27.3 Protein Targeting and Degradation
The eukaryotic cell is made up of many structures, compartments, and organelles, each with specific functions that require distinct sets of proteins and enzymes. These proteins (with the exception of those produced in mitochondria and plastids) are synthesized on ribosomes in the cytosol, so how are they directed to their final cellular destinations?
We are now beginning to understand this complex and fascinating process. Proteins destined for secretion, integration in the plasma membrane, or inclusion in lysosomes generally share the first few steps of a pathway that begins in the endoplasmic reticulum. Proteins destined for mitochondria, chloroplasts, or the nucleus use three separate mechanisms. And proteins destined for the cytosol simply remain where they are synthesized. The thermodynamic cost of protein synthesis is magnified by the processes used by cells to transport proteins to their correct cellular locations.
The most important element in many of these targeting pathways is a short sequence of amino acids called a signal sequence, whose function was first postulated by Günter Blobel and colleagues in 1970. The signal sequence directs a protein to its appropriate location in the cell and, for many proteins, is removed during transport or after the protein has reached its final destination. In proteins slated for transport into mitochondria, chloroplasts, or the ER, the signal sequence is at the amino terminus of a newly synthesized polypeptide. In many cases, the targeting capacity of particular signal sequences has been confirmed by fusing the signal sequence from one protein to a second protein and showing that the signal directs the second protein to the location where the first protein is normally found. The selective degradation of proteins no longer needed by the cell also relies largely on a set of molecular signals embedded in each protein’s structure.
In this concluding section we examine protein targeting and degradation, emphasizing the underlying signals and molecular regulation that are so crucial to cellular metabolism. Except where noted, the focus is now on eukaryotic cells.
Posttranslational Modification of Many Eukaryotic Proteins Begins in the Endoplasmic Reticulum
Perhaps the best-characterized targeting system begins in the ER. Most lysosomal, membrane, or secreted proteins have an amino-terminal signal sequence (Fig. 27-37) that marks them for translocation into the lumen of the ER; hundreds of such signal sequences have been determined. The carboxyl terminus of the signal sequence is defined by a cleavage site, where protease action removes the sequence after the protein is imported into the ER. Signal sequences vary in length from 13 to 36 amino acid residues, but all have the following features: (1) about 10 to 15 hydrophobic amino acid residues; (2) one or more positively charged residues, usually near the amino terminus, preceding the hydrophobic sequence; and (3) a short sequence at the carboxyl terminus (near the cleavage site) that is relatively polar, typically having amino acid residues with short side chains (especially Ala) at the positions closest to the cleavage site.
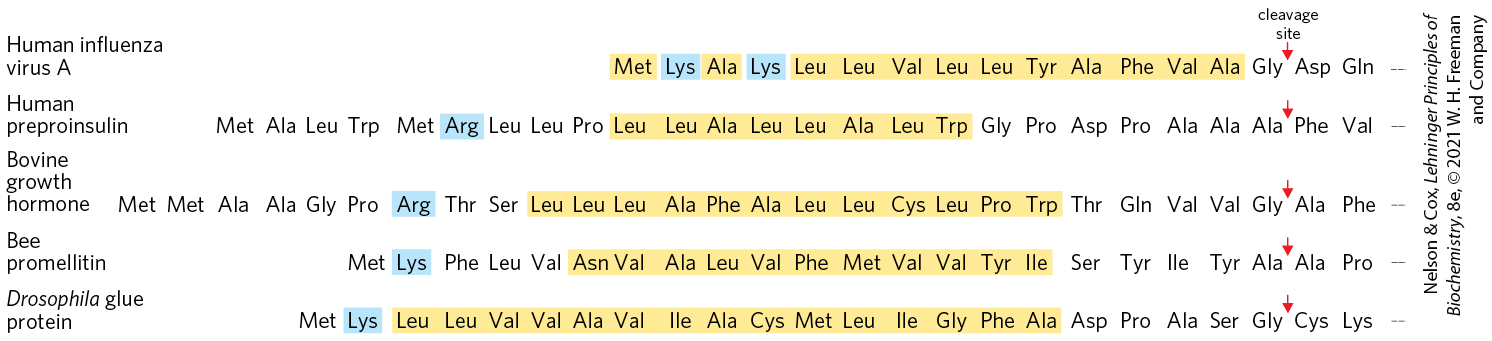
FIGURE 27-37 Amino-terminal signal sequences of some eukaryotic proteins that direct their translocation into the ER. The hydrophobic core (yellow) is preceded by one or more basic residues (blue). Polar and short-side-chain residues immediately precede (to the left, as shown here) the cleavage sites (indicated by red arrows).
As originally demonstrated by the cell biologist George Palade, proteins with these signal sequences are synthesized on ribosomes attached to the ER. The signal sequence itself helps to direct the ribosome to the ER, as illustrated in Figure 27-38. The targeting pathway begins in step , with initiation of protein synthesis on free ribosomes. The signal sequence appears early in the synthetic process (step ), because it is at the amino terminus, which, as we have seen, is synthesized first. As it emerges from the ribosome (step ), the signal sequence — and the ribosome itself — is bound by the large signal recognition particle (SRP). The SRP is a rod-shaped complex containing a 300 nucleotide RNA (7SL-RNA) and six different proteins (combined ). The SRP then binds GTP and halts elongation of the polypeptide when it is about 70 amino acids long and the signal sequence has completely emerged from the ribosome. In step , the GTP-bound SRP directs the ribosome (still bound to the mRNA) and the incomplete polypeptide to GTP-bound SRP receptors in the cytosolic face of the ER; the nascent polypeptide is delivered to a peptide translocation complex in the ER, which interacts directly with the ribosome. In step , the SRP dissociates from the ribosome, accompanied by hydrolysis of GTP in both the SRP and the SRP receptor. The SRP receptor is a heterodimer of and subunits, both of which bind and hydrolyze multiple GTP molecules during this process.
Elongation of the polypeptide now resumes (step ), with the ATP-driven translocation complex feeding the growing polypeptide into the ER lumen until the complete protein has been synthesized. In step , the signal sequence is removed by a signal peptidase within the ER lumen. The ribosome dissociates (step ) and is recycled (step ).
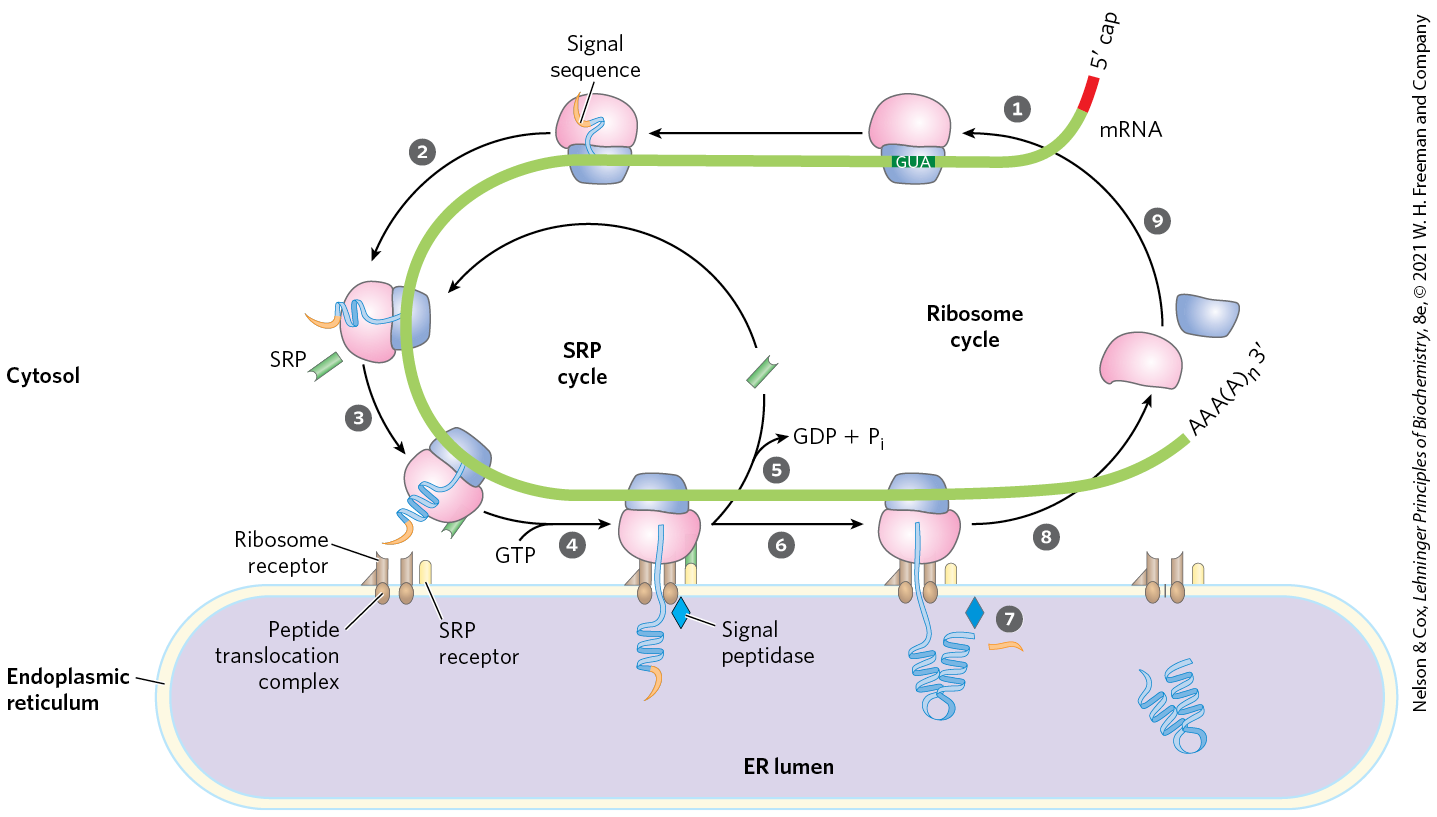
FIGURE 27-38 Directing eukaryotic proteins with the appropriate signals to the endoplasmic reticulum. This process involves the SRP cycle and the translocation and cleavage of the nascent polypeptide. One protein subunit of SRP binds directly to the signal sequence, obstructing elongation by sterically blocking the entry of aminoacyl-tRNAs and inhibiting peptidyl transferase. Another protein subunit binds and hydrolyzes GTP.
Glycosylation Plays a Key Role in Protein Targeting
In the ER lumen, newly synthesized proteins are further modified in several ways. Following the removal of signal sequences, polypeptides are folded, disulfide bonds are formed, and many proteins are glycosylated to form glycoproteins. In many glycoproteins, the linkage to their oligosaccharides is through Asn residues. These N-linked oligosaccharides are diverse (Chapter 7), but the pathways by which they form have a common first step. A 14 residue core oligosaccharide is built up stepwise, first on the cytosolic face of the membrane and then on the lumenal face. Once completed, it is transferred from a dolichol phosphate donor molecule to certain Asn residues in the protein (Fig. 27-39). The transferase is on the lumenal face of the ER and thus cannot catalyze glycosylation of cytosolic proteins. After transfer, the core oligosaccharide is trimmed and elaborated in different ways on different proteins, but all N-linked oligosaccharides retain a pentasaccharide core derived from the original 14 residue oligosaccharide.
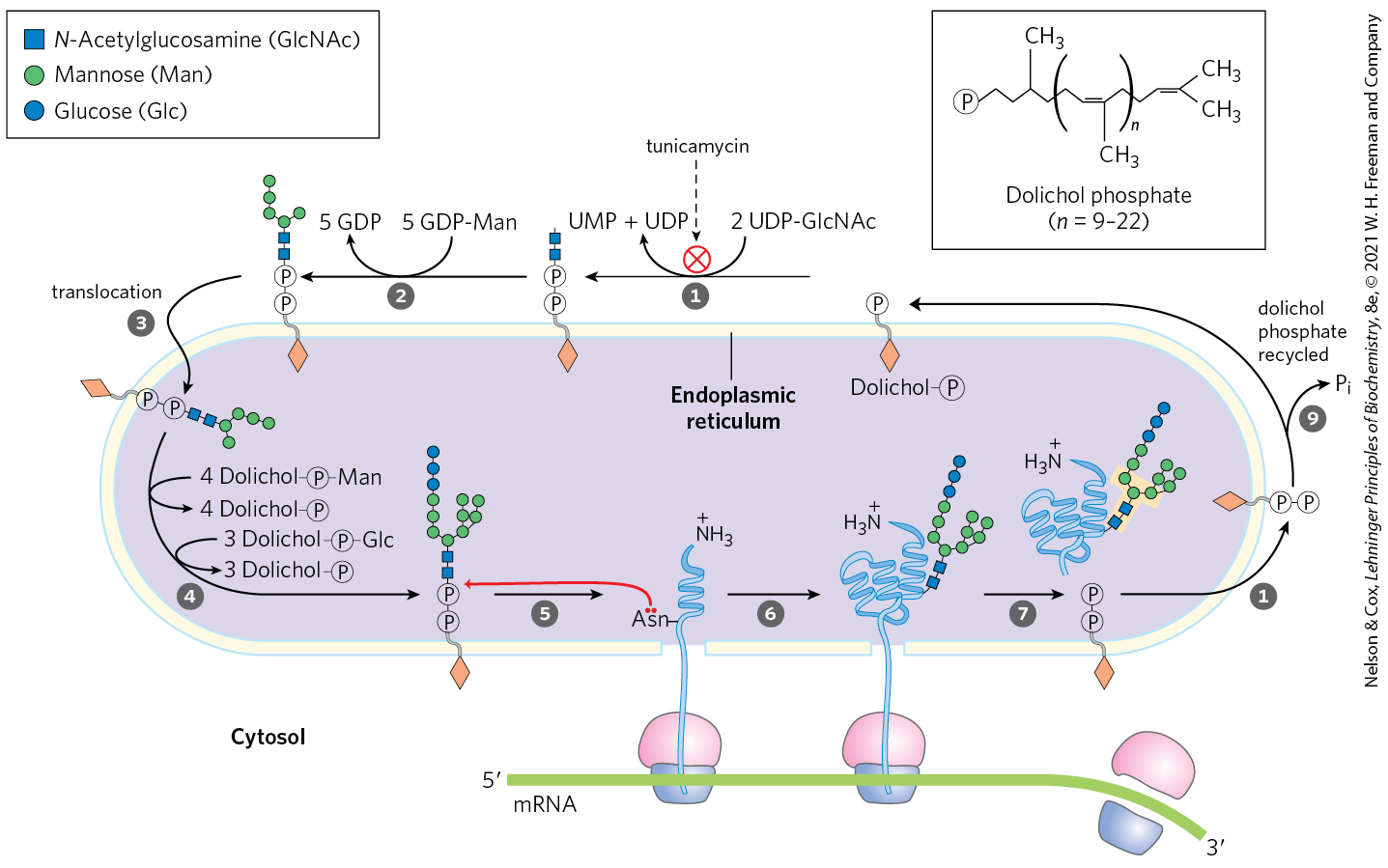
FIGURE 27-39 Synthesis of the core oligosaccharide of glycoproteins. The core oligosaccharide is built up by the successive addition of monosaccharide units. , The first steps occur on the cytosolic face of the ER. Translocation moves the incomplete oligosaccharide across the membrane (mechanism not shown), and completion of the core oligosaccharide occurs within the lumen of the ER. The precursors that contribute additional mannose and glucose residues to the growing oligosaccharide in the lumen are dolichol phosphate derivatives. In the first step in construction of the N-linked oligosaccharide moiety of a glycoprotein, the core oligosaccharide is transferred from dolichol phosphate to an Asn residue of the protein, and protein synthesis continues. The core oligosaccharide is then further modified in the ER and the Golgi complex in pathways that differ for different proteins. The five sugar residues shown surrounded by a beige screen, after step , are retained in the final structure of all N-linked oligosaccharides. The released dolichol pyrophosphate is again translocated so that the pyrophosphate is on the cytosolic face of the ER, then a phosphate is hydrolytically removed to regenerate dolichol phosphate.
Several antibiotics act by interfering with one or more steps in this process and have aided in elucidating the steps of protein glycosylation. The best characterized is tunicamycin, which mimics the structure of UDP-N-acetylglucosamine and blocks the first step of the process (Fig. 27-39, step ). A few proteins are O-glycosylated in the ER, but most O-glycosylation occurs in the Golgi complex or in the cytosol (for proteins that do not enter the ER).
Suitably modified proteins can now be moved to a variety of intracellular destinations. Proteins travel from the ER to the Golgi complex in transport vesicles (Fig. 27-40). In the Golgi complex, oligosaccharides are O-linked to some proteins, and N-linked oligosaccharides are further modified. By mechanisms not yet fully understood, the Golgi complex also sorts proteins and sends them to their final destinations. The processes that segregate proteins targeted for secretion from those targeted for the plasma membrane or lysosomes must distinguish among these proteins on the basis of structural features other than signal sequences, which were removed in the ER lumen.
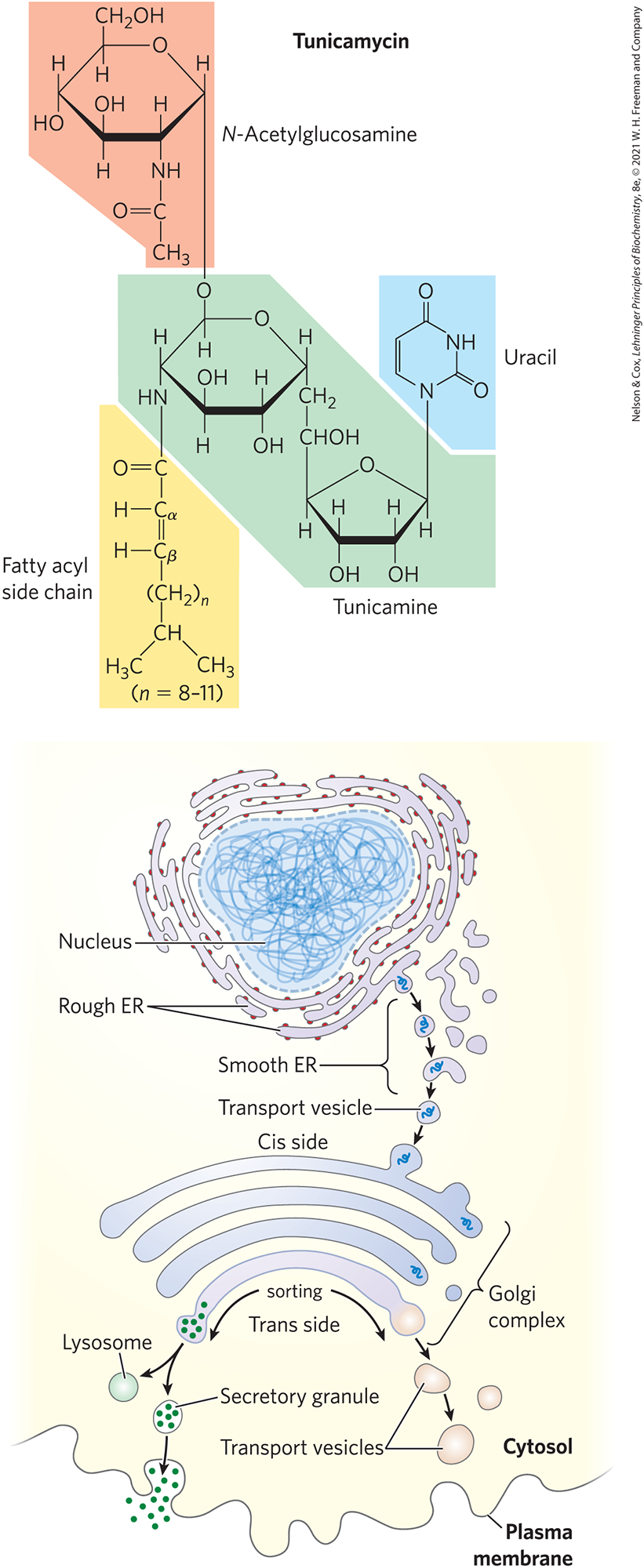
FIGURE 27-40 Pathway taken by proteins destined for lysosomes, the plasma membrane, or secretion. Proteins are moved from the ER to the cis side of the Golgi complex in transport vesicles. Sorting occurs primarily in the trans side of the Golgi complex.
This sorting process is best understood in the case of hydrolases destined for transport to lysosomes. On arrival of a hydrolase (a glycoprotein) in the Golgi complex, an as yet undetermined feature (sometimes called a signal patch) of the three-dimensional structure of the hydrolase is recognized by a phosphotransferase, which phosphorylates terminal mannose residues in the oligosaccharides (Fig. 27-41). The presence of one or more mannose 6-phosphate residues in its N-linked oligosaccharide is the structural signal that targets a protein to lysosomes. A receptor protein in the membrane of the Golgi complex recognizes the mannose 6-phosphate signal and binds the hydrolase so marked. Vesicles containing these receptor-hydrolase complexes bud from the trans side of the Golgi complex and make their way to sorting vesicles. Here, the receptor-hydrolase complex dissociates in a process facilitated by the lower pH in the vesicle and by phosphatase-catalyzed removal of phosphate groups from the mannose 6-phosphate residues. The receptor is then recycled to the Golgi complex, and vesicles containing the hydrolases bud from the sorting vesicles and move to the lysosomes. In cells treated with tunicamycin (Fig. 27-39, step ), hydrolases that should be targeted to lysosomes are instead secreted, confirming that the N-linked oligosaccharide plays a key role in targeting these enzymes to lysosomes.
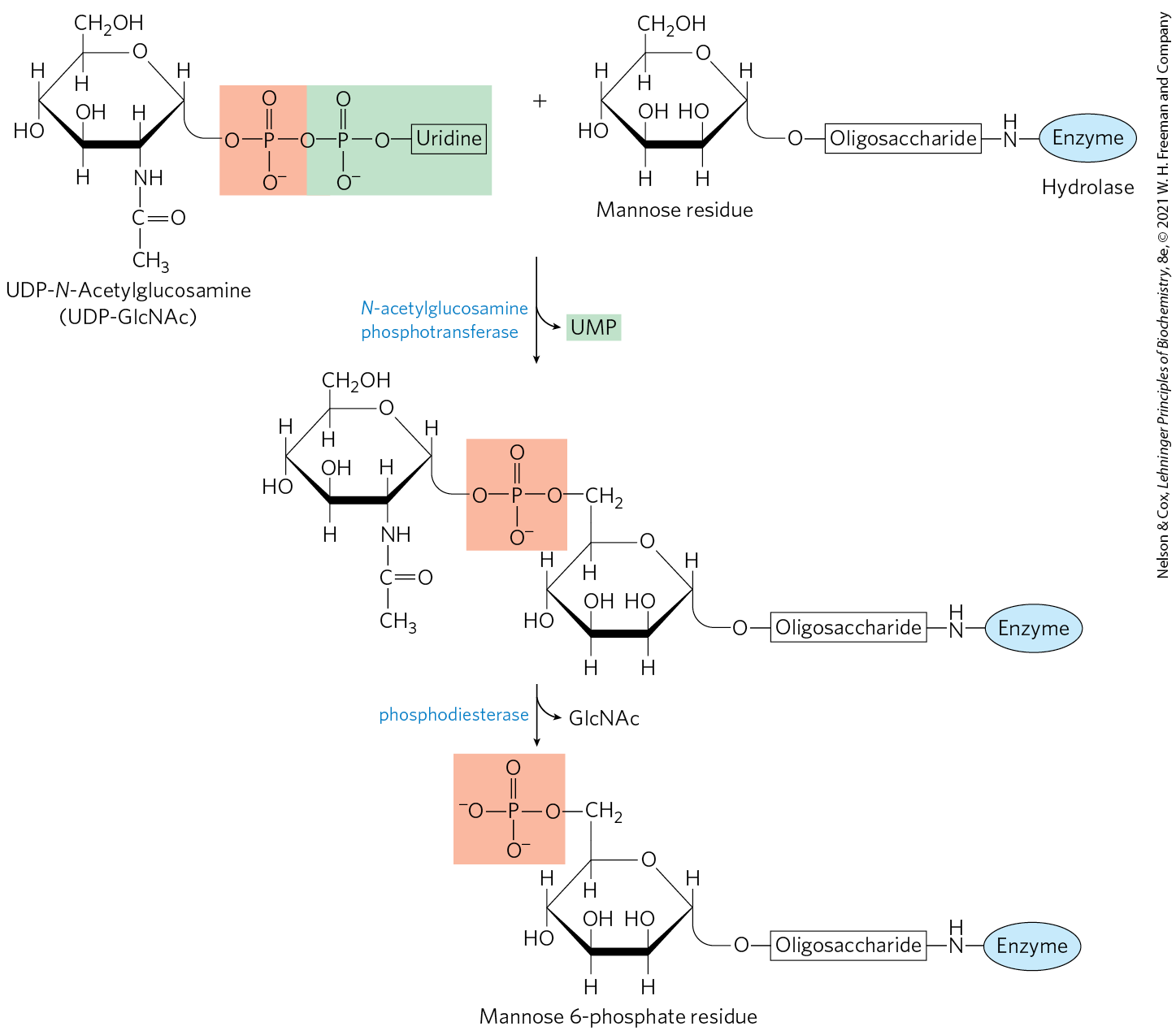
FIGURE 27-41 Phosphorylation of mannose residues on lysosome-targeted enzymes. N-Acetylglucosamine phosphotransferase recognizes some as yet unidentified structural feature of hydrolases destined for lysosomes.
The pathways that target proteins to mitochondria and chloroplasts also rely on amino-terminal signal sequences. Although mitochondria and chloroplasts contain DNA, most of their proteins are encoded by nuclear DNA and must be targeted to the appropriate organelle. Unlike other targeting pathways, however, the mitochondrial and chloroplast pathways begin only after a precursor protein has been completely synthesized and released from the ribosome. Precursor proteins destined for mitochondria or chloroplasts are bound by cytosolic chaperone proteins and delivered to receptors on the exterior surface of the target organelle. Specialized translocation mechanisms then transport the protein to its final destination in the organelle, after which the signal sequence is removed.
Signal Sequences for Nuclear Transport Are Not Cleaved
Molecular communication between the nucleus and the cytosol requires the movement of macromolecules through nuclear pores. RNA molecules synthesized in the nucleus are exported to the cytosol. Ribosomal proteins synthesized on cytosolic ribosomes are imported into the nucleus and assembled into 60S and 40S ribosomal subunits in the nucleolus; completed subunits are then exported back to the cytosol (Fig. 27-17). A variety of nuclear proteins (RNA and DNA polymerases, histones, topoisomerases, proteins that regulate gene expression, and so forth) are synthesized in the cytosol and imported into the nucleus. This traffic is modulated by a complex system of molecular signals and transport proteins that is gradually being elucidated.
In most multicellular eukaryotes, the nuclear envelope breaks down at each cell division, and once division is completed and the nuclear envelope reestablished, the dispersed nuclear proteins must be reimported. To allow this repeated nuclear importation, the signal sequence that targets a protein to the nucleus — the nuclear localization sequence (NLS) — is not removed after the protein arrives at its destination. An NLS, unlike other signal sequences, may be located almost anywhere along the primary sequence of the protein. NLSs can vary considerably in structure, but many consist of four to eight amino acid residues and include several consecutive basic (Arg or Lys) residues.
Nuclear importation is mediated by several proteins that cycle between the cytosol and the nucleus (Fig. 27-42), including importin α and β and a small GTPase known as Ran (Ras-related nuclear protein). A heterodimer of importin α and β functions as a soluble receptor for proteins targeted to the nucleus, with the α subunit binding NLS-bearing proteins in the cytosol. The complex of the NLS-bearing protein and the importin docks at a nuclear pore and is translocated through the pore by an energy-dependent mechanism. In the nucleus, the importin β is bound by Ran GTPase, releasing importin β from the imported protein. Importin β is bound by Ran and by CAS (cellular apoptosis susceptibility protein) and separated from the NLS-bearing protein. Importin α and β, in their complexes with Ran and CAS, are then exported from the nucleus. Ran hydrolyzes GTP in the cytosol to release the importins, which are then free to begin another importation cycle. Ran itself is also cycled back into the nucleus by the binding of Ran-GDP to nuclear transport factor 2 (NTF2). Inside the nucleus, the GDP bound to Ran is replaced with GTP through the action of Ran guanosine nucleotide–exchange factor (Ran-GEF).
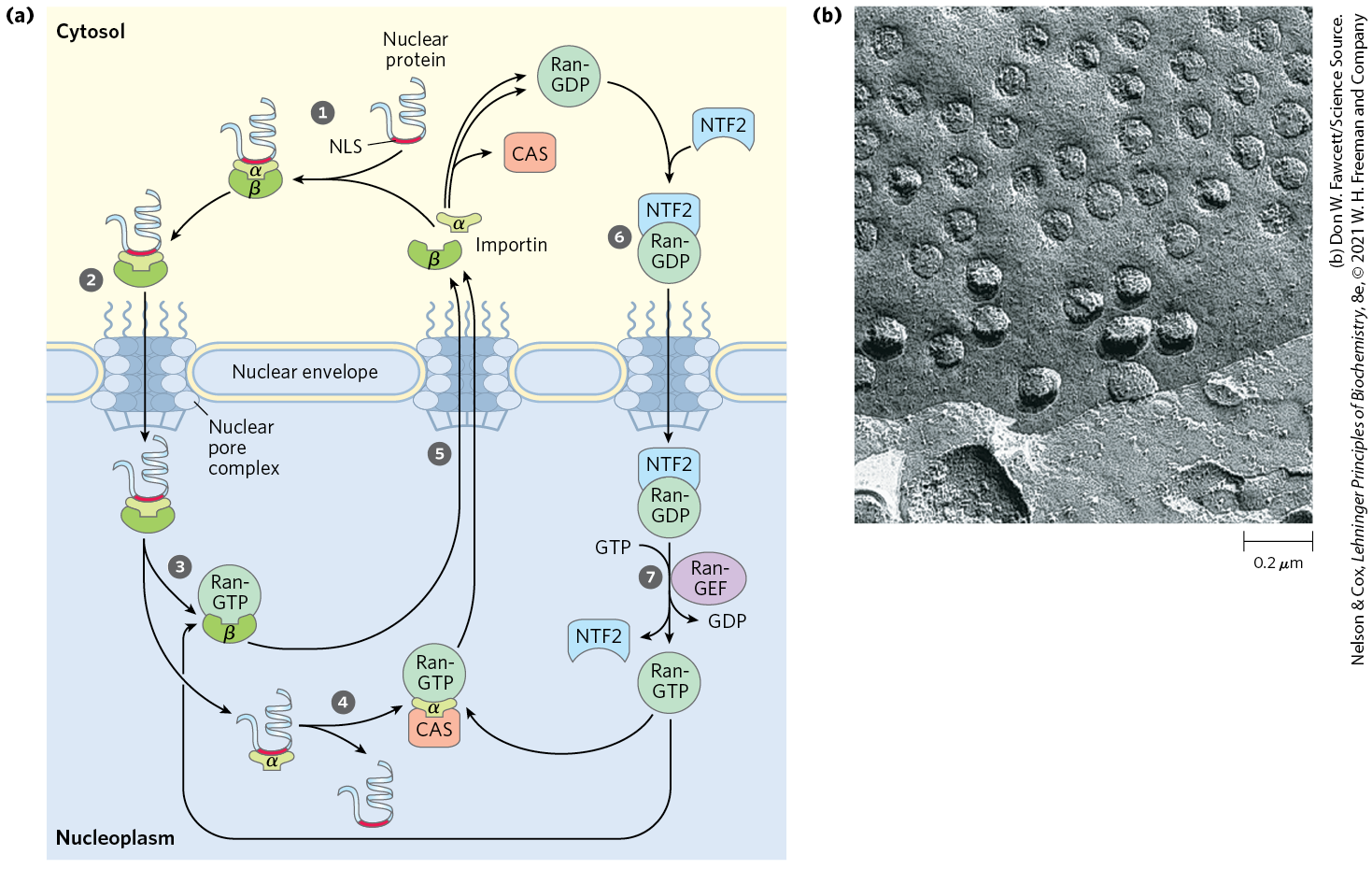
FIGURE 27-42 Targeting of nuclear proteins. (a) A protein with an appropriate nuclear localization signal (NLS) is bound by a complex of importins α and β. The resulting complex binds to a nuclear pore and translocates. Inside the nucleus, dissociation of importin β is promoted by the binding of Ran-GTP. Importin α binds to Ran-GTP and CAS (cellular apoptosis susceptibility protein), releasing the nuclear protein. Importins α and β and CAS are transported out of the nucleus and recycled. They are released in the cytosol when Ran hydrolyzes its bound GTP. Ran-GDP is bound by NTF2, and transported back into the nucleus. Ran-GEF promotes the exchange of GDP for GTP in the nucleus, and Ran-GTP is ready to process another NLS-bearing protein-importin complex. (b) Transmission electron micrograph of a freeze-fractured nucleus, showing numerous nuclear pores. The nuclear pore complex is one of the largest molecular aggregates in the cell . It is made up of multiple copies of more than 30 different proteins. [(a) Information from C. Strambio-De-Castillia et al., Nat. Rev. Mol. Cell Biol. 11:490, 2010, Fig. 1.]
During mitosis, when the nuclear envelope transiently breaks down, the Ran GTPase and the importins play additional roles. The Ran GTPase–importin β complex helps to position the spindle microtubules on the cell perimeter to facilitate chromosome segregation as the cell divides, and this complex also regulates microtubule interaction with other cellular structures.
Bacteria Also Use Signal Sequences for Protein Targeting
Bacteria can target proteins to their inner or outer membranes, to the periplasmic space between these membranes, or to the extracellular medium. They use signal sequences at the amino terminus of the proteins (Fig. 27-43), much like those on eukaryotic proteins targeted to the ER, mitochondria, and chloroplasts.
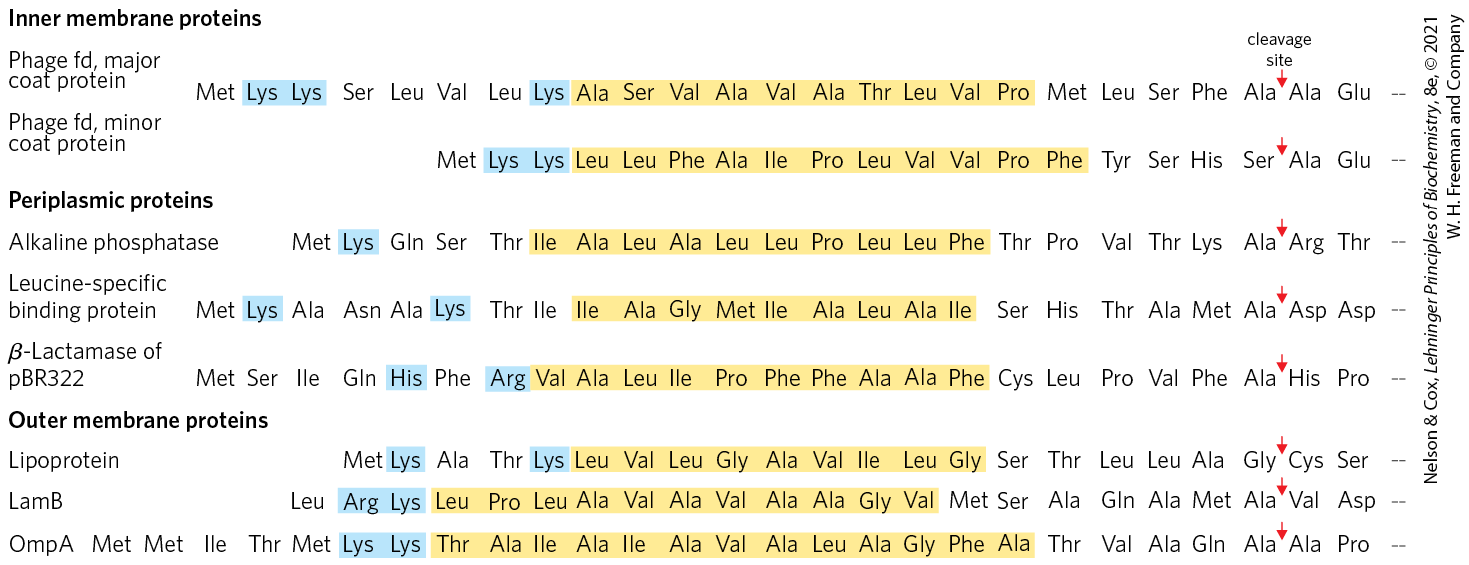
FIGURE 27-43 Signal sequences that target proteins to different locations in bacteria. Basic amino acids near the amino terminus are highlighted in blue, hydrophobic core amino acids in yellow. Cleavage sites marking the ends of the signal sequences are indicated by red arrows. Note that the inner bacterial cell membrane is where phage fd coat proteins and DNA are assembled into phage particles. OmpA is outer membrane protein A; LamB is a cell surface receptor protein for λ phage.
Most proteins exported from E. coli make use of the pathway shown in Figure 27-44. Following translation, a protein to be exported may fold only slowly, the amino-terminal signal sequence impeding the folding. The soluble chaperone protein SecB binds to the protein’s signal sequence or other features of its incompletely folded structure. The bound protein is then delivered to SecA, a protein associated with the inner surface of the plasma membrane. SecA acts as both a receptor and a translocating ATPase. Released from SecB and bound to SecA, the protein is delivered to a translocation complex in the membrane, made up of SecY, E, and G, and is translocated stepwise through the membrane at the SecYEG complex in lengths of about 20 amino acid residues. Each step requires the hydrolysis of ATP, catalyzed by SecA.
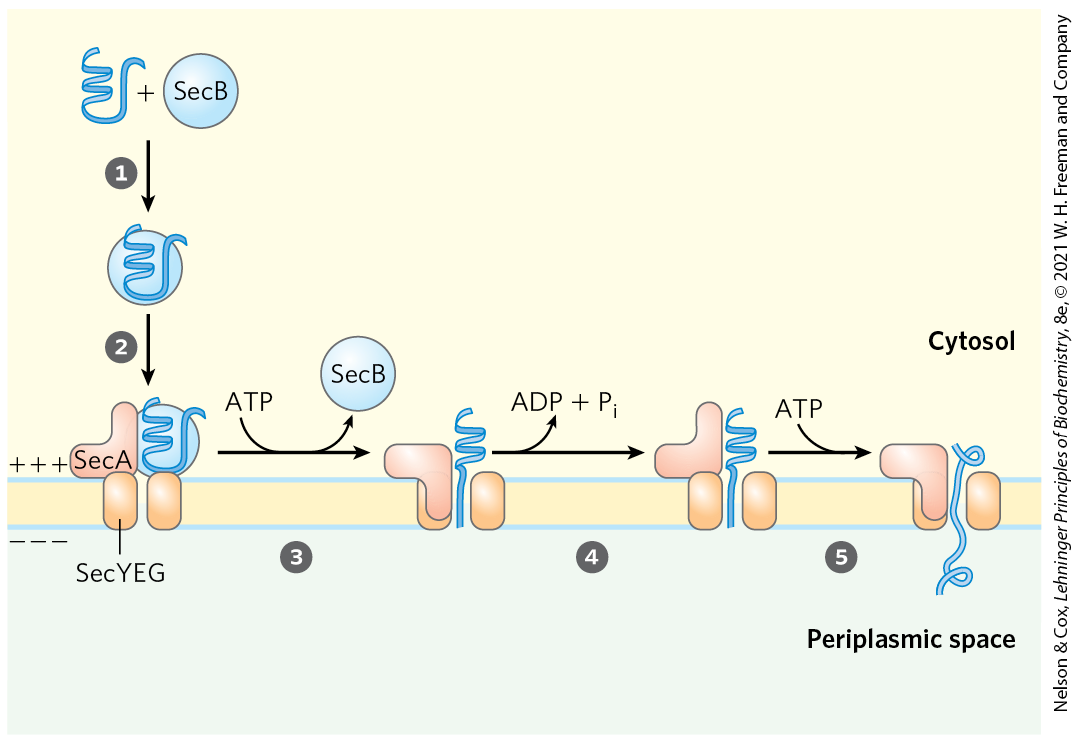
FIGURE 27-44 Model for protein export in bacteria. A newly translated polypeptide binds to the cytosolic chaperone protein SecB, which delivers it to SecA, a protein associated with the translocation complex (SecYEG) in the bacterial cell membrane. SecB is released, and SecA inserts itself into the membrane, forcing about 20 amino acid residues of the protein to be exported through the translocation complex. Hydrolysis of an ATP by SecA provides the energy for a conformational change that causes SecA to withdraw from the membrane, releasing the polypeptide. SecA binds another ATP, and the next stretch of 20 amino acid residues is pushed across the membrane through the translocation complex. Steps and are repeated until the entire protein has passed through and is released to the periplasm. The electrochemical potential across the membrane (denoted by + and −) also provides some of the driving force required for protein translocation.
Although most exported bacterial proteins use this pathway, some follow an alternative pathway that uses signal recognition and receptor proteins homologous to components of the eukaryotic SRP and the SRP receptor (see Fig. 27-38).
Cells Import Proteins by Receptor-Mediated Endocytosis
Some proteins are imported into eukaryotic cells from the surrounding medium; examples include low-density lipoprotein (LDL), the iron-carrying protein transferrin, peptide hormones, and circulating proteins destined for degradation. There are several importation pathways (Fig. 27-45). In one path, proteins bind to receptors in invaginations of the membrane called coated pits, which concentrate endocytic receptors in preference to other cell-surface proteins. The pits are coated on their cytosolic side with a lattice of the protein clathrin, which forms closed polyhedral structures (Fig. 27-46). The clathrin lattice grows as more receptors are occupied by target proteins. Eventually, a complete membrane-bounded endocytic vesicle is pinched off the plasma membrane with the aid of the large GTPase dynamin, and it enters the cytoplasm. The clathrin is quickly removed by uncoating enzymes, and the vesicle fuses with an endosome. ATPase activity in the endosomal membranes reduces the pH therein, facilitating dissociation of receptors from their target proteins. In a related pathway, caveolin causes invagination of patches of membrane containing lipid rafts associated with certain types of receptors (see Fig. 11-23). These endocytic vesicles then fuse with caveolin-containing internal structures called caveosomes, where the internalized molecules are sorted and redirected to other parts of the cell and the caveolins are prepared for recycling to the membrane surface. There are also clathrin- and caveolin-independent pathways; some make use of dynamin and others do not.
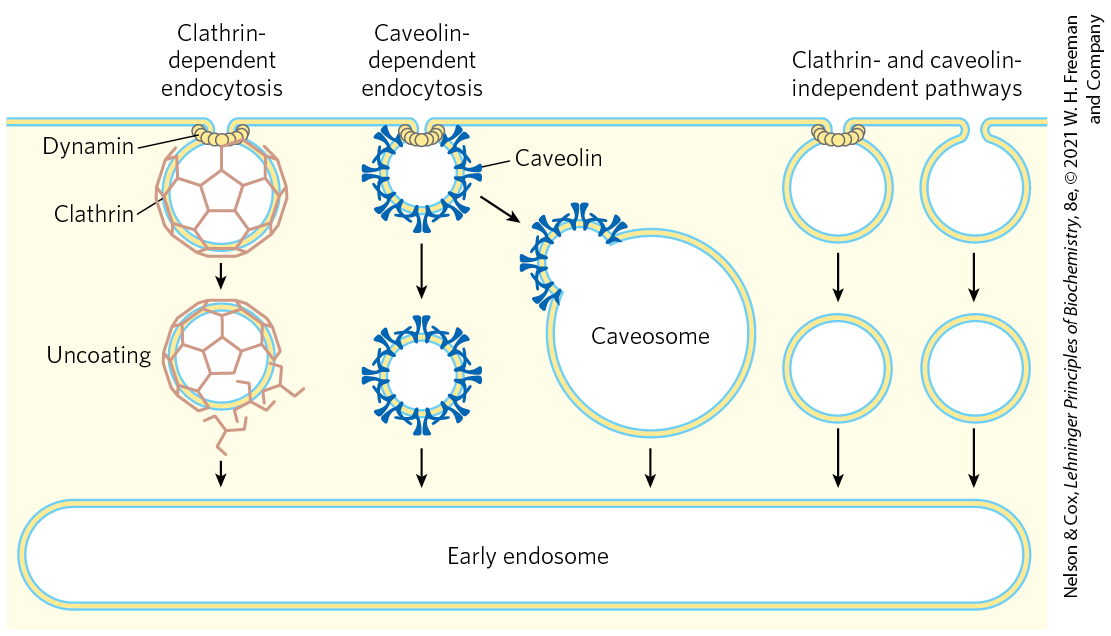
FIGURE 27-45 Summary of endocytosis pathways in eukaryotic cells. Pathways dependent on clathrin or caveolin make use of the GTPase dynamin to pinch vesicles from the plasma membrane. Some pathways do not use clathrin or caveolin; some of these make use of dynamin and some do not.
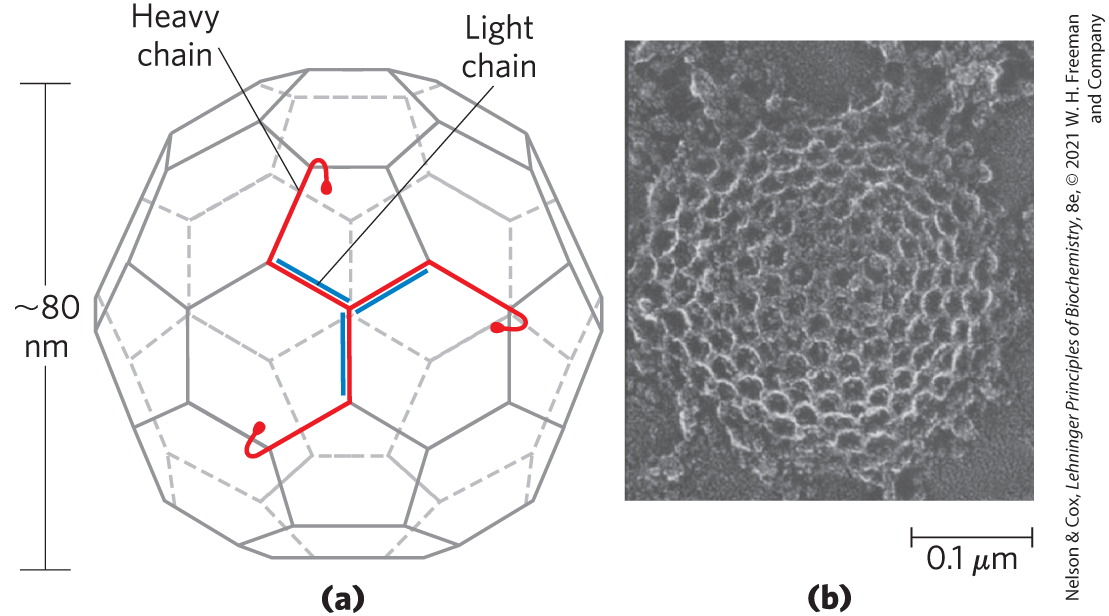
FIGURE 27-46 Clathrin. (a) The protein has three light (L) chains and three heavy (H) chains of the clathrin unit, organized as a three-legged structure called a triskelion. Triskelions tend to assemble into polyhedral lattices. (b) Electron micrograph of a coated pit on the cytosolic face of the plasma membrane of a fibroblast. [(a) Information from S. Mayor and R. E. Pagano, Nat. Rev. Mol. Cell Biol. 8:603, 2007. (b) ©1980 Heuser. The Rockefeller University Press. J. Heuser, J. Cell Biol. 84:560, 1980.]
The imported proteins and receptors then go their separate ways, their fates varying with the cell and protein type. Transferrin and its receptor are eventually recycled. Some hormones, growth factors, and immune complexes, after eliciting the appropriate cellular response, are degraded along with their receptors. LDL is degraded after the associated cholesterol has been delivered to its destination, but the LDL receptor is recycled (see Fig. 21-42).
Receptor-mediated endocytosis is exploited by some toxins and viruses to gain entry to cells. Influenza virus, diphtheria toxin, SARS-CoV-2 (the virus that causes COVID-19), and cholera toxin all enter cells in this way.
Protein Degradation Is Mediated by Specialized Systems in All Cells
Protein degradation is critical to overall cellular proteostasis, preventing the buildup of abnormal or unwanted proteins and permitting the recycling of amino acids. The half-lives of eukaryotic proteins vary from 30 seconds to many days. Most proteins turn over rapidly relative to the lifetime of a cell, although a few (such as hemoglobin) can last for the life of the cell (about 110 days for an erythrocyte). Rapidly degraded proteins include those that are defective because of incorrectly inserted amino acids or because of damage accumulated during normal functioning. And enzymes that act at key regulatory points in metabolic pathways often turn over rapidly.
Defective proteins and those with characteristically short half-lives are generally degraded in both bacterial and eukaryotic cells by selective ATP-dependent cytosolic systems. A second system in vertebrates, operating in lysosomes, recycles the amino acids of membrane proteins, extracellular proteins, and proteins with characteristically long half-lives.
In E. coli, many proteins are degraded by one of several proteolytic systems that contain (see Chapter 25), including Lon (the name refers to the “long form” of proteins, observed only when this protease is absent), ClpXP, ClpAP, ClpCP, ClpYQ, and FtsH. Each system targets particular proteins distinguished by their structure or subcellular location or both. Typically, ATP hydrolysis is used to maneuver a target protein through a pore into a proteolytic chamber, unfolding the protein in the process. Proteins are cleaved within the chamber. Once a protein has been reduced to small, inactive peptides, other ATP-independent proteases complete their degradation.
The ATP-dependent pathway in eukaryotic cells is quite different, involving the protein ubiquitin, which, as its name suggests, occurs throughout the eukaryotic kingdoms. One of the most highly conserved proteins known, ubiquitin (76 amino acid residues) is essentially identical in organisms as different as yeasts and humans and is key to proteostasis (see Figs. 4-23 and 13-29) and cell cycle regulation (see Fig. 12-38). Ubiquitin is covalently linked to proteins slated for destruction via an ATP-dependent pathway that includes three separate types of enzymes: E1 activating enzymes, E2 conjugating enzymes, and E3 ligases (Fig. 27-47).
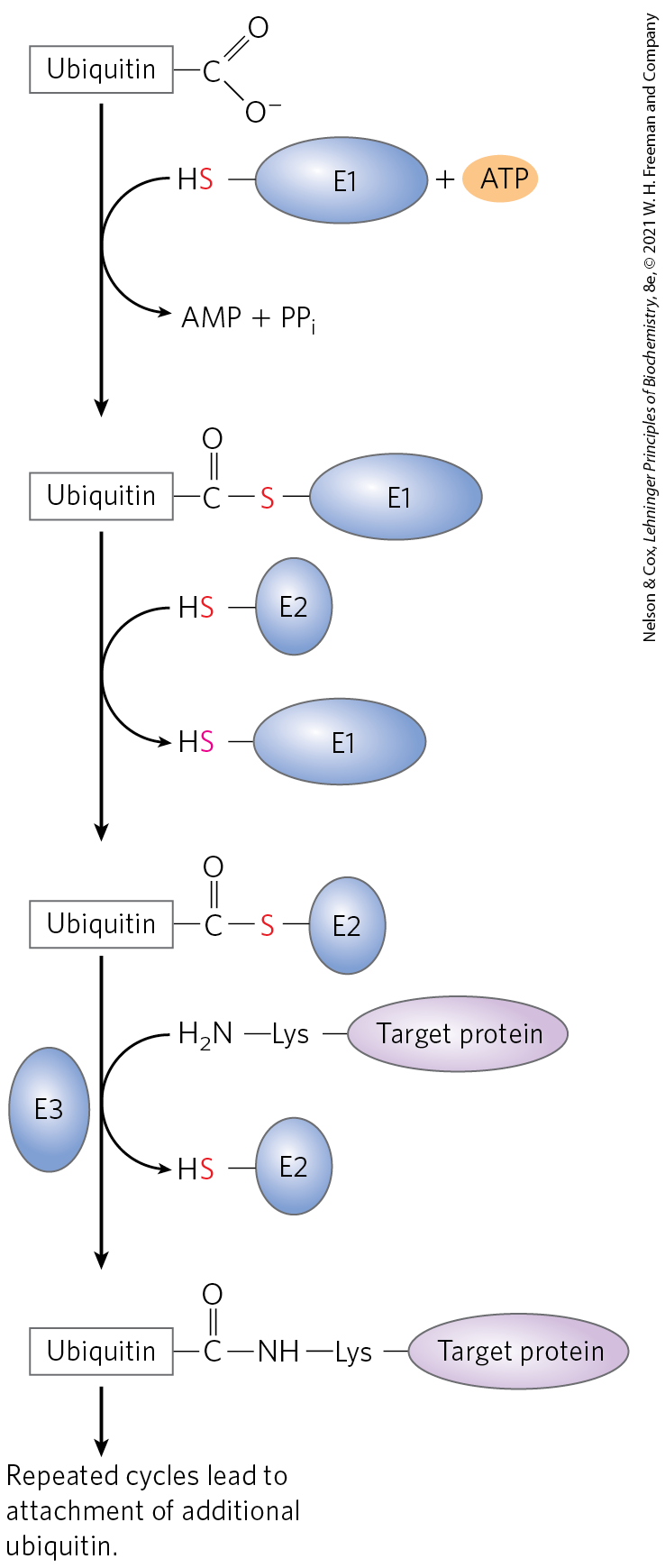
FIGURE 27-47 Three-step pathway by which ubiquitin is attached to a protein. The pathway includes two different enzyme-ubiquitin intermediates. First, the free carboxyl group of ubiquitin’s carboxyl-terminal Gly residue becomes linked to an E1-class activating enzyme through a thioester. The ubiquitin is then transferred to an E2 conjugating enzyme. An E3 ligase ultimately catalyzes transfer of the ubiquitin from E2 to the target, linking ubiquitin through an amide (isopeptide) bond to the ε-amino group of a Lys residue in the target protein. Additional cycles produce polyubiquitin, a covalent polymer of ubiquitin subunits that targets the attached protein for destruction in eukaryotes. Multiple pathways of this sort, with different protein targets, are present in most eukaryotic cells.
Ubiquitinated proteins are degraded by a large complex known as the 26S proteasome (Fig. 27-48). The eukaryotic proteasome consists of two copies each of at least 32 different subunits, most of which are highly conserved from yeasts to humans. The proteasome contains two main types of subcomplexes: a barrel-like core particle and regulatory particles at each end of the barrel. The 19S regulatory particle on each end of the core particle contains approximately 18 subunits, including some that recognize and bind to ubiquitinated proteins. Six of the subunits are that probably function in unfolding the ubiquitinated proteins and translocating the unfolded polypeptide into the core particle for degradation. The 19S particle also deubiquitinates the proteins as they are degraded in the proteasome. Most cells have additional regulatory complexes that can replace the 19S particle. These alternative regulators do not hydrolyze ATP and do not bind to ubiquitin, but they are important for the degradation of particular cellular proteins. The 26S proteasome can be effectively “accessorized” with regulatory complexes that change with changing cellular conditions.
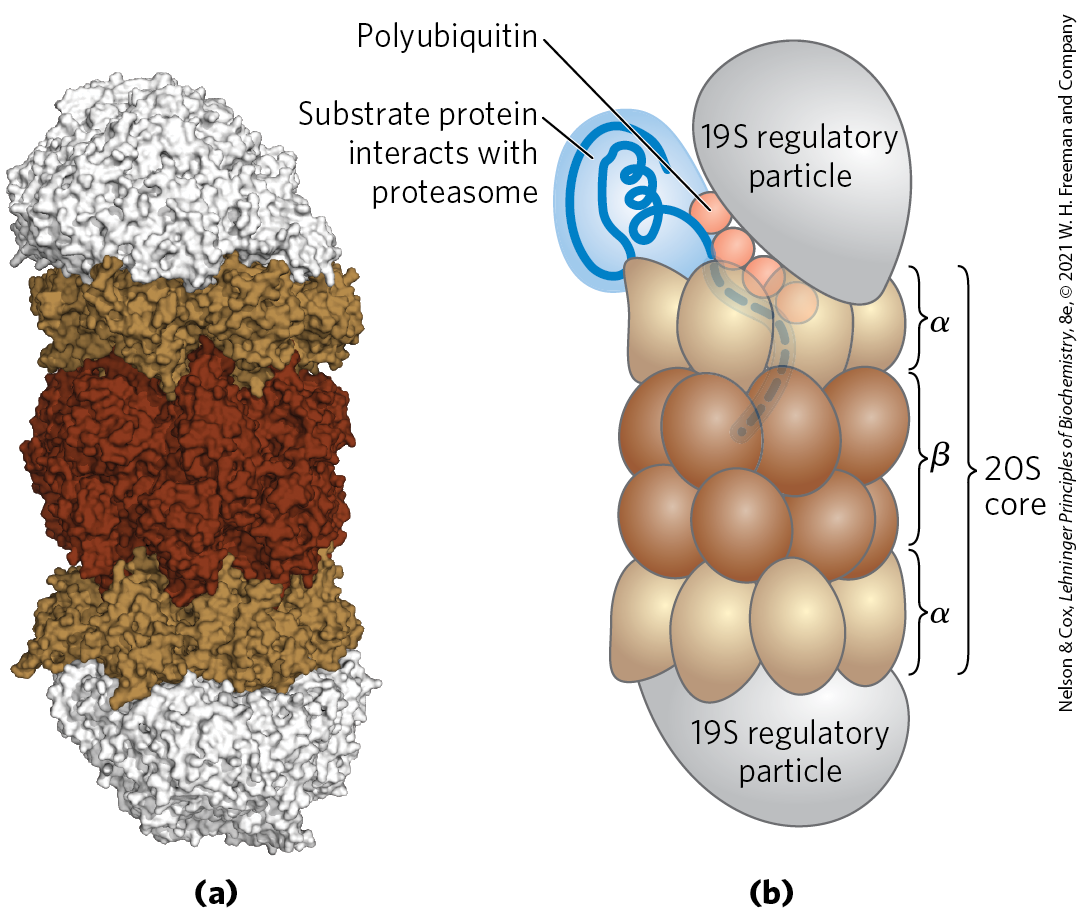
FIGURE 27-48 Three-dimensional structure of the eukaryotic proteasome. The 20S core particle and the 19S regulatory particle, or cap, are shown (a) as a molecular structure and (b) in schematic form. The core particle consists of four rings arranged to form a barrel-like structure. The outer rings are formed from seven α subunits, and the inner rings from seven β subunits. Three of the β subunits have protease activities, each with different substrate specificity. A regulatory particle forms a cap on each end of the core particle. The regulatory particle binds ubiquitinated proteins, unfolds them, and translocates them into the core particle, where they are degraded to peptides of 3 to 25 amino acid residues. [(a) Data from PDB ID 3L5Q, K. Sadre-Bazzaz et al., Mol. Cell 37:728, 2010.]
Although we do not yet understand all the signals that trigger ubiquitination, one simple signal has been found. For many proteins, the identity of the first residue that remains after removal of the amino-terminal Met residue, and any other posttranslational proteolytic processing of the amino-terminal end, has a profound influence on half-life (Table 27-9). These amino-terminal signals have been conserved over billions of years of evolution and are the same in bacterial protein degradation systems and in the human ubiquitination pathway. More complex signals, such as the destruction box discussed in Chapter 12 (see Fig. 12-38), are also being identified.
| Amino-terminal residue | Half-lifea |
|---|---|
Stabilizing |
|
Ala, Gly, Met, Ser, Thr, Val |
>20 h |
Destabilizing |
|
Gln, Ile |
∼30 min |
Glu, Tyr |
∼10 min |
Pro |
∼7 min |
Asp, Leu, Lys, Phe |
∼3 min |
Arg |
∼2 min |
Information from A. Bachmair et al., Science 234:179, 1986. aHalf-lives were measured in yeast for the β-galactosidase protein modified so that in each experiment it had a different amino-terminal residue. Half-lives may vary for different proteins and in different organisms, but this general pattern appears to hold for all organisms. |
|
Ubiquitin-dependent proteolysis is as important for the regulation of cellular processes as it is for the elimination of defective proteins. Many proteins required at only one stage of the eukaryotic cell cycle are rapidly degraded by the ubiquitin-dependent pathway after fulfilling their function. Ubiquitin-dependent destruction of cyclin is critical to cell-cycle regulation. The E1, E2, and E3 components of the ubiquitination pathway (Fig. 27-47) are large families of proteins. Different E1, E2, and E3 enzymes exhibit different specificities for target proteins and thus regulate different cellular processes. Some of these enzymes are highly localized in certain cellular compartments, reflecting a specialized function.
Not surprisingly, defects in the ubiquitination pathway have been implicated in a wide range of disease states. An inability to degrade certain proteins that activate cell division (the products of oncogenes) can lead to tumor formation, and a too-rapid degradation of proteins that act as tumor suppressors can have the same effect. The ineffective or overly rapid degradation of cellular proteins also seems to play a role in a range of other conditions, including renal diseases, asthma, and neurodegenerative disorders such as Alzheimer and Parkinson diseases that are associated with the formation of characteristic proteinaceous structures in neurons. Cystic fibrosis is caused in some cases by a too-rapid degradation of a chloride ion channel, with resultant loss of function (see Box 11-2). Liddle syndrome, in which a sodium channel in the kidney is not degraded, leads to excessive absorption and early-onset hypertension. Drugs designed to inhibit proteasome function are being developed as potential treatments for some of these conditions. Many bacterial pathogens have found ways to hijack the eukaryotic ubiquitination system, evolving enzymes that ubiquitinate and thus eliminate host proteins as required to facilitate infection. In a changing metabolic environment, protein degradation is as important to a cell’s survival as is protein synthesis, and much remains to be learned about these interesting pathways.
SUMMARY 27.3 Protein Targeting and Degradation
- After synthesis, many proteins are directed to particular locations in the cell, a process mediated by signal sequences embedded in the polypeptide chain. In eukaryotic cells, one class of signal sequences is recognized by the signal recognition particle (SRP), which binds the signal sequence as soon as it appears on the ribosome and transfers the entire ribosome and incomplete polypeptide to the endoplasmic reticulum. Polypeptides with these signal sequences are moved into the endoplasmic reticulum lumen as they are synthesized.
- Once in the lumen of the ER, many proteins are glycosylated. So modified, they are moved to the Golgi complex, then sorted and sent to lysosomes, the plasma membrane, or transport vesicles.
- Proteins targeted to the nucleus have an internal signal sequence that, unlike other signal sequences, is not cleaved once the protein is successfully targeted.
- Proteins targeted to mitochondria and chloroplasts in eukaryotic cells, and those destined for export in bacteria, also make use of an amino-terminal signal sequence.
- Some eukaryotic cells import proteins by receptor-mediated endocytosis.
- All cells eventually degrade proteins, using specialized proteolytic systems. Defective proteins and those slated for rapid turnover are generally degraded by an ATP-dependent system. In eukaryotic cells, the proteins are first tagged by linkage to ubiquitin, a highly conserved protein. Ubiquitin-dependent proteolysis, critical to the regulation of many cellular processes, is carried out by proteasomes, which also are highly conserved.
 The thermodynamic cost of protein synthesis is magnified by the processes used by cells to transport proteins to their correct cellular locations.
The thermodynamic cost of protein synthesis is magnified by the processes used by cells to transport proteins to their correct cellular locations. , with initiation of protein synthesis on free ribosomes. The signal sequence appears early in the synthetic process (step
, with initiation of protein synthesis on free ribosomes. The signal sequence appears early in the synthetic process (step  ), because it is at the amino terminus, which, as we have seen, is synthesized first. As it emerges from the ribosome (step
), because it is at the amino terminus, which, as we have seen, is synthesized first. As it emerges from the ribosome (step  ), the signal sequence — and the ribosome itself — is bound by the large signal recognition particle (SRP). The SRP is a rod-shaped complex containing a 300 nucleotide RNA (7SL-RNA) and six different proteins (combined ). The SRP then binds GTP and halts elongation of the polypeptide when it is about 70 amino acids long and the signal sequence has completely emerged from the ribosome. In step
), the signal sequence — and the ribosome itself — is bound by the large signal recognition particle (SRP). The SRP is a rod-shaped complex containing a 300 nucleotide RNA (7SL-RNA) and six different proteins (combined ). The SRP then binds GTP and halts elongation of the polypeptide when it is about 70 amino acids long and the signal sequence has completely emerged from the ribosome. In step  , the GTP-bound SRP directs the ribosome (still bound to the mRNA) and the incomplete polypeptide to GTP-bound SRP receptors in the cytosolic face of the ER; the nascent polypeptide is delivered to a peptide translocation complex in the ER, which interacts directly with the ribosome. In step
, the GTP-bound SRP directs the ribosome (still bound to the mRNA) and the incomplete polypeptide to GTP-bound SRP receptors in the cytosolic face of the ER; the nascent polypeptide is delivered to a peptide translocation complex in the ER, which interacts directly with the ribosome. In step  , the SRP dissociates from the ribosome, accompanied by hydrolysis of GTP in both the SRP and the SRP receptor. The SRP receptor is a heterodimer of and subunits, both of which bind and hydrolyze multiple GTP molecules during this process.
, the SRP dissociates from the ribosome, accompanied by hydrolysis of GTP in both the SRP and the SRP receptor. The SRP receptor is a heterodimer of and subunits, both of which bind and hydrolyze multiple GTP molecules during this process. ), with the ATP-driven translocation complex feeding the growing polypeptide into the ER lumen until the complete protein has been synthesized. In step
), with the ATP-driven translocation complex feeding the growing polypeptide into the ER lumen until the complete protein has been synthesized. In step  , the signal sequence is removed by a signal peptidase within the ER lumen. The ribosome dissociates (step
, the signal sequence is removed by a signal peptidase within the ER lumen. The ribosome dissociates (step  ) and is recycled (step
) and is recycled (step  ).
). Not surprisingly, defects in the ubiquitination pathway have been implicated in a wide range of disease states. An inability to degrade certain proteins that activate cell division (the products of oncogenes) can lead to tumor formation, and a too-rapid degradation of proteins that act as tumor suppressors can have the same effect. The ineffective or overly rapid degradation of cellular proteins also seems to play a role in a range of other conditions, including renal diseases, asthma, and neurodegenerative disorders such as Alzheimer and Parkinson diseases that are associated with the formation of characteristic proteinaceous structures in neurons. Cystic fibrosis is caused in some cases by a too-rapid degradation of a chloride ion channel, with resultant loss of function (see
Not surprisingly, defects in the ubiquitination pathway have been implicated in a wide range of disease states. An inability to degrade certain proteins that activate cell division (the products of oncogenes) can lead to tumor formation, and a too-rapid degradation of proteins that act as tumor suppressors can have the same effect. The ineffective or overly rapid degradation of cellular proteins also seems to play a role in a range of other conditions, including renal diseases, asthma, and neurodegenerative disorders such as Alzheimer and Parkinson diseases that are associated with the formation of characteristic proteinaceous structures in neurons. Cystic fibrosis is caused in some cases by a too-rapid degradation of a chloride ion channel, with resultant loss of function (see 
 After synthesis, many proteins are directed to particular locations in the cell, a process mediated by signal sequences embedded in the polypeptide chain. In eukaryotic cells, one class of signal sequences is recognized by the signal recognition particle (SRP), which binds the signal sequence as soon as it appears on the ribosome and transfers the entire ribosome and incomplete polypeptide to the endoplasmic reticulum. Polypeptides with these signal sequences are moved into the endoplasmic reticulum lumen as they are synthesized.
After synthesis, many proteins are directed to particular locations in the cell, a process mediated by signal sequences embedded in the polypeptide chain. In eukaryotic cells, one class of signal sequences is recognized by the signal recognition particle (SRP), which binds the signal sequence as soon as it appears on the ribosome and transfers the entire ribosome and incomplete polypeptide to the endoplasmic reticulum. Polypeptides with these signal sequences are moved into the endoplasmic reticulum lumen as they are synthesized.