7.4 Carbohydrates as Informational Molecules: The Sugar Code
Glycobiology, the study of the structure and function of glycoconjugates, is one of the most active and exciting areas of biochemistry and cell biology. The challenge is to understand how cells use specific oligosaccharides to encode important information about intracellular targeting of proteins, cell-cell interactions, cell differentiation and tissue development, and extracellular signals. Our discussion uses just a few examples to illustrate the diversity of structure and the range of biological activity of the glycoconjugates. In Chapter 20 we discuss the biosynthesis of polysaccharides, and in Chapter 27, the assembly of oligosaccharide chains on glycoproteins.
Oligosaccharide Structures Are Information-Dense
Improved methods for the analysis of oligosaccharide and polysaccharide structure have revealed remarkable complexity and diversity in the oligosaccharides of glycoproteins and glycolipids. Consider the oligosaccharide chains in Figure 7-27, typical of those found in many glycoproteins. The most complex of those shown contains 14 monosaccharide residues of four different kinds, variously linked as , , , , , and , some with the α and some with the β configuration. Branched structures, not found in nucleic acids or proteins, are common in oligosaccharides. With the reasonable assumption that 20 different monosaccharide subunits are available for construction of oligosaccharides, we can calculate that many billions of different hexameric oligosaccharides are possible; this compares with different hexapeptides possible for the 20 common amino acids, and 4,096 different hexanucleotides for the four nucleotide subunits. If we also allow for variations in oligosaccharides resulting from sulfation of one or more residues, the number of possible oligosaccharides increases by two orders of magnitude. In reality, only a subset of possible combinations is found, given the restrictions imposed by the biosynthetic enzymes and the availability of precursors. Nevertheless, the enormously rich structural information in glycans does not merely rival but far surpasses that of nucleic acids in the density of information contained in a molecule of modest size. Each of the oligosaccharides represented in Figures 7-20 and 7-27 presents a unique, three-dimensional face — a word in the sugar code — readable by the proteins that interact with it.
Lectins Are Proteins That Read the Sugar Code and Mediate Many Biological Processes
Lectins bind carbohydrates with high specificity and with moderate to high affinity. These proteins serve in a wide variety of cell-cell recognition, signaling, and adhesion processes and in intracellular targeting of newly synthesized proteins. Plant lectins, abundant in seeds, probably serve as deterrents to insects and other predators. In the laboratory, purified plant lectins are useful reagents for detecting and separating glycans and glycoproteins with different oligosaccharide moieties. Here we discuss just a few examples of the roles of lectins in animal cells.
Some peptide hormones that circulate in the blood have oligosaccharide moieties that strongly influence their circulatory half-life. Luteinizing hormone and thyrotropin (polypeptide hormones produced in the pituitary) have N-linked oligosaccharides that end with the disaccharide GalNAc4SNAc, which is recognized by a lectin (receptor) of hepatocytes. (GalNAc4S is N-acetylgalactosamine sulfated on the —OH group at C-4.) Receptor-hormone interaction mediates the uptake and destruction of luteinizing hormone and thyrotropin, reducing their concentration in the blood. Thus the blood levels of these hormones undergo a periodic rise (due to pulsatile secretion by the pituitary) and fall (due to constant destruction by hepatocytes).
Residues of Neu5Ac (a sialic acid) situated at the ends of the oligosaccharide chains of many plasma glycoproteins (Fig. 7-20) protect these proteins from uptake and degradation in the liver. For example, ceruloplasmin, a copper-containing serum glycoprotein, has several oligosaccharide chains ending in Neu5Ac. The mechanism that removes sialic acid residues from serum glycoproteins is unclear. It may be due to the activity of the enzyme neuraminidase (also called sialidase) produced by invading organisms or to a steady, slow release of the residues by extracellular enzymes. The plasma membrane of hepatocytes has lectin molecules (asialoglycoprotein receptors; “asialo-” indicating “without sialic acid”) that specifically bind oligosaccharide chains with galactose residues no longer “protected” by a terminal Neu5Ac residue. Receptor-ceruloplasmin interaction triggers endocytosis and destruction of the ceruloplasmin.
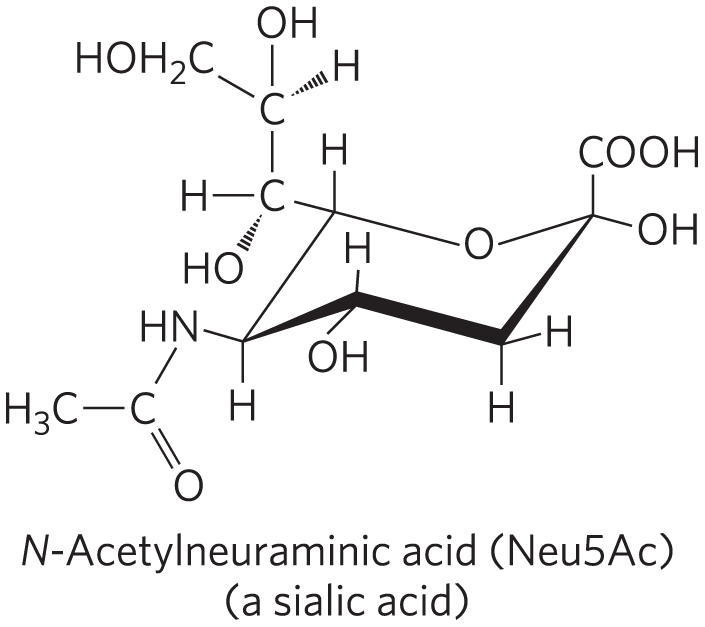
A similar mechanism is apparently responsible for removing “old” erythrocytes from the mammalian bloodstream. Newly synthesized erythrocytes have several membrane glycoproteins with oligosaccharide chains that end in Neu5Ac. In the laboratory, when the sialic acid residues are removed by withdrawing a sample of blood from experimental animals, treating it with neuraminidase in vitro, and reintroducing it into the circulation, the treated erythrocytes disappear from the bloodstream within a few hours; erythrocytes with intact oligosaccharides (withdrawn and reintroduced without neuraminidase treatment) continue to circulate for days.
Selectins are a family of plasma membrane lectins that mediate cell-cell recognition and adhesion in a wide range of cellular processes. One such process is the movement of immune cells (leukocytes) through the capillary wall, from blood to tissues, at sites of infection or inflammation (Fig. 7-29). At an infection site, P-selectin on the surface of capillary endothelial cells interacts with a specific oligosaccharide of the surface glycoproteins of circulating leukocytes. This interaction slows the leukocytes as they roll along the endothelial lining of the capillaries. A second interaction, between integrin molecules in the leukocyte plasma membrane and an adhesion protein on the endothelial cell surface, now stops the leukocyte and allows it to move through the capillary wall into the infected tissues to initiate the immune attack. Two other selectins participate in this “lymphocyte homing”: E-selectin on the endothelial cell and l-selectin on the leukocyte bind their cognate oligosaccharides on the leukocyte and endothelial cell, respectively. Several of the selectins essential to lymphocyte homing bind specifically to the tetrasaccharide , called sialyl Lewis x or sialyl .
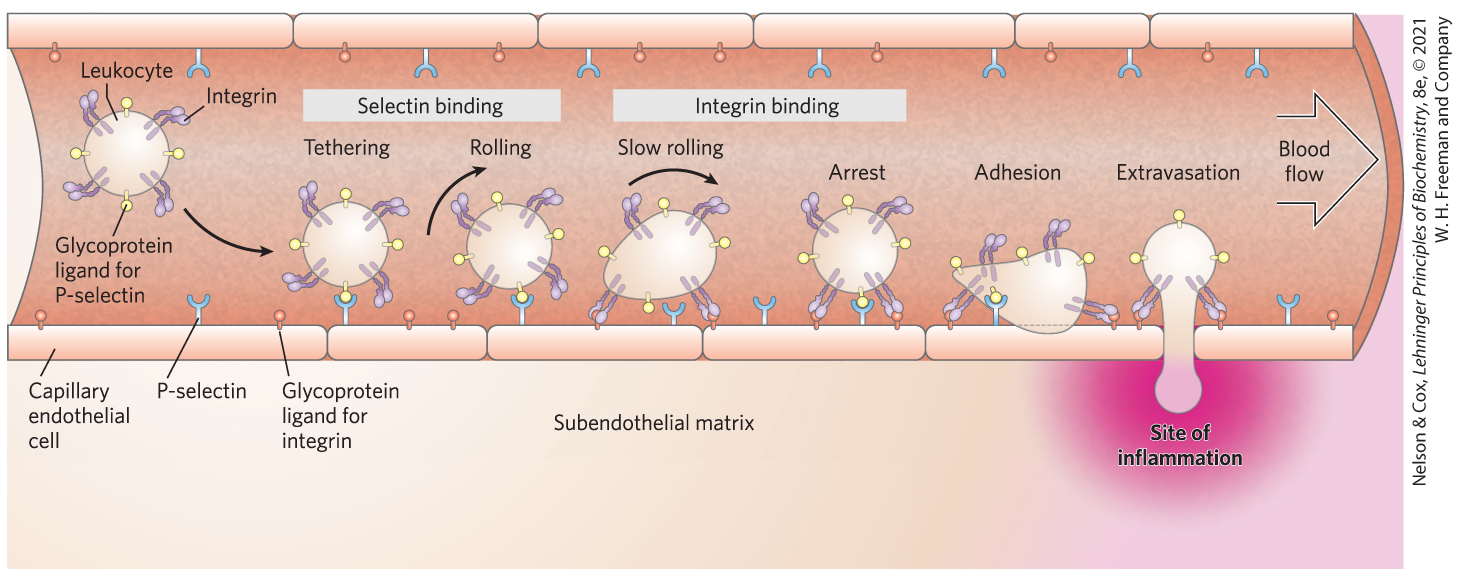
FIGURE 7-29 Role of lectin-ligand interactions in leukocyte movement to the site of an infection or injury. A leukocyte circulating through a capillary is slowed by transient interactions between P-selectin molecules in the plasma membrane of the capillary endothelial cells and glycoprotein ligands for P-selectin on the leukocyte surface. As the leukocyte interacts with successive P-selectin molecules, it rolls along the capillary surface. Near a site of inflammation, stronger interactions between integrin in the leukocyte surface and its ligand in the capillary surface lead to tight adhesion. The leukocyte stops rolling and, under the influence of signals from the site of inflammation (such as sialyl Lewis x), escapes through the capillary wall as it moves toward the region of inflammation.
Human selectins mediate the inflammatory responses in rheumatoid arthritis, asthma, psoriasis, multiple sclerosis, and the rejection of transplanted organs, and thus there is great interest in developing drugs that inhibit selectin-mediated cell adhesion. Many carcinomas express sialyl Lewis x, which, when shed into the circulation, facilitates tumor cell survival and metastasis. Carbohydrate derivatives that mimic the sialyl Lewis x portion of sialoglycoproteins and compete for specific selectin-binding sites, or that alter the biosynthesis of the oligosaccharide, might prove effective as selectin-specific drugs for treating chronic inflammation or metastatic disease.
Several animal viruses, including the influenza virus, attach to their host cells through interactions with oligosaccharides displayed on the host cell surface. The lectin of the influenza virus, known as the HA (hemagglutinin) protein, is essential for viral entry and infection. After the virus has entered a host cell and has been replicated, the newly synthesized viral particles bud out of the cell, wrapped in a portion of its plasma membrane. A viral sialidase (neuraminidase) trims the terminal sialic acid residue from the host cell’s oligosaccharides, releasing the viral particles from their interaction with the cell and preventing their aggregation with one another. Another round of infection can now begin. The antiviral drugs oseltamivir (Tamiflu) and zanamivir (Relenza) are used clinically in the treatment of influenza. These drugs are sugar analogs; they inhibit the viral sialidase by competing with the host cell’s oligosaccharides for binding (Fig. 7-30). This prevents the release of viruses from the infected cell by sialidase, and also causes viral particles to aggregate, both of which block another cycle of infection.
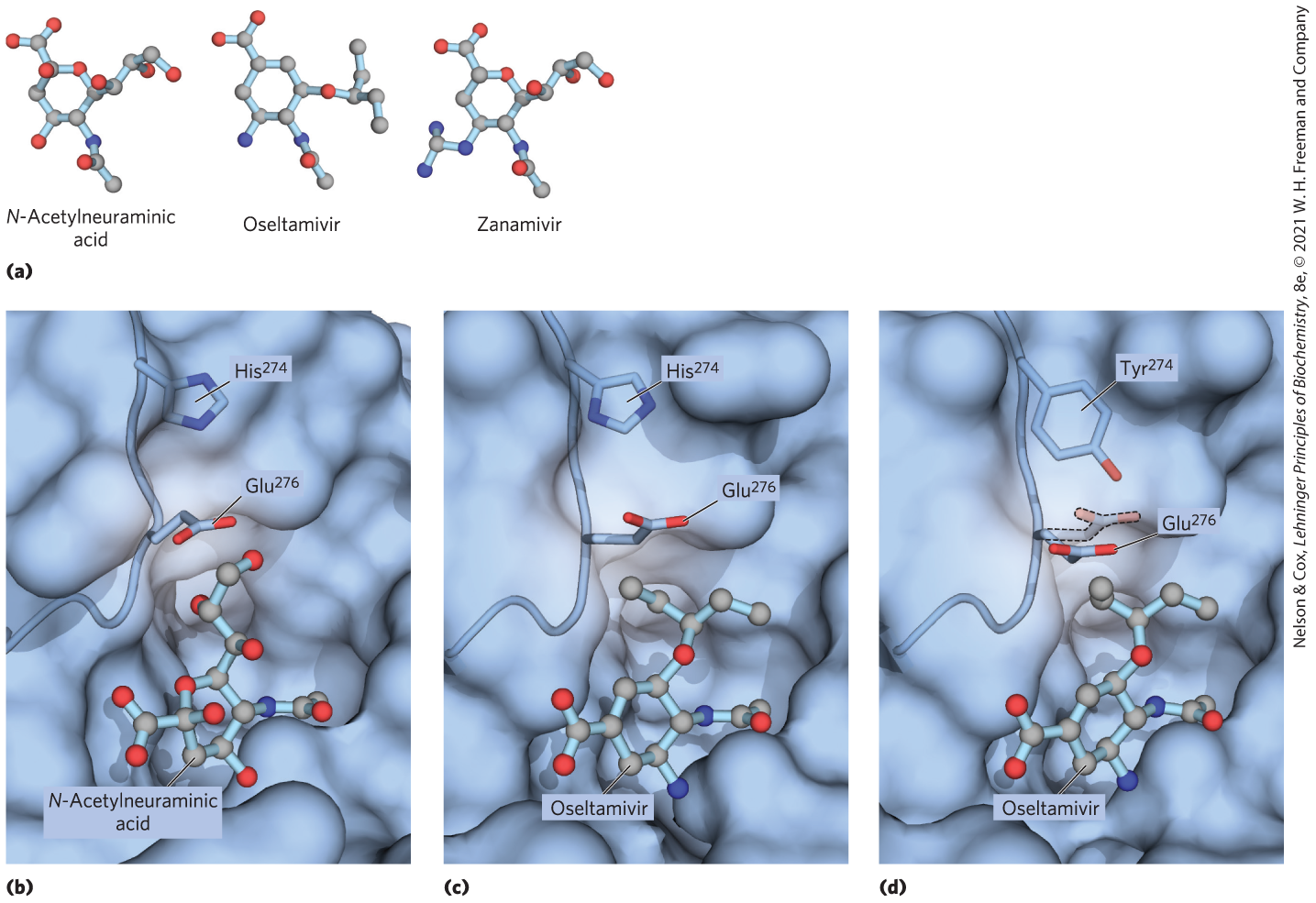
FIGURE 7-30 Binding site on influenza neuraminidase for N-acetylneuraminic acid and an antiviral drug. (a) The normal binding ligand for influenza neuraminidase is a sialic acid, N-acetylneuraminic acid. The drugs oseltamivir and zanamivir occupy the same site on the enzyme, competitively inhibiting it and blocking viral release from the host cell. (b) The normal interaction with N-acetylneuraminic acid in the binding site. (c) Oseltamivir can fit into this site by pushing a nearby Glu residue out of the way. (d) A mutation in the influenza virus’s gene for neuraminidase replaces a His near this Glu residue with the larger side chain of a Tyr. Now, oseltamivir is not as effective at pushing the Glu out of its way, and the drug binds much less well to the binding site, making the mutant virus effectively resistant to oseltamivir. [Data from (b) PDB ID 2BAT, J. N. Varghese et al., Proteins 14:327, 1992; (c) PDB ID 2HU4, R. J. Russell et al., Nature 443:45, 2006; (d) PDB ID 3CL0, P. J. Collins et al., Nature 453:1258, 2008.]
Some of the most devastating of the human parasitic diseases, widespread in much of the developing world, are caused by eukaryotic microorganisms that display unusual surface oligosaccharides, which in some cases are known to be protective for the parasites. These organisms include the trypanosomes, responsible for African sleeping sickness (see Box 6-1) and Chagas disease; Plasmodium falciparum, the malaria parasite; and Entamoeba histolytica, the causative agent of amoebic dysentery. The prospect of finding drugs that interfere with the synthesis of these unusual oligosaccharide chains, and therefore with the replication of the parasites, has inspired much recent work on the biosynthetic pathways of these oligosaccharides.
Lectins also act intracellularly, in sorting proteins for transportation to specific cellular compartments (see Chapter 27). For example, an oligosaccharide containing mannose 6-phosphate, recognized by a lectin, acts as a molecular “ZIP code” that tags newly synthesized proteins in the Golgi complex for transfer to the lysosome (see Fig. 27-41).
Lectin-Carbohydrate Interactions Are Highly Specific and Often Multivalent
The high density of information in the structure of oligosaccharides provides a sugar code with an essentially unlimited number of unique “words” small enough to be read by a single protein. In their carbohydrate-binding sites, lectins have a subtle molecular complementarity that allows interaction only with their correct carbohydrate binding partners. Often a divalent metal ion such as or is part of the binding site. Lectin-ligand interactions can have extraordinarily high specificity. The affinity between an oligosaccharide and an individual carbohydrate binding domain (CBD) of a lectin is sometimes modest (micromolar to millimolar values), but the effective affinity is often greatly increased by lectin multivalency, in which a single lectin molecule has multiple CBDs. In a cluster of oligosaccharides — as is commonly found on a membrane surface, for example — each oligosaccharide can engage one of the lectin’s CBDs, strengthening the interaction. When cells express multiple lectin receptors, the avidity of the interaction can be very high, enabling highly cooperative events such as cell attachment and rolling (Fig. 7-29).
X-ray crystallographic studies of the structure of the lectin that is the receptor for mannose 6-phosphate reveal details that explain the specificity of the binding and the role of a divalent cation in the lectin-sugar interaction (Fig. 7-31). When the protein tagged with mannose 6-phosphate reaches the lysosome (which has a lower internal pH than the Golgi complex), the receptor loses its affinity for mannose 6-phosphate and the tagged protein is released into the lysosomal matrix.
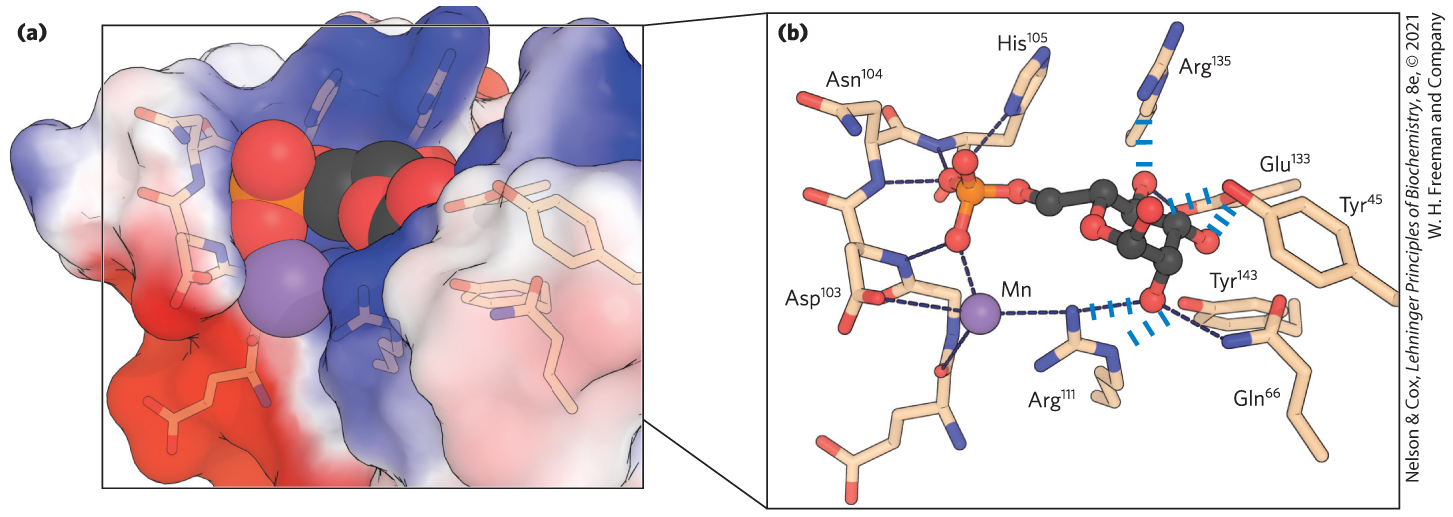
FIGURE 7-31 Details of a lectin-carbohydrate interaction. (a) Structure of the bovine mannose 6-phosphate receptor complexed with mannose 6-phosphate. The protein is represented as a surface contour image, showing the surface as predominantly negatively charged (red) or positively charged (blue). Mannose 6-phosphate is shown as a space-filling model; a manganese ion is shown as a violet sphere. (b) An enlarged view of the binding site. Mannose 6-phosphate is hydrogen-bonded to and coordinated with the manganese ion (shown smaller than its van der Waals radius, for clarity). Each hydroxyl group of mannose is hydrogen-bonded to the protein. The hydrogen-bonded to a phosphate oxygen of mannose 6-phosphate may be the residue that, when protonated at low pH, causes the receptor to release mannose 6-phosphate into the lysosome. [Data from PDB ID 1M6P, D. L. Roberts et al., Cell 93:639, 1998.]
In addition to such highly specific interactions, there are more general interactions that contribute to the binding of many carbohydrates to their lectins. For example, many sugars have a more polar side and a less polar side (Fig. 7-32); the more polar side hydrogen-bonds with the lectin, while the less polar side undergoes interactions with nonpolar amino acid residues through the hydrophobic effect. The sum of all these interactions produces high-affinity binding and high specificity of lectins for their carbohydrate ligands. This represents a kind of information transfer that is clearly central in many processes within and between cells. Figure 7-33 summarizes some of the biological interactions mediated by the sugar code.
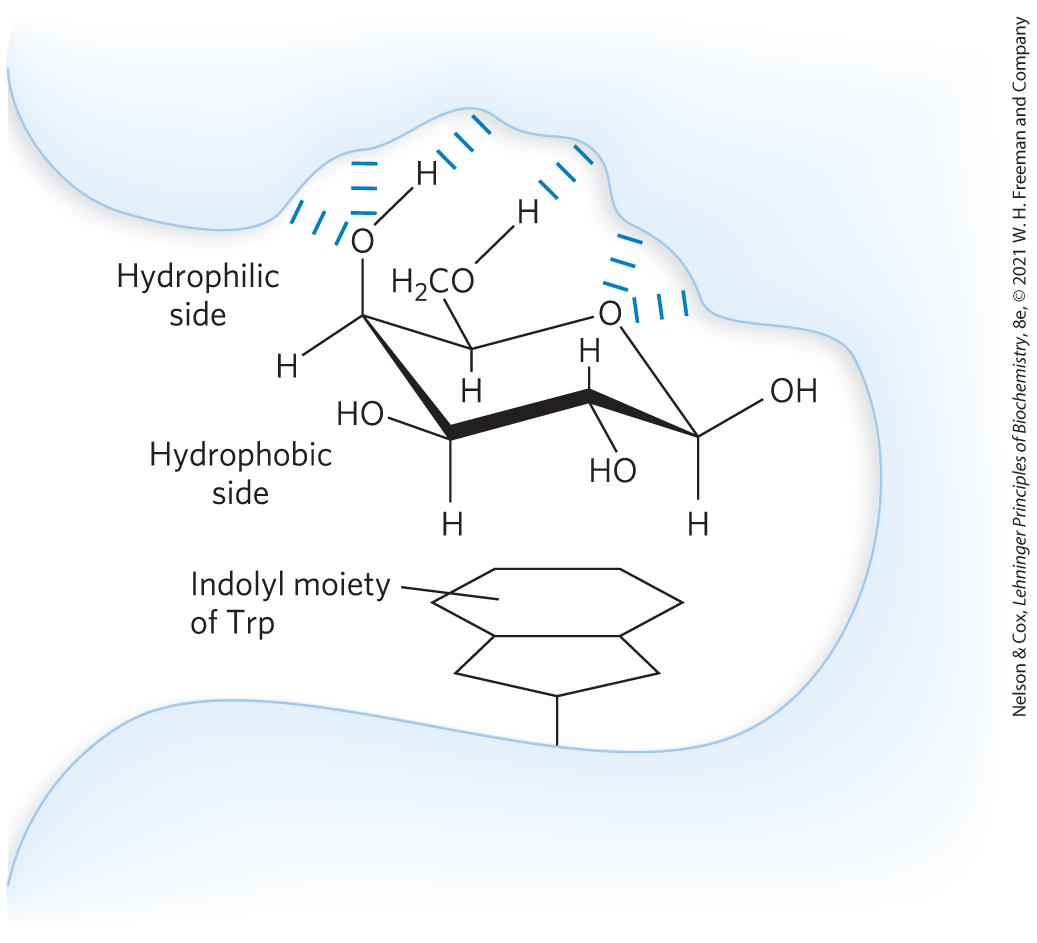
FIGURE 7-32 Interactions of sugar residues due to the hydrophobic effect. Sugar units such as galactose have a more polar side (the top of the chair as shown here, with the ring oxygen and several hydroxyls), available to hydrogen-bond with the lectin, and a less polar side that can interact with nonpolar side chains in the protein, such as the indole ring of Trp residues, through the hydrophobic effect. [Information from a figure provided by Dr. C.-W. von der Lieth, Heidelberg; H.-J. Gabius, Naturwissenschaften 87:108, 2000, Fig. 6.]
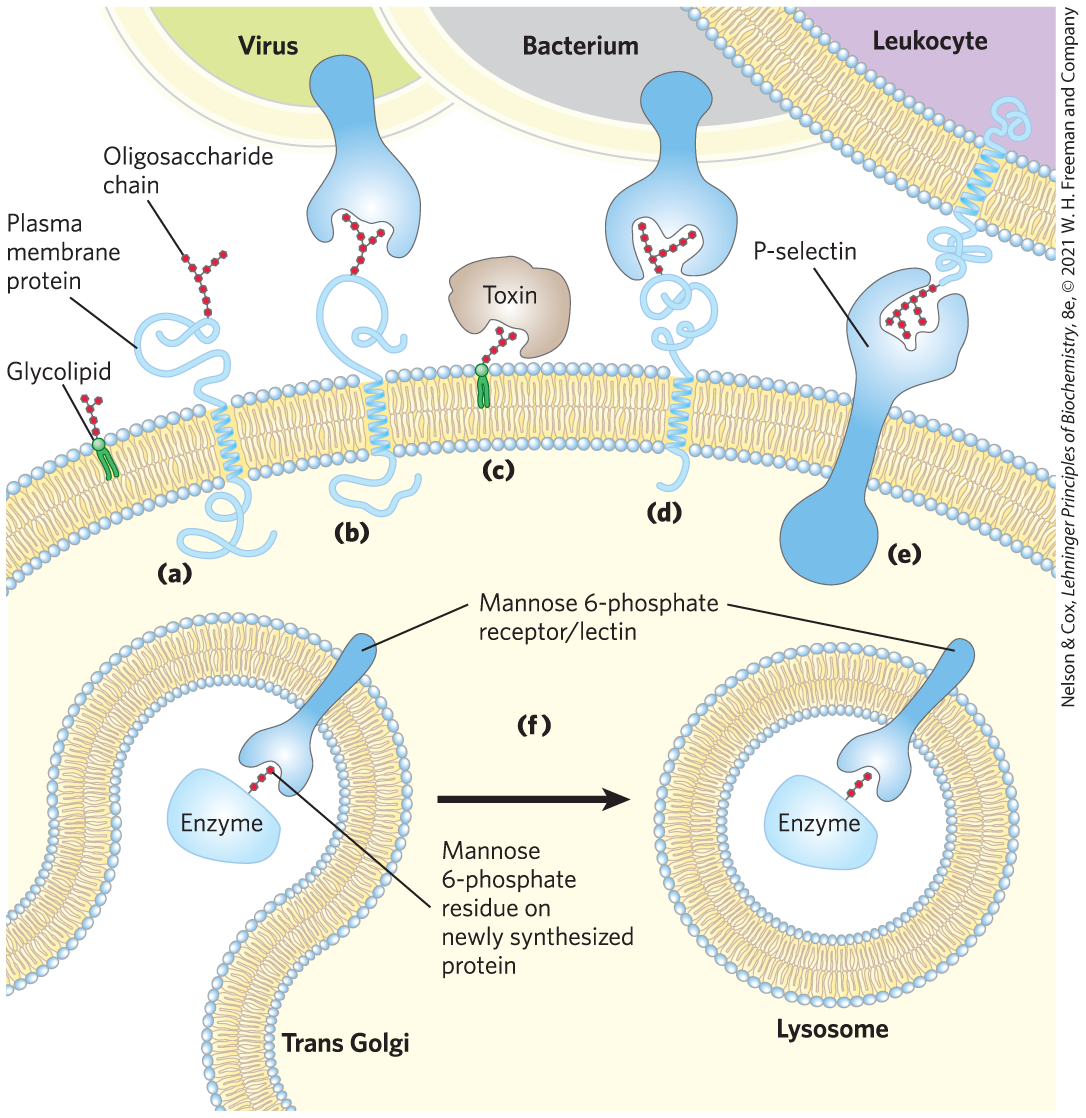
FIGURE 7-33 Role of oligosaccharides in recognition events at the cell surface and in the endomembrane system. (a) Oligosaccharides with unique structures (represented as strings of red hexagons) are components of a variety of glycoproteins or glycolipids on the outer surface of plasma membranes. Their oligosaccharide moieties are bound by extracellular lectins with high specificity and affinity. (b) Viruses that infect animal cells, such as the influenza virus, bind to cell surface glycoproteins as the first step in infection. (c) Bacterial toxins, such as the cholera and pertussis toxins, bind to a surface glycolipid before entering a cell. (d) Some bacteria adhere to and then colonize or infect animal cells. (e) Selectins (lectins) in the plasma membrane of certain cells mediate cell-cell interactions, such as those of leukocytes with the endothelial cells of the capillary wall at an infection site. (f) The mannose 6-phosphate receptor/lectin of the trans Golgi complex binds to the oligosaccharide of lysosomal enzymes, targeting them for transfer into the lysosome. [Information from N. Sharon and H. Lis, Sci. Am. 268 (January):82, 1993.]
SUMMARY 7.4 Carbohydrates as Informational Molecules: The Sugar Code
- Monosaccharides can be assembled into an almost limitless variety of oligosaccharides, which differ in the stereochemistry and position of glycosidic bonds, the type and orientation of substituent groups, and the number and type of branches. Glycans are far more information-dense than nucleic acids or proteins.
- The sugar specificity of many plant lectins makes them powerful laboratory reagents in glycobiology. Receptor/lectins in the surface of hepatocytes recognize glycoproteins that have lost their terminal sialic acid residue and mediate the normal uptake (by hepatocytes) and destruction of blood cells and of certain circulating glycoprotein hormones.
- Bacterial and viral pathogens and some eukaryotic parasites adhere to their animal cell targets through binding of lectins on the pathogens to oligosaccharides on the target cell surface, or vice versa. Interactions are often polyvalent.
- Structural studies of lectin-sugar complexes show the detailed complementarity between the two molecules, which accounts for the strength and specificity of lectin interactions with carbohydrates.
 cells use specific oligosaccharides to encode important information about intracellular targeting of proteins, cell-cell interactions, cell differentiation and tissue development, and extracellular signals. Our discussion uses just a few examples to illustrate the diversity of structure and the range of biological activity of the glycoconjugates. In
cells use specific oligosaccharides to encode important information about intracellular targeting of proteins, cell-cell interactions, cell differentiation and tissue development, and extracellular signals. Our discussion uses just a few examples to illustrate the diversity of structure and the range of biological activity of the glycoconjugates. In 

 an oligosaccharide containing mannose 6-phosphate, recognized by a lectin, acts as a molecular “ZIP code” that tags newly synthesized proteins in the Golgi complex for transfer to the lysosome (see
an oligosaccharide containing mannose 6-phosphate, recognized by a lectin, acts as a molecular “ZIP code” that tags newly synthesized proteins in the Golgi complex for transfer to the lysosome (see  Monosaccharides can be assembled into an almost limitless variety of oligosaccharides, which differ in the stereochemistry and position of glycosidic bonds, the type and orientation of substituent groups, and the number and type of branches. Glycans are far more information-dense than nucleic acids or proteins.
Monosaccharides can be assembled into an almost limitless variety of oligosaccharides, which differ in the stereochemistry and position of glycosidic bonds, the type and orientation of substituent groups, and the number and type of branches. Glycans are far more information-dense than nucleic acids or proteins.