Chapter 15 The Metabolism of Glycogen in Animals
In Chapter 14 we examined universal pathways by which hexoses are metabolized through glycolysis, fermentation, gluconeogenesis, and the pentose phosphate pathways to provide energy and components for the biosynthesis of amino acids, fats, and nucleotides. In this chapter, we focus more narrowly on the metabolism of glycogen, the polymeric storage form of glucose employed by animals. These are the principles that guide our discussion:
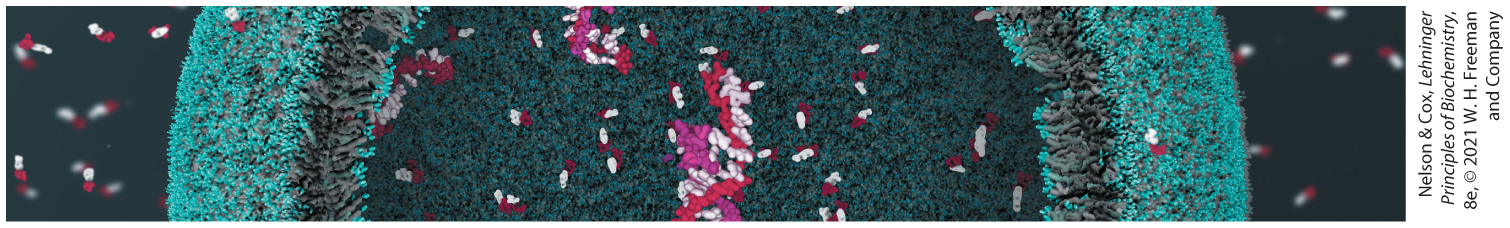
Glycogen provides vertebrate animals with a ready source of glucose to supply the brain and skeletal muscles with energy. Although animals store about 100 times more energy as fat than as glycogen, they cannot metabolize fat into glucose. The highly branched polymeric structure of glycogen granules allows cells in the liver and muscle to make large numbers of glucose and glucose phosphate monomers available quickly, without raising the osmolarity of the cytosol by storing them in monomeric form.
Monomers are released from glycogen granules by a phosphorolysis reaction that creates phosphorylated glucose molecules that can enter glycolysis to supply energy to the cell. Skeletal muscle cells especially require stores of glycogen to supply energy for bursts of activity. In the liver, the phosphate can be removed, allowing free glucose to be transported out of the cell to the blood for use in the brain and other tissues when dietary glucose is not sufficient.
Glycogen synthesis requires a protein primer and an activated glucose precursor. Individual glucose molecules activated as sugar nucleotides are added to the nonreducing end of the growing linear chains in the outer tiers of the glycogen β-granules, and a branching enzyme adds branches periodically.
Regulation of the balance between the formation of glycogen from excess glucose and the release of glucose from glycogen polymers when it is needed in metabolism is a critical function of cellular and organismal homeostasis. This balance, ultimately controlled by the hormones epinephrine, glucagon, and insulin, is achieved through allosteric regulation and phosphorylation of the synthetic and degradative enzymes. These enzymes, and the regulatory proteins that act on them, are integral parts of the glycogen granule.
Glycogen was discovered in the mid-1800s by Claude Bernard. The French physiologist also found that a liver “ferment” (enzyme) released a reducing sugar from liver tissue. He named this reducing sugar matière glycogène — sugar-forming substance. In the first half of the twentieth century, scientists in laboratories around the world followed up this early work, purifying the “ferments” that synthesize and degrade glycogen and characterizing the regulation of these enzymes by insulin and epinephrine. These studies characterized the enzymes and also uncovered multiple regulatory mechanisms that proved to be universal: second messengers responsive to extracellular signals, protein kinase cascades, and protein phosphorylation, for example. In this chapter, we begin by exploring the structure and function of glycogen particles, describe the pathways of glycogen breakdown and synthesis, and finally, dig into the complex web of regulatory controls that exquisitely deliver the necessary amount of energy from glucose that each organ system requires to function in the moment.
 Glycogen provides vertebrate animals with a ready source of glucose to supply the brain and skeletal muscles with energy. Although animals store about 100 times more energy as fat than as glycogen, they cannot metabolize fat into glucose. The highly branched polymeric structure of glycogen granules allows cells in the liver and muscle to make large numbers of glucose and glucose phosphate monomers available quickly, without raising the osmolarity of the cytosol by storing them in monomeric form.
Glycogen provides vertebrate animals with a ready source of glucose to supply the brain and skeletal muscles with energy. Although animals store about 100 times more energy as fat than as glycogen, they cannot metabolize fat into glucose. The highly branched polymeric structure of glycogen granules allows cells in the liver and muscle to make large numbers of glucose and glucose phosphate monomers available quickly, without raising the osmolarity of the cytosol by storing them in monomeric form. Monomers are released from glycogen granules by a phosphorolysis reaction that creates phosphorylated glucose molecules that can enter glycolysis to supply energy to the cell. Skeletal muscle cells especially require stores of glycogen to supply energy for bursts of activity. In the liver, the phosphate can be removed, allowing free glucose to be transported out of the cell to the blood for use in the brain and other tissues when dietary glucose is not sufficient.
Monomers are released from glycogen granules by a phosphorolysis reaction that creates phosphorylated glucose molecules that can enter glycolysis to supply energy to the cell. Skeletal muscle cells especially require stores of glycogen to supply energy for bursts of activity. In the liver, the phosphate can be removed, allowing free glucose to be transported out of the cell to the blood for use in the brain and other tissues when dietary glucose is not sufficient. Glycogen synthesis requires a protein primer and an activated glucose precursor. Individual glucose molecules activated as sugar nucleotides are added to the nonreducing end of the growing linear chains in the outer tiers of the glycogen β-granules, and a branching enzyme adds branches periodically.
Glycogen synthesis requires a protein primer and an activated glucose precursor. Individual glucose molecules activated as sugar nucleotides are added to the nonreducing end of the growing linear chains in the outer tiers of the glycogen β-granules, and a branching enzyme adds branches periodically. Regulation of the balance between the formation of glycogen from excess glucose and the release of glucose from glycogen polymers when it is needed in metabolism is a critical function of cellular and organismal homeostasis. This balance, ultimately controlled by the hormones epinephrine, glucagon, and insulin, is achieved through allosteric regulation and phosphorylation of the synthetic and degradative enzymes. These enzymes, and the regulatory proteins that act on them, are integral parts of the glycogen granule.
Regulation of the balance between the formation of glycogen from excess glucose and the release of glucose from glycogen polymers when it is needed in metabolism is a critical function of cellular and organismal homeostasis. This balance, ultimately controlled by the hormones epinephrine, glucagon, and insulin, is achieved through allosteric regulation and phosphorylation of the synthetic and degradative enzymes. These enzymes, and the regulatory proteins that act on them, are integral parts of the glycogen granule.