthase has at least nine separate sites in five designated regions that are susceptible to phosphorylation by one of the cellular protein kinases. Thus, regulation of this enzyme is a matter not of binary (on/off) switching but of finely tuned modulation of activity over a wide range in response to a variety of signals.
As is the case for kinases, there are many different enzymes that remove phosphoryl groups from proteins. Cells contain a family of phosphoprotein phosphatases that each hydrolyze specific –Ser, –Thr, and –Tyr esters, releasing . The phosphoprotein phosphatases generally act on only a subset of phosphorylated proteins, but they show less substrate specificity than protein kinases.
Some Enzymes and Other Proteins Are Regulated by Proteolytic Cleavage of an Enzyme Precursor
For some enzymes, an inactive precursor called a zymogen is cleaved to form the active enzyme. Many proteolytic enzymes (proteases) of the stomach and pancreas are regulated in this way. Chymotrypsin and trypsin are initially synthesized as chymotrypsinogen and trypsinogen (Fig. 6-42). Specific cleavage causes conformational changes that expose the enzyme active site. This type of activation is irreversible, and additional regulation requires a different mechanism. When necessary, a previously activated protease is inactivated by inhibitor proteins that bind very tightly to the enzyme active site. For example, pancreatic trypsin inhibitor binds to and inhibits trypsin. -Antiproteinase primarily inhibits neutrophil elastase (neutrophils are a type of leukocyte, or white blood cell; elastase is a protease that acts on elastin, a component of some connective tissues). An insufficiency of -antiproteinase, which can be caused by exposure to cigarette smoke, has been associated with lung damage, including emphysema.
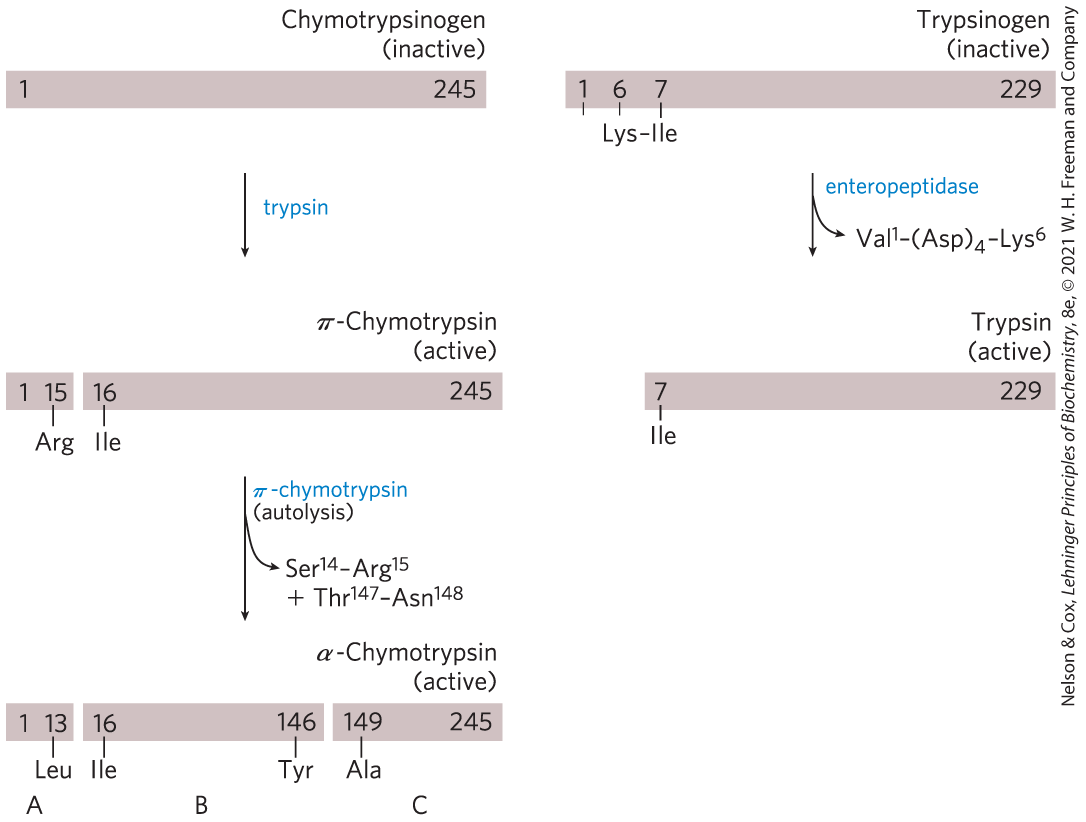
FIGURE 6-42 Activation of zymogens by proteolytic cleavage. Shown here is the formation of active chymotrypsin (formally, α-chymotrypsin) and trypsin from their zymogens, chymotrypsinogen and trypsinogen. The π-chymotrypsin intermediate generated by trypsin cleavage has a somewhat altered specificity relative to the mature α-chymotrypsin. The bars represent the amino acid sequences of the polypeptide chains, with numbers indicating the positions of the residues (the amino-terminal residue is number 1). Residues at the termini of the polypeptide fragments generated by cleavage are indicated below the bars. Note that in the final active forms, some numbered residues are missing. Recall that the three polypeptide chains (A, B, and C) of chymotrypsin are linked by disulfide bonds (see Fig. 6-24).
Proteases are not the only proteins activated by proteolysis. In other cases, however, the precursors are called not zymogens but, more generally, proproteins or proenzymes, as appropriate. For example, the connective tissue protein collagen is initially synthesized as the soluble precursor procollagen.
A Cascade of Proteolytically Activated Zymogens Leads to Blood Coagulation
The formation of a blood clot provides a well-studied example of a regulatory cascade, a mechanism that allows a very sensitive response to — and amplification of — a molecular signal. The blood coagulation pathways also bring together several other types of regulation, including proteolytic activation and regulatory proteins. We have not yet encountered the latter type of regulation. It employs proteins whose only function is to regulate the activity of other proteins by interacting with them noncovalently.
In a regulatory cascade, a signal leads to the activation of protein X. Protein X catalyzes the activation of protein Y. Protein Y catalyzes the activation of protein Z, and so on. Since proteins X, Y, and Z are catalysts and activate multiple copies of the next protein in the chain, the signal is amplified in each step. In some cases, the activation steps involve proteolytic cleavage and are thus effectively irreversible. In other cases, activation entails readily reversible protein modification steps such as phosphorylation. Regulatory cascades govern a wide range of biological processes, including, besides blood coagulation, some aspects of cell fate determination during development, the detection of light by retinal rods, and programmed cell death (apoptosis). A regulatory cascade is also one of the strategies governing the overall activity of glycogen phosphorylase (see Fig. 15-13).
A blood clot is an aggregate of specialized cell fragments that lack nuclei, called platelets. They are cross-linked and stabilized by proteinaceous fibers consisting mainly of the protein fibrin (Fig. 6-43a). Fibrin is derived from a soluble zymogen called fibrinogen. After albumins and globulins, fibrinogen is usually the third most abundant type of protein in blood plasma. Blood clotting begins with the activation of circulating platelets at the site of a wound. Tissue damage causes collagen molecules present beneath the epithelial cell layer that lines each blood vessel to become exposed to the blood. Platelet activation is primarily triggered by interaction with this collagen. Activation leads to the presentation of anionic phospholipids on the surface of each platelet and the release of signaling molecules such as thromboxanes (p. 355) that help stimulate the activation of additional platelets. The activated platelets aggregate at the site of a wound, forming a loose clot. Stabilization of the clot requires fibrin, generated by fibrinogen cleavage as the endpoint of regulatory cascades.

FIGURE 6-43 The function of fibrin in blood clots. (a) A blood clot consists of aggregated platelets (small, light-colored cells) tied together with strands of cross-linked fibrin. Erythrocytes (red in this colorized scanning electron micrograph) are also trapped in the matrix. (b) The soluble plasma protein fibrinogen consists of two complexes of α, β, and γ subunits . The removal of amino-terminal peptides from the α and β subunits (not shown) leads to the formation of higher-order complexes and eventual covalent cross-linking that results in the formation of fibrin fibers. The “knobs” are globular domains at the ends of the proteolyzed subunits.
Fibrinogen is a dimer of heterotrimers with three different but evolutionarily related types of subunits (Fig. 6-43b). Fibrinogen is converted to fibrin and thereby activated for blood clotting, by the proteolytic removal of 16 amino acid residues from the amino-terminal end (the A peptide) of each α subunit and 14 amino acid residues from the amino-terminal end (the B peptide) of each β subunit. Peptide removal is catalyzed by the serine protease thrombin. The newly exposed amino termini of the α and β subunits fit neatly into binding sites in the carboxyl-terminal globular portions of the γ and β subunits, respectively, of another fibrin protein. Fibrin thus polymerizes into a gel-like matrix to generate a soft clot. Covalent cross-links between the associated fibrins are generated by the condensation of particular Lys residues in one fibrin heterotrimer with Gln residues in another, catalyzed by a transglutaminase, factor XIIIa. The covalent cross-links convert the soft clot into a hard clot.
Fibrinogen activation to produce fibrin is the end point of not one but two parallel but intertwined regulatory cascades (Fig. 6-44). One of these is referred to as the contact activation pathway (“contact” refers to interaction of key components of this system with anionic phospholipids presented on the surface of platelets at the site of a wound). As all components of this pathway are found in the blood plasma, it is also called the intrinsic pathway. The second path is the tissue factor or extrinsic pathway. A major component of this pathway, the protein tissue factor (TF), is not present in the blood. Most of the protein factors in both pathways are designated by roman numerals. Many of these factors are either chymotrypsin-like serine proteases or regulatory proteins, most with zymogen precursors that are synthesized in the liver and exported to the blood. For those with both inactive and active forms, the roman numeral designates the inactive zymogen (VII, X, for example). An “a” is added to designate the cleaved and active form (VIIa, Xa). The regulatory proteins bind to particular serine proteases and help to activate them.
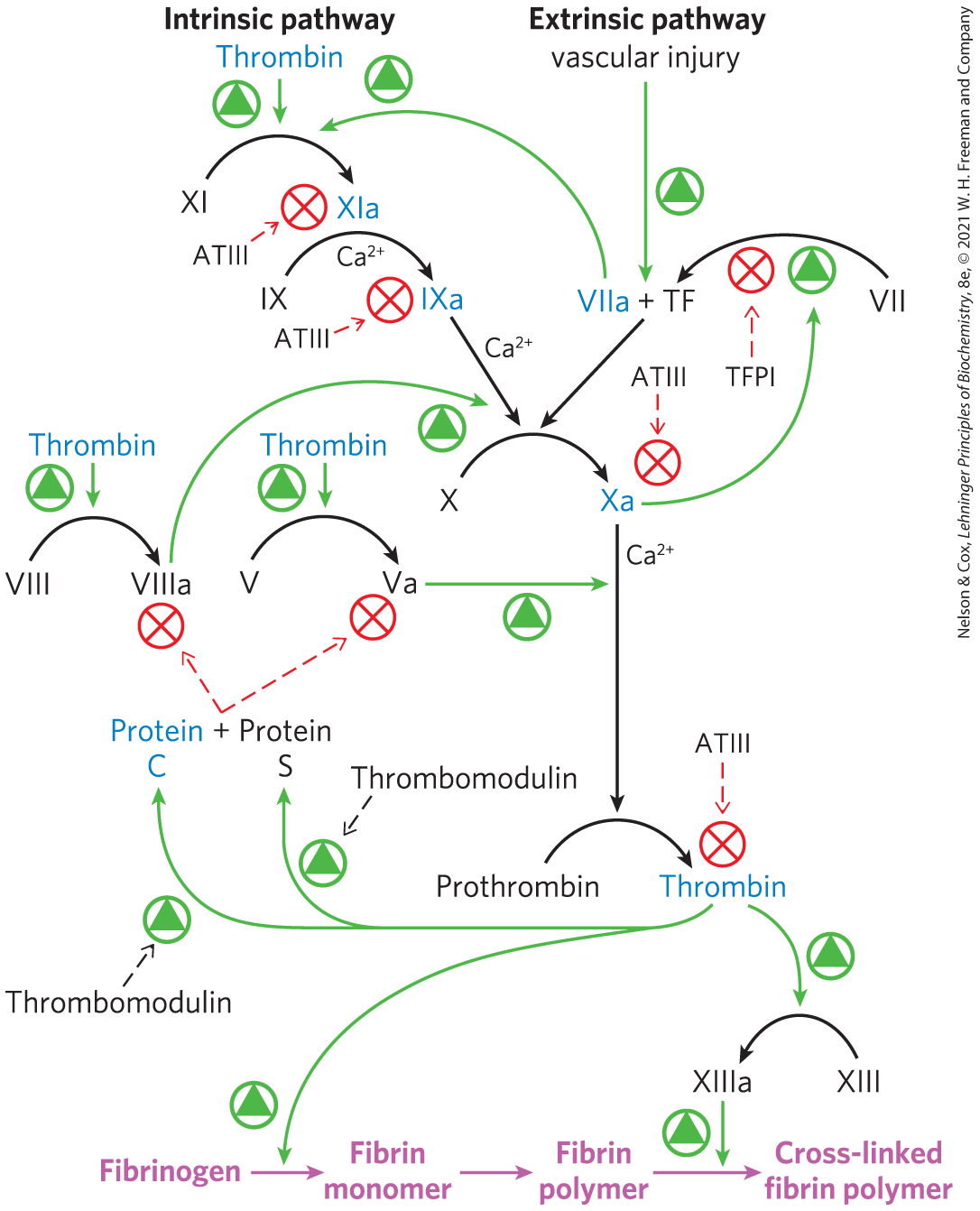
FIGURE 6-44 The coagulation cascades. The interlinked intrinsic and extrinsic pathways leading to the cleavage of fibrinogen to form active fibrin are shown. Active serine proteases in the pathways are shown in blue. Green arrows denote activating steps, and red arrows indicate inhibitory processes.
The extrinsic pathway comes into play first. Tissue damage exposes the blood plasma to TF embedded largely in the membranes of fibroblasts and smooth muscle cells beneath the endothelial layer. An initiating complex is formed between TF and factor VII, present in the blood plasma. Factor VII is a zymogen of a serine protease, and TF is a regulatory protein required for its function. Factor VII is converted to its active form, factor VIIa, by proteolytic cleavage catalyzed by factor Xa (another serine protease). The TF-VIIa complex then cleaves factor X, creating the active form, factor Xa.
If TF-VIIa is needed to cleave X, and Xa is needed to cleave TF-VII, how does the process ever get started? A very small amount of factor VIIa is present in the blood at all times, enough to form a small amount of the active TF-VIIa complex immediately after tissue is damaged. This allows formation of factor Xa and establishes the initiating feedback loop. Once levels of factor Xa begin to build up, Xa (in a complex with another regulatory protein, factor Va) cleaves prothrombin to form active thrombin, and thrombin cleaves fibrinogen.
The extrinsic pathway thus provides a burst of thrombin. However, the TF-VIIa complex is quickly shut down by the protein tissue factor pathway inhibitor (TFPI). Clot formation is sustained by the activation of components of the second cascade, the intrinsic pathway. Factor IX is converted to the active serine protease factor IXa by the TF-VIIa protease during initiation of the clotting sequence. Factor IXa, in a complex with the regulatory protein VIIIa, is relatively stable and provides an alternative enzyme for the proteolytic conversion of factor X to Xa. Activated IXa can also be produced by the serine protease factor XIa. Most of the XIa is generated by cleavage of factor XI zymogen by thrombin in a feedback loop.
Left uncontrolled, blood coagulation could eventually lead to blockage of blood vessels, causing heart attacks or strokes. More regulation is thus needed. As a hard clot forms, regulatory pathways are already acting to limit the time during which the coagulation cascade is active. In addition to cleaving fibrinogen, thrombin forms a complex with a protein embedded in the vascular surface of endothelial cells, thrombomodulin. The thrombin-thrombomodulin complex cleaves the serine protease zymogen protein C. Activated protein C, in a complex with the regulatory protein S, cleaves and inactivates factors Va and VIIIa, leading to suppression of the overall cascade. Another protein, antithrombin III (ATIII), is a serine protease inhibitor. ATIII makes a covalent 1:1 complex between an Arg residue on ATIII and the active-site Ser residue of serine proteases, particularly thrombin and factor Xa. These two regulatory systems, in concert with TFPI, help to establish a threshold or level of exposure to TF that is needed to activate the coagulation cascade. Individuals with genetic defects that eliminate or decrease levels of protein C or ATIII in the blood have a greatly elevated risk of thrombosis (inappropriate formation of blood clots).
The control of blood coagulation has important roles in medicine, particularly in the prevention of blood clotting during surgery and in patients at risk for heart attacks or strokes. Several different medical approaches to anticoagulation are available. The first takes advantage of another feature of several proteins in the coagulation cascade that we have not yet considered. The factors VII, IX, X, and prothrombin, along with proteins C and S, have calcium-binding sites that are critical to their function. In each case, the calcium-binding sites are formed by modification of multiple Glu residues near the amino terminus of each protein to γ-carboxyglutamate residues (abbreviated Gla; p. 76). The Glu-to-Gla modifications are carried out by enzymes that depend on the function of the fat-soluble vitamin K (p. 359). Bound calcium functions to adhere these proteins to the anionic phospholipids that appear on the surface of activated platelets, effectively localizing the coagulation factors to the area where the clot is to form. Vitamin K antagonists such as warfarin (Coumadin) have proven highly effective as anticoagulants. A second approach to anticoagulation is the administration of heparins. Heparins are highly sulfated polysaccharides (see Fig. 7-19). They act as anticoagulants by increasing the affinity of ATIII for factor Xa and thrombin, thus facilitating the inactivation of key cascade elements (see Figs 7-23 and 7-24). Finally, aspirin (acetylsalicylate; Fig. 21-15b) is effective as an anticoagulant. Aspirin inhibits the enzyme cyclooxygenase, required for the production of thromboxanes. As aspirin reduces thromboxane release from platelets, the capacity of the platelets to aggregate declines.
Humans born with a deficiency in any one of most components of the clotting cascade have an increased tendency to bleed that varies from mild to essentially uncontrollable, a fatal condition. Genetic defects in the genes encoding the proteins required for blood clotting result in diseases referred to as hemophilias. Hemophilia B is a sex-linked trait resulting from a deficiency in factor IX. This type of hemophilia affects about one in 25,000 males worldwide. The most famous example of the inheritance of hemophilia B occurred among European royalty. Queen Victoria (1819–1901) was evidently a carrier. Prince Leopold, her eighth child, suffered from hemophilia B and died at the age of 31 after a minor fall. At least two of her daughters were carriers and passed the defective gene to other royal families of Europe (Fig. 6-45).
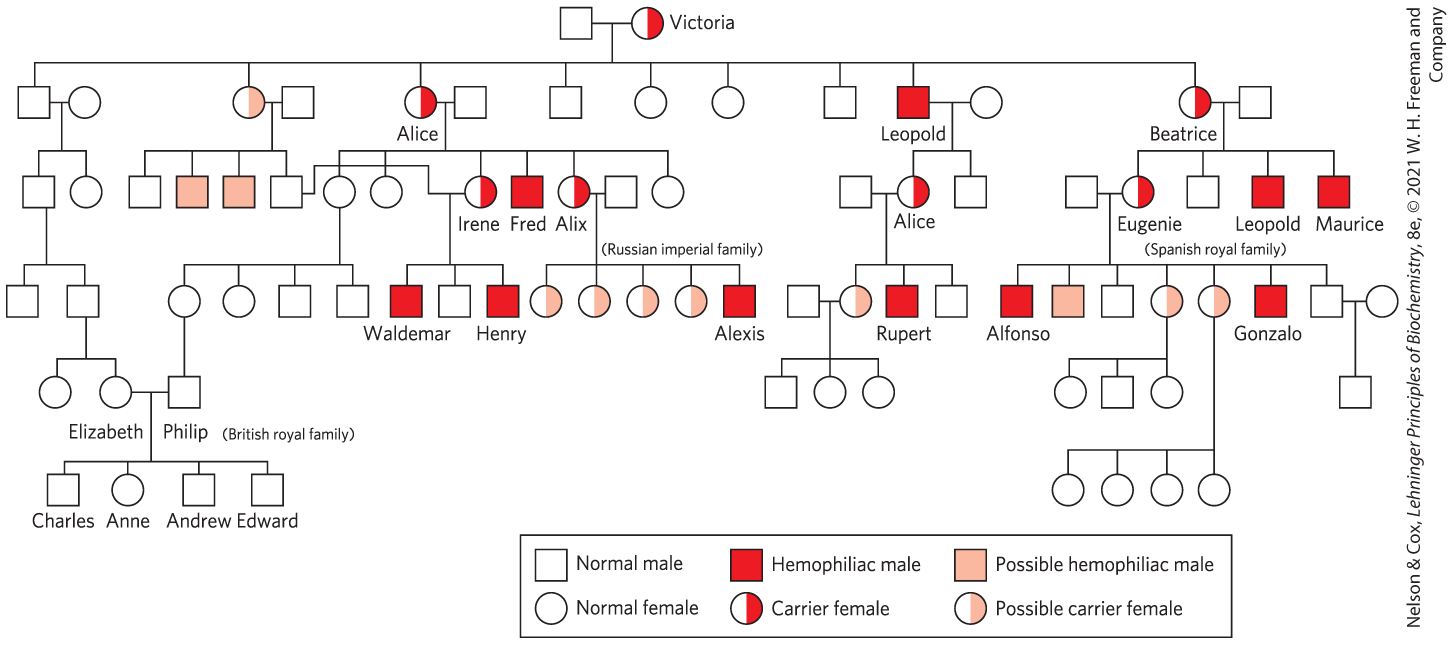
FIGURE 6-45 The royal families of Europe, and inheritance of hemophilia B. Males are indicated by squares and females by circles. Males who suffered from hemophilia are represented by red squares, presumed female carriers by half-red circles.
Some Regulatory Enzymes Use Several Regulatory Mechanisms
Glycogen phosphorylase and the blood clotting cascade are not the only examples of complex regulatory patterns. Additional examples of regulatory complexity are found at key metabolic crossroads. Bacterial glutamine synthetase, which catalyzes a reaction that introduces reduced nitrogen into cellular metabolism (Chapter 22), is among the most complex regulatory enzymes known. It is regulated allosterically, with at least eight different modulators; by reversible covalent modification; and by the association of other regulatory proteins, a mechanism examined in detail when we consider the regulation of specific metabolic pathways in Part II of this book.
What is the advantage of such complexity in the regulation of enzymatic activity? We began this chapter by stressing the central importance of catalysis to the existence of life. The control of catalysis is also critical to life. If all possible reactions in a cell were catalyzed simultaneously, macromolecules and metabolites would quickly be broken down to much simpler chemical forms. Instead, cells catalyze only the reactions they need at a given moment. When chemical resources are plentiful, cells synthesize and store glucose and other metabolites. When chemical resources are scarce, cells use these stores to fuel cellular metabolism. Chemical energy is used economically, parceled out to various metabolic pathways as cellular needs dictate. The availability of powerful catalysts, each specific for a given reaction, makes the regulation of these reactions possible. This, in turn, gives rise to the complex, highly regulated symphony we call life.
SUMMARY 6.5 Regulatory Enzymes
- The activities of metabolic pathways in cells are regulated by control of the activities of certain enzymes.
- The activity of an allosteric enzyme is adjusted by reversible binding of a specific modulator to a regulatory site. A modulator may be the substrate itself or some other metabolite, and the effect of the modulator may be inhibitory or stimulatory.
- The kinetic behavior of allosteric enzymes reflects cooperative interactions among enzyme subunits.
- Other regulatory enzymes are modulated by covalent modification of a specific functional group necessary for activity.
- The phosphorylation of specific amino acid residues is a particularly common way to regulate enzyme activity.
- Many proteolytic enzymes are synthesized as inactive precursors called zymogens, which are activated by cleavage to release small peptide fragments.
- Blood clotting is mediated by two interlinked regulatory cascades of proteolytically activated zymogens.
- Enzymes at important metabolic intersections may be regulated by complex combinations of effectors, allowing coordination of the activities of interconnected pathways.
 Adjustments in the rate of reactions catalyzed by regulatory enzymes, and therefore in the rate of entire metabolic sequences, allow the cell to meet changing needs for energy and for biomolecules required in growth and repair.
Adjustments in the rate of reactions catalyzed by regulatory enzymes, and therefore in the rate of entire metabolic sequences, allow the cell to meet changing needs for energy and for biomolecules required in growth and repair. –Ser and several Arg side chains (phosphorylase a). In this conformation, the enzyme is much more active.
–Ser and several Arg side chains (phosphorylase a). In this conformation, the enzyme is much more active. The control of blood coagulation has important roles in medicine, particularly in the prevention of blood clotting during surgery and in patients at risk for heart attacks or strokes. Several different medical approaches to anticoagulation are available. The first takes advantage of another feature of several proteins in the coagulation cascade that we have not yet considered. The factors VII, IX, X, and prothrombin, along with proteins C and S, have calcium-binding sites that are critical to their function. In each case, the calcium-binding sites are formed by modification of multiple Glu residues near the amino terminus of each protein to γ-carboxyglutamate residues (abbreviated Gla;
The control of blood coagulation has important roles in medicine, particularly in the prevention of blood clotting during surgery and in patients at risk for heart attacks or strokes. Several different medical approaches to anticoagulation are available. The first takes advantage of another feature of several proteins in the coagulation cascade that we have not yet considered. The factors VII, IX, X, and prothrombin, along with proteins C and S, have calcium-binding sites that are critical to their function. In each case, the calcium-binding sites are formed by modification of multiple Glu residues near the amino terminus of each protein to γ-carboxyglutamate residues (abbreviated Gla; 
 The activities of metabolic pathways in cells are regulated by control of the activities of certain enzymes.
The activities of metabolic pathways in cells are regulated by control of the activities of certain enzymes.