17.1 Digestion, Mobilization, and Transport of Fats
Cells can obtain fatty acid fuels from four sources: fats consumed in the diet, fats stored in cells as lipid droplets, fats synthesized in one organ for export to another, and fats obtained by autophagy (which degrades the cell’s own organelles). Some species use all four sources under various circumstances; others use one or two. Vertebrates, for example, obtain fats in the diet; mobilize fats stored in specialized tissue (adipose tissue, consisting of cells called adipocytes); and, in the liver, convert excess dietary carbohydrates to fats for export to other tissues. During starvation, they can recycle lipids by autophagy. On average, 40% or more of the daily energy requirement of humans in highly industrialized countries is supplied by dietary triacylglycerols. Triacylglycerols provide more than half the energy requirements of some organs, particularly the liver, heart, and resting skeletal muscle. Stored triacylglycerols are virtually the sole source of energy in hibernating animals and migrating birds. Vascular plants mobilize fats stored in seeds during germination, but do not otherwise depend on fats for energy.
Dietary Fats Are Absorbed in the Small Intestine
In vertebrates, before ingested triacylglycerols can be absorbed through the intestinal wall they must be converted from insoluble macroscopic fat particles to finely dispersed microscopic micelles. This solubilization is carried out by bile salts, such as taurocholic acid (p. 352), which are synthesized from cholesterol in the liver, stored in the gallbladder, and released into the small intestine after ingestion of a fatty meal. Bile salts are amphipathic compounds that act as biological detergents, emulsifying dietary fats into mixed micelles of bile salts and triacylglycerols (Fig. 17-1, step ). Micelle formation enormously increases the fraction of lipid molecules accessible to the action of water-soluble lipases in the intestine, and lipase action converts triacylglycerols to monoacylglycerols (monoglycerides) and diacylglycerols (diglycerides) and free fatty acids (step ). These products of lipase action diffuse or are transported into the epithelial cells lining the intestinal surface (the intestinal mucosa) (step ), where they are reconverted to triacylglycerols and packaged with dietary cholesterol and specific apolipoproteins (from the Greek apo, meaning “detached” or “separate,” designating the protein in its lipid-free form) into lipoprotein aggregates called chylomicrons (step ). It is the protein moieties that target triacylglycerols, phospholipids, cholesterol, and cholesteryl esters for transport between organs.
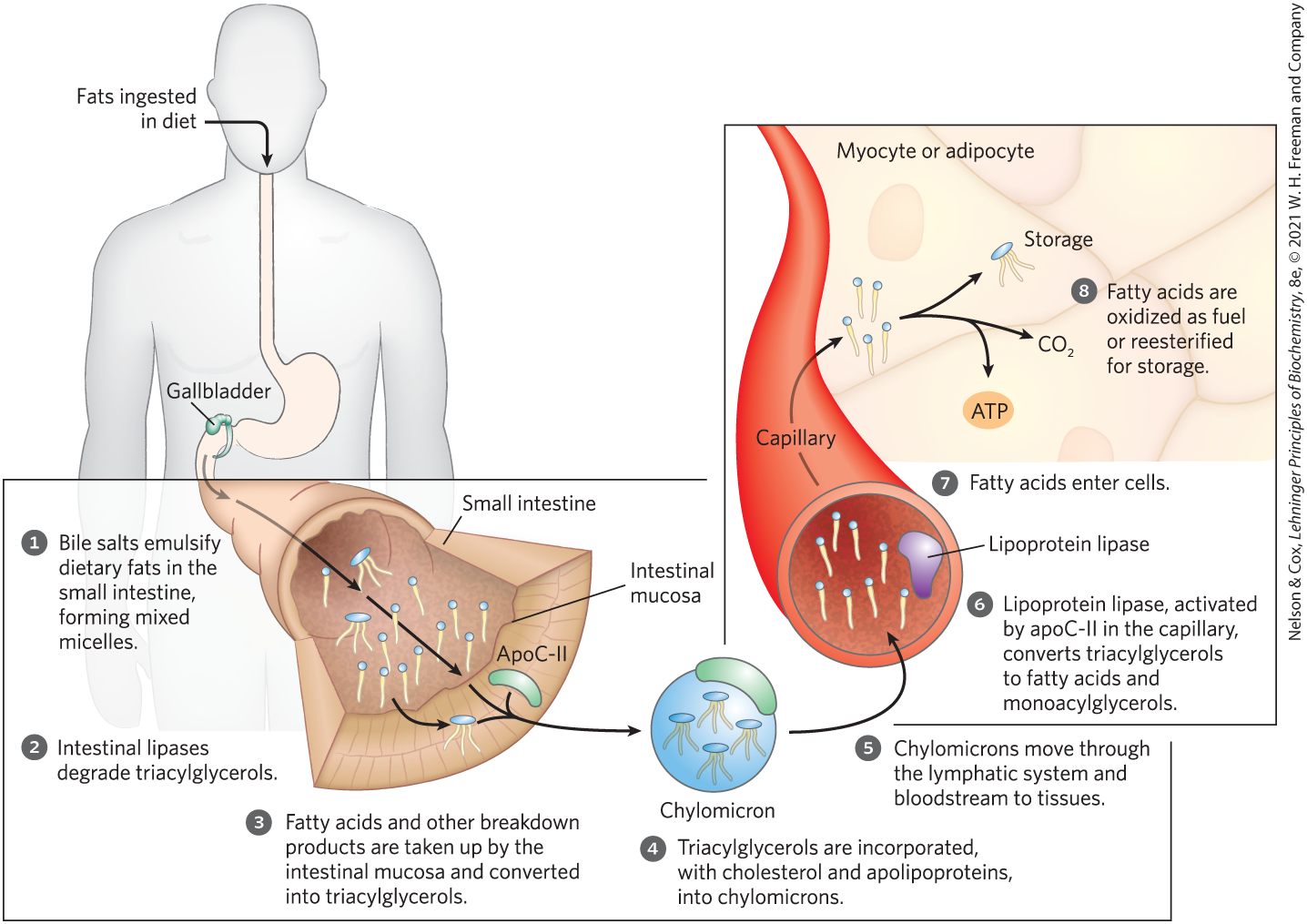
FIGURE 17-1 Processing of dietary lipids in vertebrates. Digestion and absorption of dietary lipids occur in the small intestine, and the fatty acids released from triacylglycerols are packaged and delivered to muscle and adipose tissues. The eight steps are discussed in the text.
Apolipoproteins combine with lipids to form several classes of lipoprotein particles, spherical aggregates with hydrophobic lipids at the core and hydrophilic protein side chains and lipid head groups at the surface. Various combinations of lipid and protein produce particles of different densities, ranging from chylomicrons and very-low-density lipoproteins (VLDL) to very-high-density lipoproteins (VHDL). These particles can be separated by ultracentrifugation. The structures of these lipoprotein particles and their roles in lipid transport are detailed in Chapter 21 (Fig. 21-39).
Apolipoprotein B-48 (apoB-48) is the primary protein component of chylomicrons. In lipid uptake from the intestine, chylomicrons move from the intestinal mucosa into the lymphatic system, and then enter the blood, where they can exchange apolipoproteins with other types of circulating lipoprotein. In the blood, chylomicrons pick up apolipoprotein C-II (apoC-II) from high-density lipoprotein (HDL) particles and are carried to muscle and adipose tissue (Fig. 17-1, step ). In the capillaries of these tissues, the extracellular enzyme lipoprotein lipase, activated by apoC-II, hydrolyzes triacylglycerols to free fatty acids and monoacylglycerols (step ), which are taken up by specific transporters in the plasma membranes of cells in the target tissues (step ). In muscle, the fatty acids are oxidized for energy; in adipose tissue, they are reesterified for storage as triacylglycerols (step ).
The remnants of chylomicrons, depleted of most of their triacylglycerols but still containing cholesterol and apolipoproteins, travel in the blood to the liver, where they are taken up by endocytosis mediated by receptors for their apolipoproteins. Triacylglycerols that enter the liver by this route may be oxidized to provide energy or to provide precursors for the synthesis of ketone bodies, as described in Section 17.3.
When the diet contains more fatty acids than are needed immediately for fuel or as precursors, the liver converts them to triacylglycerols, which are packaged with specific apolipoproteins into VLDLs. These VLDLs are secreted by hepatocytes and transported in the blood to adipose tissue, where the triacylglycerols are removed and stored in lipid droplets within adipocytes.
Hormones Trigger Mobilization of Stored Triacylglycerols
Neutral lipids (triacylglycerols, sterols, and steroyl esters) are stored in adipocytes (and in steroid-synthesizing cells of the adrenal cortex, ovary, and testis) in lipid droplets, which have a core of triacylglycerols and sterol esters surrounded by a monolayer of phospholipids. The surface of these droplets is coated with perilipins, a family of proteins that restrict access to lipid droplets, preventing untimely lipid mobilization. When hormones signal the need for metabolic energy, triacylglycerols stored in adipose tissue are mobilized (brought out of storage) and transported to tissues (skeletal muscle, heart, and renal cortex) in which fatty acids can be oxidized for energy production. The hormones epinephrine and glucagon, secreted in response to low blood glucose levels or a fight-or-flight situation, stimulate the enzyme adenylyl cyclase in the adipocyte plasma membrane (Fig. 17-2), which produces the intracellular second messenger cyclic AMP (cAMP; see Fig. 12-4). Cyclic AMP–dependent protein kinase (PKA) triggers changes that open the lipid droplet to the action of three cytosolic lipases, which act on tri-, di-, and monoacylglycerols, releasing fatty acids and glycerol.
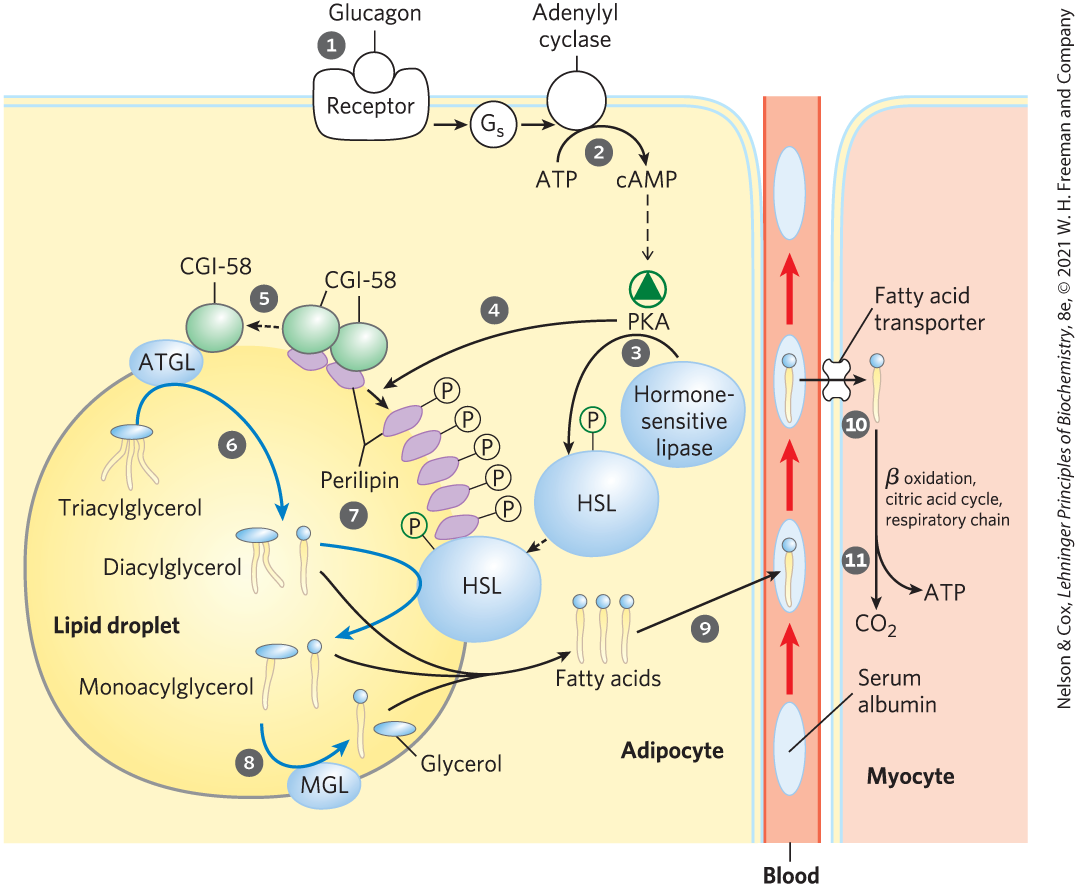
FIGURE 17-2 Mobilization of triacylglycerols stored in adipose tissue. When low levels of glucose in the blood trigger the release of glucagon, the hormone binds its receptor in the adipocyte membrane and thus stimulates adenylyl cyclase, via a G protein, to produce cAMP. This activates PKA, which phosphorylates the hormone-sensitive lipase (HSL) and perilipin molecules on the surface of the lipid droplet. Phosphorylation of perilipin causes dissociation of the protein CGI-58 from perilipin. CGI-58 (comparative gene identification-58), a protein closely associated with lipid droplets, then recruits adipose triacylglycerol lipase (ATGL) to the droplet surface and stimulates its lipase activity. Active ATGL converts triacylglycerols to diacylglycerols. The phosphorylated perilipin associates with phosphorylated HSL, allowing it access to the surface of the lipid droplet, where it converts diacylglycerols to monoacylglycerols. A third lipase, monoacylglycerol lipase (MGL), hydrolyzes monoacylglycerols. Fatty acids leave the adipocyte, and are transported in the blood bound to serum albumin. They are released from the albumin and enter a myocyte via a specific fatty acid transporter. In the myocyte, fatty acids are oxidized to , and the energy of oxidation is conserved in ATP, which fuels muscle contraction and other energy-requiring metabolism in the myocyte.
The fatty acids thus released (free fatty acids, FFAs) pass from the adipocyte into the blood, where they bind to the blood protein serum albumin (Fig. 17-3). This protein , which makes up about half of the total serum protein, noncovalently binds as many as seven fatty acids. Bound to this soluble protein, the otherwise insoluble fatty acids are carried to tissues such as skeletal muscle, heart, and renal cortex. In these target tissues, fatty acids dissociate from albumin and are moved by plasma membrane transporters into cells to serve as fuel.
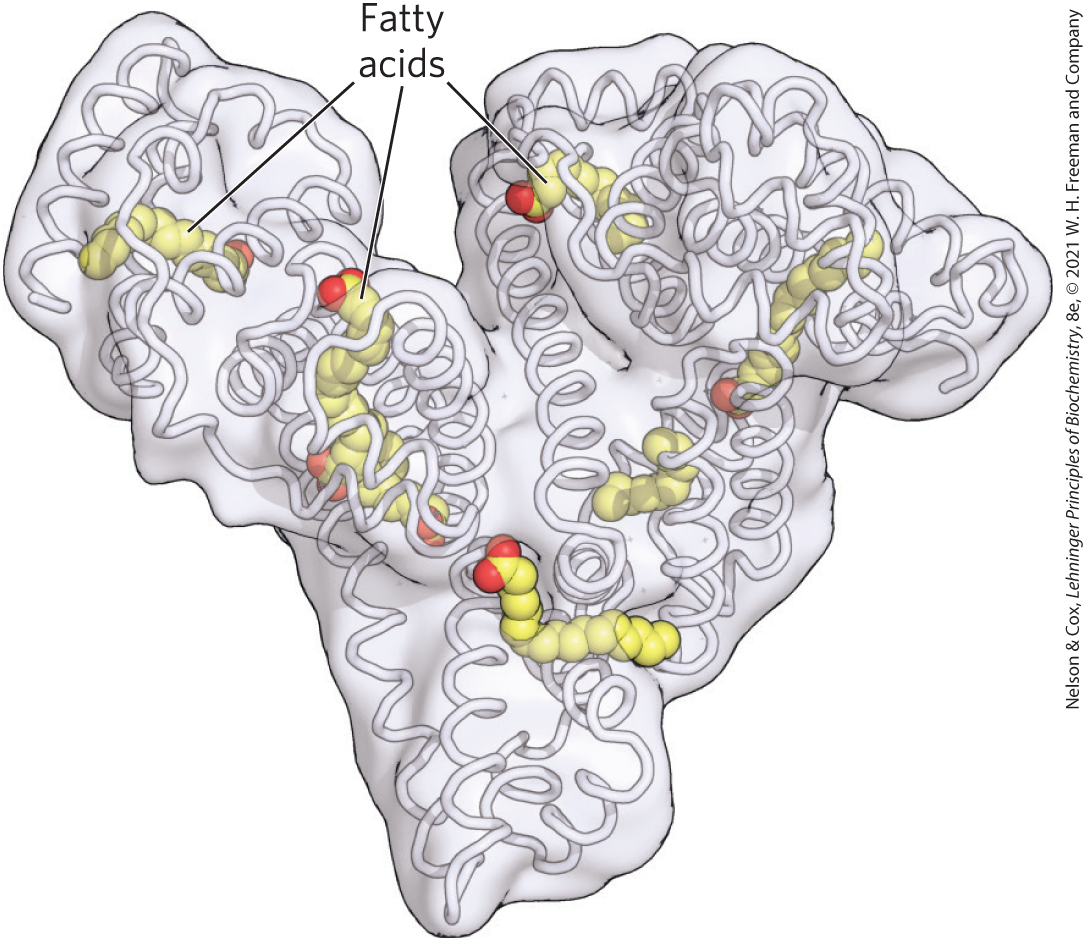
FIGURE 17-3 Human serum albumin complexed with stearate. Each day the human liver releases 10–15 g of serum albumin into the bloodstream, where this workhorse protein transports many ligands, drugs, and particularly fatty acids through the blood. The structure has nooks and crannies that can carry up to seven fatty acids. Its passengers are both hydrophobic and hydrophilic, and include steroid hormones, the blood thinner warfarin, the antibiotic penicillin, the anti-inflammatory drug ibuprofen, and the anxiolytic diazepam.
About 95% of the biologically available energy of triacylglycerols resides in their three long-chain fatty acids; only 5% is contributed by the glycerol moiety. The glycerol released by lipase action is phosphorylated by glycerol kinase (Fig. 17-4), and the resulting glycerol 3-phosphate is oxidized to dihydroxyacetone phosphate. The glycolytic enzyme triose phosphate isomerase converts this compound to glyceraldehyde 3-phosphate, which is oxidized via glycolysis.
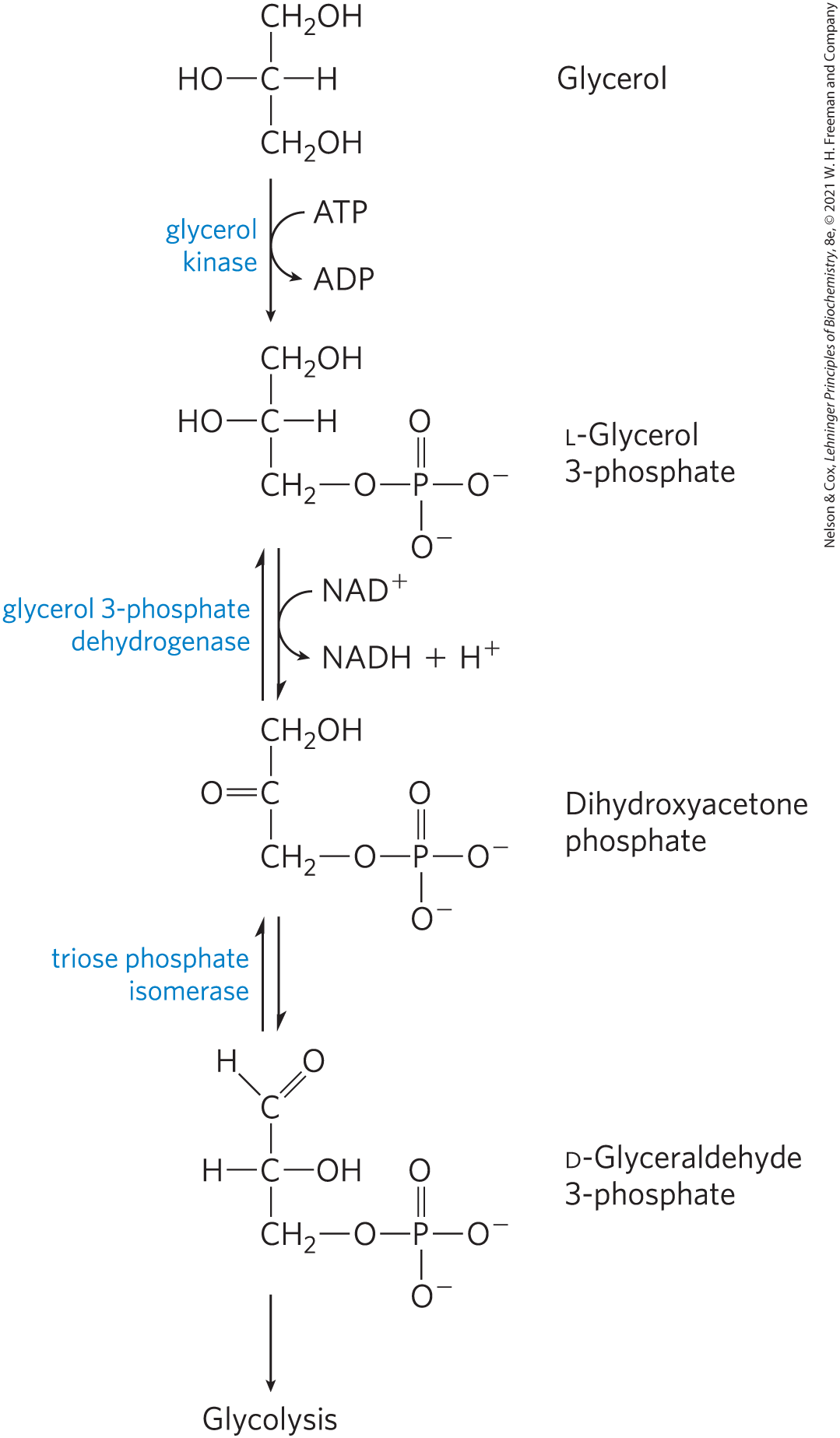
FIGURE 17-4 Entry of glycerol into the glycolytic pathway.
Fatty Acids Are Activated and Transported into Mitochondria
The enzymes of fatty acid oxidation in animal cells are located in the mitochondrial matrix, as demonstrated in 1948 by Eugene P. Kennedy and Albert Lehninger. Short- and medium-chain fatty acids, those with chain lengths of 12 or fewer carbons, enter mitochondria without the help of membrane transporters. Long-chain fatty acids, those with 14 or more carbons, which constitute the majority of the FFAs obtained in the diet or released from adipose tissue, cannot pass directly through the mitochondrial membranes: they must be transported through the carnitine shuttle. First, the fatty acid must be activated by a fatty acyl–CoA synthetase isozyme specific for long-chain fatty acids. The isozymes are present in the outer mitochondrial membrane, where they promote the general reaction
Fatty acyl–CoA synthetases catalyze the formation of a thioester linkage between the fatty acid carboxyl group and the thiol group of coenzyme A to yield a fatty acyl–CoA, coupled to the cleavage of ATP to AMP and . (Recall the description of this reaction in Chapter 13, which illustrates how the free energy released by cleavage of phosphoanhydride bonds in ATP could be coupled to the formation of a high-energy compound; p. 485.) The reaction catalyzed by a fatty acyl–CoA synthetase occurs in two steps and involves a fatty acyl–adenylate intermediate (Fig. 17-5).
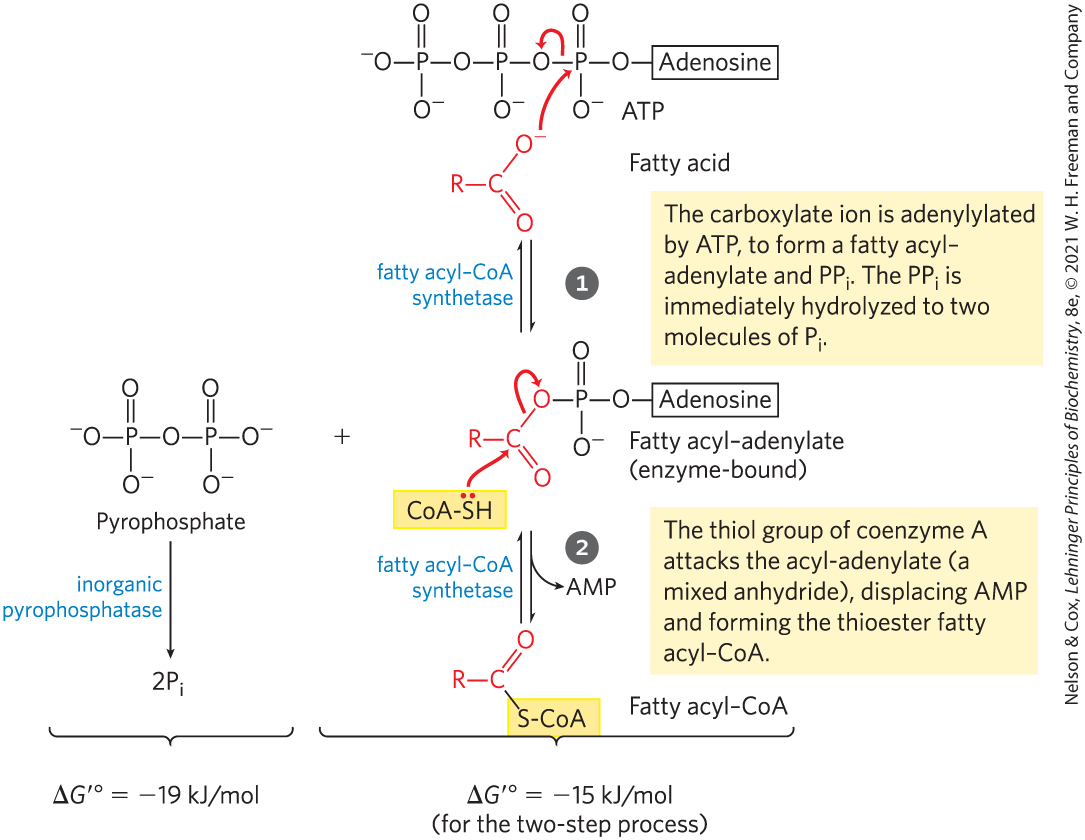
MECHANISM FIGURE 17-5 Activation of a fatty acid by conversion to a fatty acyl–CoA. Formation of the fatty acyl–CoA derivative occurs in two steps, catalyzed by fatty acyl–CoA synthetase. Hydrolysis of the pyrophosphate created in the first step of that reaction is catalyzed by inorganic pyrophosphatase. The overall reaction is highly exergonic.
Fatty acyl–CoAs, like acetyl-CoA, are high-energy compounds; their hydrolysis to FFAs and CoA has a large, negative standard free-energy change. The formation of a fatty acyl–CoA is made more favorable by the hydrolysis of two high-energy bonds in ATP; the pyrophosphate formed in the activation reaction is immediately hydrolyzed by inorganic pyrophosphatase (left side of Fig. 17-5), which pulls the preceding activation reaction in the direction of fatty acyl–CoA formation. The overall reaction (three steps) is
(17-1)
Fatty acyl–CoA esters formed on the cytosolic side of the outer mitochondrial membrane can be transported into the mitochondrion and oxidized to produce ATP, or they can be used in the cytosol to synthesize membrane lipids.
Fatty acyl–CoAs destined for mitochondrial oxidation must be attached to carnitine to be shuttled across the inner mitochondrial membrane.

In a transesterification catalyzed by carnitine acyltransferase 1, CAT1 (also called carnitine palmitoyltransferase 1, CPT1) in the outer mitochondrial membrane, the fatty acyl–CoA is transiently attached to the hydroxyl group of carnitine to form fatty acyl–carnitine (Fig. 17-6). The fatty acyl–carnitine ester then diffuses across the intermembrane space and enters the matrix by passive transport through the acyl-carnitine/carnitine cotransporter of the inner mitochondrial membrane. This cotransporter moves one molecule of carnitine from the matrix to the intermembrane space as one molecule of fatty acyl–carnitine moves into the matrix. Once inside the matrix, the fatty acyl group is transferred from carnitine back to coenzyme A from the intramitochondrial pool by carnitine acyltransferase 2 (CAT2, or CPT2). This isozyme, located on the inner face of the inner mitochondrial membrane, regenerates fatty acyl–CoA and releases it, along with free carnitine, into the matrix. The carnitine is then available to be transferred back through the acyl-carnitine/carnitine cotransporter to be used to shuttle the next fatty acid across. Once inside the mitochondrion, the fatty acyl–CoA is acted upon by a set of enzymes in the matrix.
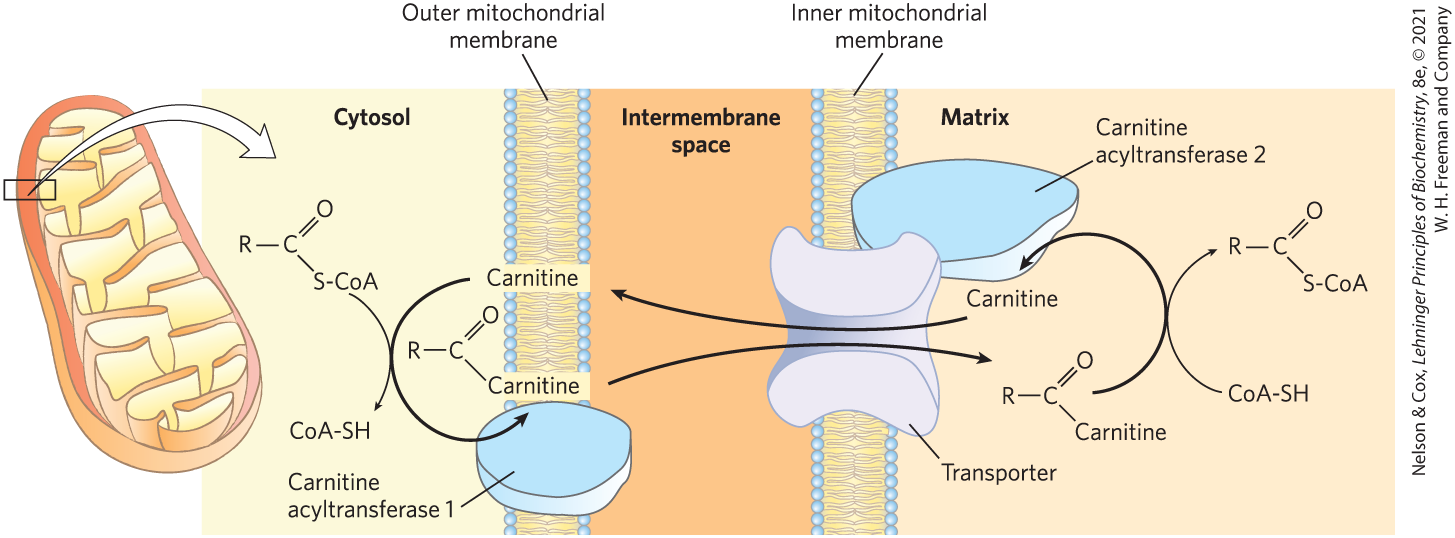
FIGURE 17-6 Fatty acid entry into mitochondria via the acyl-carnitine/carnitine transporter. Fatty acyl–carnitine formed on the outer mitochondrial membrane moves into the matrix by passive cotransport through the inner membrane. In the matrix, the acyl group is transferred to mitochondrial coenzyme A, freeing carnitine to leave the matrix through the same transporter.
This process for transferring fatty acids into the mitochondrion — esterification to CoA, transesterification to carnitine, translocation across the inner membrane, and transesterification back to CoA — links two separate pools of coenzyme A and of fatty acyl–CoA, one in the cytosol, the other in mitochondria. These pools have different functions. Coenzyme A in the mitochondrial matrix is largely used in oxidative degradation of pyruvate, fatty acids, and some amino acids, whereas cytosolic coenzyme A is used in the biosynthesis of fatty acids (see Fig. 21-10). Fatty acyl–CoA in the cytosolic pool can be used there for membrane lipid synthesis or can be moved into the mitochondrial matrix for oxidation and ATP production. Conversion to the carnitine ester commits the fatty acyl moiety to the oxidative fate. The carnitine-mediated entry process is the rate-limiting step for oxidation of fatty acids in mitochondria and, as discussed later, is a control point. Carnitine acyltransferase 1 is inhibited by malonyl-CoA, the first intermediate in fatty acid synthesis (see Fig. 21-1). This inhibition prevents the simultaneous synthesis and degradation of fatty acids, a wasteful futile cycle.
SUMMARY 17.1 Digestion, Mobilization, and Transport of Fats
- Dietary triacylglycerols are emulsified in the small intestine by bile salts, hydrolyzed by intestinal lipases, absorbed by intestinal epithelial cells, and reconverted into triacylglycerols. They are combined with specific apolipoproteins for passage through the blood and lymph to adipose tissue, where they are stored as lipid droplets.
- Triacylglycerols stored in adipose tissue are mobilized by a hormone-sensitive triacylglycerol lipase. The released fatty acids bind to serum albumin and are carried in the blood to the heart, skeletal muscle, and other tissues that use fatty acids for fuel.
- Once inside cells, fatty acids are activated at the outer mitochondrial membrane by conversion to fatty acyl–CoA thioesters. Fatty acyl–CoA that is to be oxidized enters mitochondria via the carnitine shuttle, which is a major control point. Malonyl-CoA, the first intermediate in fatty acid synthesis, inhibits carnitine acyltransferase 1, assuring that fatty acid oxidation and fatty acid synthesis do not occur simultaneously.
 ). Micelle formation enormously increases the fraction of lipid molecules accessible to the action of water-soluble lipases in the intestine, and lipase action converts triacylglycerols to monoacylglycerols (monoglycerides) and diacylglycerols (diglycerides) and free fatty acids (step
). Micelle formation enormously increases the fraction of lipid molecules accessible to the action of water-soluble lipases in the intestine, and lipase action converts triacylglycerols to monoacylglycerols (monoglycerides) and diacylglycerols (diglycerides) and free fatty acids (step  ). These products of lipase action diffuse or are transported into the epithelial cells lining the intestinal surface (the intestinal mucosa) (step
). These products of lipase action diffuse or are transported into the epithelial cells lining the intestinal surface (the intestinal mucosa) (step  ), where they are reconverted to triacylglycerols and packaged with dietary cholesterol and specific
), where they are reconverted to triacylglycerols and packaged with dietary cholesterol and specific  ). It is the protein moieties that target triacylglycerols, phospholipids, cholesterol, and cholesteryl esters for transport between organs.
). It is the protein moieties that target triacylglycerols, phospholipids, cholesterol, and cholesteryl esters for transport between organs. ). In the capillaries of these tissues, the extracellular enzyme lipoprotein lipase, activated by apoC-II, hydrolyzes triacylglycerols to free fatty acids and monoacylglycerols (step
). In the capillaries of these tissues, the extracellular enzyme lipoprotein lipase, activated by apoC-II, hydrolyzes triacylglycerols to free fatty acids and monoacylglycerols (step  ), which are taken up by specific transporters in the plasma membranes of cells in the target tissues (step
), which are taken up by specific transporters in the plasma membranes of cells in the target tissues (step  ). In muscle, the fatty acids are oxidized for energy; in adipose tissue, they are reesterified for storage as triacylglycerols (step
). In muscle, the fatty acids are oxidized for energy; in adipose tissue, they are reesterified for storage as triacylglycerols (step  ).
). When hormones signal the need for metabolic energy, triacylglycerols stored in adipose tissue are mobilized (brought out of storage) and transported to tissues (skeletal muscle, heart, and renal cortex) in which fatty acids can be oxidized for energy production. The hormones epinephrine and glucagon, secreted in response to low blood glucose levels or a fight-or-flight situation, stimulate the enzyme adenylyl cyclase in the adipocyte plasma membrane (
When hormones signal the need for metabolic energy, triacylglycerols stored in adipose tissue are mobilized (brought out of storage) and transported to tissues (skeletal muscle, heart, and renal cortex) in which fatty acids can be oxidized for energy production. The hormones epinephrine and glucagon, secreted in response to low blood glucose levels or a fight-or-flight situation, stimulate the enzyme adenylyl cyclase in the adipocyte plasma membrane ( Fatty acids leave the adipocyte, and are transported in the blood bound to serum albumin. They are released from the albumin and
Fatty acids leave the adipocyte, and are transported in the blood bound to serum albumin. They are released from the albumin and  enter a myocyte via a specific fatty acid transporter.
enter a myocyte via a specific fatty acid transporter.  In the myocyte, fatty acids are oxidized to
In the myocyte, fatty acids are oxidized to  Fatty acyl–CoA synthetases catalyze the formation of a thioester linkage between the fatty acid carboxyl group and the thiol group of coenzyme A to yield a fatty acyl–CoA, coupled to the cleavage of ATP to AMP and . (Recall the description of this reaction in
Fatty acyl–CoA synthetases catalyze the formation of a thioester linkage between the fatty acid carboxyl group and the thiol group of coenzyme A to yield a fatty acyl–CoA, coupled to the cleavage of ATP to AMP and . (Recall the description of this reaction in  The carnitine-mediated entry process is the rate-limiting step for oxidation of fatty acids in mitochondria and, as discussed later, is a control point. Carnitine acyltransferase 1 is inhibited by malonyl-CoA, the first intermediate in fatty acid synthesis (see
The carnitine-mediated entry process is the rate-limiting step for oxidation of fatty acids in mitochondria and, as discussed later, is a control point. Carnitine acyltransferase 1 is inhibited by malonyl-CoA, the first intermediate in fatty acid synthesis (see  Dietary triacylglycerols are emulsified in the small intestine by bile salts, hydrolyzed by intestinal lipases, absorbed by intestinal epithelial cells, and reconverted into triacylglycerols. They are combined with specific apolipoproteins for passage through the blood and lymph to adipose tissue, where they are stored as lipid droplets.
Dietary triacylglycerols are emulsified in the small intestine by bile salts, hydrolyzed by intestinal lipases, absorbed by intestinal epithelial cells, and reconverted into triacylglycerols. They are combined with specific apolipoproteins for passage through the blood and lymph to adipose tissue, where they are stored as lipid droplets.