Part II BIOENERGETICS AND METABOLISM

Every enzyme-catalyzed reaction and reaction sequence serves an important role in an organism’s physiology: to (1) obtain chemical energy by capturing solar energy or degrading energy-rich nutrients from the environment; (2) convert nutrient molecules into the cell’s own characteristic molecules, including precursors of macromolecules; (3) polymerize monomeric precursors into macromolecules: proteins, nucleic acids, and polysaccharides; and (4) synthesize and degrade biomolecules required for specialized cellular functions, such as membrane lipids, intracellular messengers, and pigments.
Although metabolism embraces many thousands of different enzyme-catalyzed reactions, our major concern in Part II is the central metabolic pathways, which are few in number and remarkably similar in all forms of life. Living organisms can be divided into two large groups according to the chemical form in which they obtain carbon from the environment. Autotrophs (such as photosynthetic bacteria, green algae, and vascular plants) can use carbon dioxide from the atmosphere as their sole source of carbon, from which they construct all their carbon-containing biomolecules (see Fig. 1). Heterotrophs cannot use atmospheric carbon dioxide and must obtain carbon from their environment in the form of relatively complex organic molecules such as glucose. Multicellular animals and most microorganisms are heterotrophic. Autotrophic cells and organisms are relatively self-sufficient, whereas heterotrophic cells and organisms, with their requirements for carbon in more complex forms, must subsist on the products of other organisms.
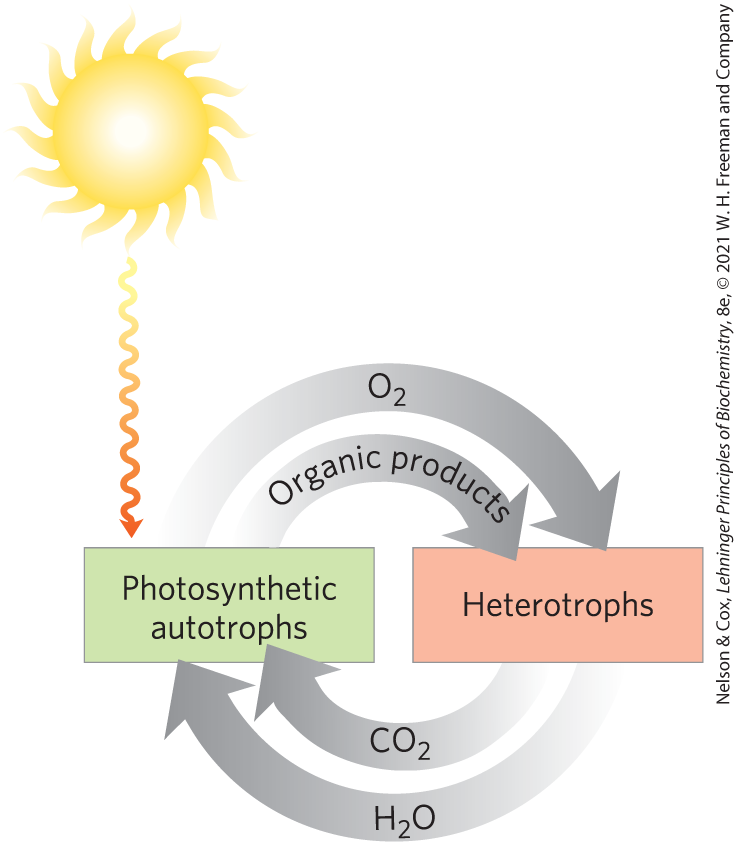
FIGURE 1 Cycling of carbon dioxide and oxygen between the autotrophic (photosynthetic) and heterotrophic domains in the biosphere. The flow of mass through this cycle is enormous; about metric tons of carbon are turned over in the biosphere annually.
Many autotrophic organisms are photosynthetic and obtain their energy from sunlight, whereas heterotrophic organisms obtain their energy from the degradation of organic nutrients produced by autotrophs. In our biosphere, autotrophs and heterotrophs live together in a vast, interdependent cycle in which autotrophic organisms use atmospheric carbon dioxide to build their organic biomolecules, some of them generating oxygen from water in the process. Heterotrophs, in turn, use the organic products of autotrophs as nutrients, which they oxidize, releasing to the atmosphere the produced by oxidation. Thus carbon, oxygen, and water are constantly cycled between the heterotrophic and autotrophic worlds, with solar energy as the driving force for this global process. A similarly complex global web connects molecular nitrogen () with nitrogen in its other oxidation states, including (the form in which nitrogen enters metabolism). The cycling of carbon, oxygen, and nitrogen, which ultimately involves all species, depends on a proper balance between the activities of the producers (autotrophs) and consumers (heterotrophs) in our biosphere. Global warming by the greenhouse effect (the result of rising concentrations in our atmosphere) is a biochemical phenomenon, occurring on a very large scale.
These cycles of matter are driven by an enormous flow of energy into and through the biosphere, beginning with the capture of solar energy by photosynthetic organisms and use of this energy to generate energy-rich carbohydrates and other organic nutrients; these nutrients are then used as energy sources by heterotrophic organisms.
Precursors are converted into products through a series of intermediates called metabolites. The term intermediary metabolism is often applied to the combined activities of all the metabolic pathways that interconvert precursors, metabolites, and products of low molecular weight (generally, ). Catabolism is the degradative phase of metabolism in which organic nutrient molecules (carbohydrates, fats, and proteins) are converted into smaller, simpler end products (such as lactic acid, , and ). Catabolic pathways release energy, some of which is conserved in the formation of ATP and reduced electron carriers (NADH or NADPH); the rest is lost as heat. In anabolism, also called biosynthesis, small, simple precursors are built up into larger and more complex molecules, including lipids, polysaccharides, proteins, and nucleic acids. Anabolic reactions require an input of energy, generally in the form of the phosphoryl group transfer potential of ATP and the reducing power of NADH and NADPH (Fig. 2).
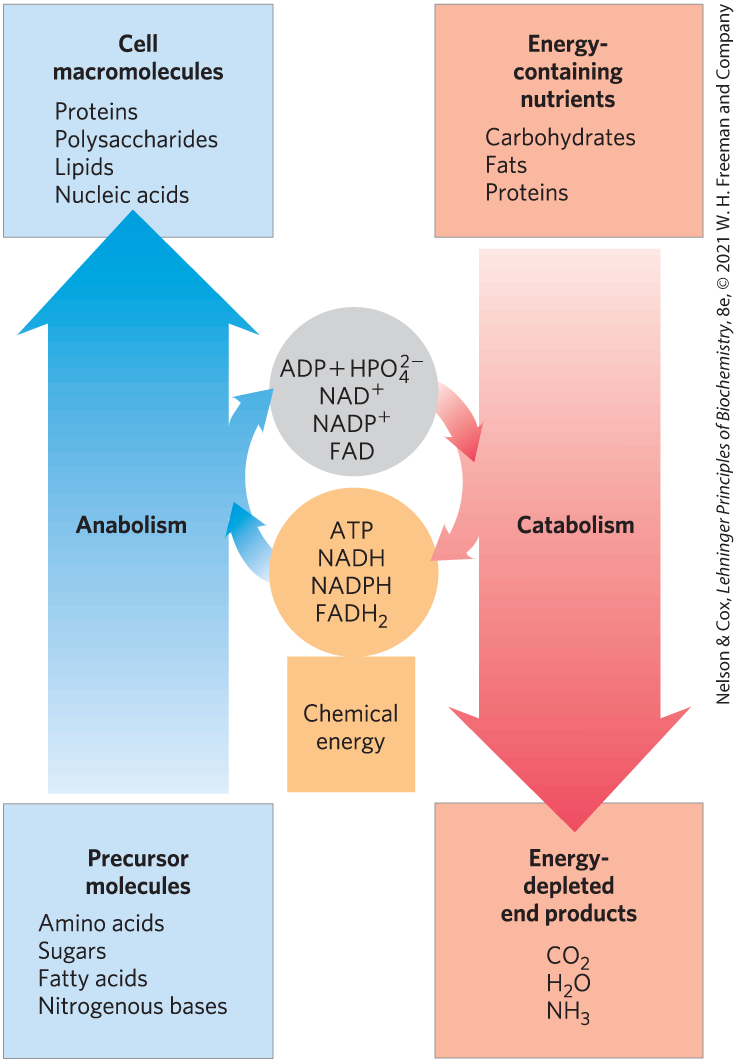
FIGURE 2 The big picture: energy relationships between catabolic and anabolic pathways. Catabolic pathways deliver chemical energy in the form of ATP, NADH, NADPH, and . These energy carriers are used in anabolic pathways to convert small precursor molecules into cellular macromolecules.
Some metabolic pathways are linear, and some are branched, yielding multiple useful end products from a single precursor or converting several starting materials into a single product. In general, catabolic pathways are convergent and anabolic pathways are divergent (Fig. 3). Some pathways are cyclic: one starting component of the pathway is regenerated in a series of reactions that converts another starting component into a product. We shall see examples of each type of pathway in the following chapters.
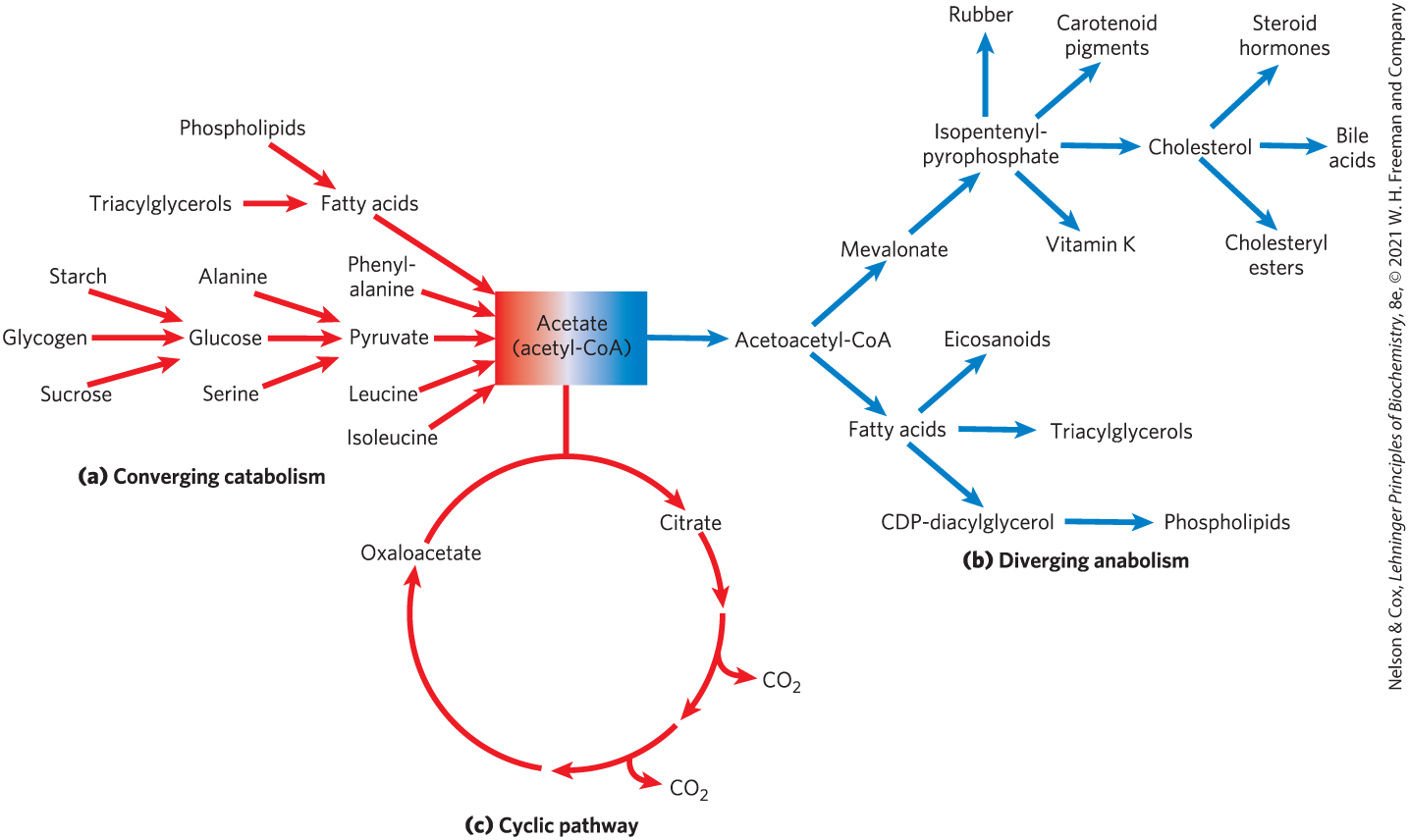
FIGURE 3 Three types of nonlinear metabolic pathways. (a) Converging, catabolic, (b) diverging, anabolic, and (c) cyclic pathways. In (c), one of the starting materials (oxaloacetate in this case) is regenerated and reenters the pathway. Acetate, a key metabolic intermediate, is the breakdown product of a variety of fuels (a), serves as the precursor for an array of products (b), and is consumed in the catabolic pathway known as the citric acid cycle (c).
Most cells have the enzymes to carry out both the degradation and the synthesis of the important categories of biomolecules — fatty acids, for example. The simultaneous synthesis and degradation of fatty acids would be wasteful, however. This is prevented by reciprocally regulating the anabolic and catabolic reaction sequences: when one sequence is active, the other is suppressed. Such regulation could not occur if anabolic and catabolic pathways were catalyzed by exactly the same set of enzymes, operating in one direction for anabolism, the opposite direction for catabolism: inhibition of an enzyme involved in catabolism would also inhibit the reaction sequence in the anabolic direction. Catabolic and anabolic pathways that connect the same two end points (glucose →→ pyruvate, and pyruvate →→ glucose, for example) may employ many of the same enzymes; but, invariably, at least one of the steps is catalyzed by different enzymes in the catabolic and anabolic directions, and these enzymes are the sites of separate regulation. Moreover, for both anabolic and catabolic pathways to be essentially irreversible, the reactions unique to each direction must include at least one that is thermodynamically very favorable — in other words, a reaction for which the reverse reaction is very unfavorable.
As a further contribution to the separate regulation of catabolic and anabolic reaction sequences, paired catabolic and anabolic pathways commonly take place in different cellular compartments: for example, fatty acid catabolism occurs in animal mitochondria, whereas fatty acid synthesis occurs in the cytosol. The concentrations of intermediates, enzymes, and regulators can be maintained at different levels in these different compartments. Because metabolic pathways are subject to kinetic control by substrate concentration, separate pools of anabolic and catabolic intermediates also contribute to the control of metabolic rates. Devices that separate anabolic and catabolic processes will be of particular interest in our discussions of metabolism.
Metabolic pathways are regulated at several levels, from within the cell and from outside. A key enzyme in a pathway may be activated allosterically, or its amount may be changed by the rates of synthesis and breakdown of the enzyme. In multicellular organisms, the metabolic activities of different tissues are regulated and integrated by growth factors and hormones that act from outside the cell.
We begin Part II with a discussion of the basic energetic principles that govern all metabolism, as well as a refresher on the reactions from organic chemistry that we will see in metabolism (Chapter 13). We then consider the major catabolic pathways by which cells obtain energy from the oxidation of various fuels (Chapters 14 through 20). Chapters 19 and 20 represent the pivotal point of our discussion of metabolism; they concern chemiosmotic energy coupling, a universal mechanism in which a transmembrane electrochemical potential, produced either by substrate oxidation or by light absorption, drives the synthesis of ATP.
Chapters 20 through 22 describe the major anabolic pathways by which cells use the energy in ATP to produce carbohydrates, lipids, amino acids, and nucleotides from simpler precursors. In Chapter 23 we step back from our detailed look at the metabolic pathways — as they occur in all organisms, from E. coli to humans — and consider how they are regulated and integrated in mammals by hormonal mechanisms.
As we undertake our study of intermediary metabolism, a final word. Keep in mind that the many reactions described in these pages take place in, and play crucial roles in, living organisms. As you encounter each reaction and each pathway, ask: Where does this piece fit in the big picture? What does this chemical transformation do for the organism? How does this pathway interconnect with the other pathways operating simultaneously in the same cell to produce the energy and products required for cell maintenance and growth? What would be the expected result of a defect in this enzyme or that pathway? Studied with this perspective, metabolism provides fascinating and revealing insights into life, with countless applications in medicine, agriculture, and biotechnology.