17.3 Ketone Bodies
In humans and most other mammals, acetyl-CoA formed in the liver during oxidation of fatty acids can either enter the citric acid cycle (stage 2 of Fig. 17-7) or undergo conversion to the “ketone bodies,” acetone, acetoacetate, and d-β-hydroxybutyrate, for export to other tissues. (The term “bodies” is an historical artifact; the compounds are soluble in blood and urine, not particulate, and not all of them are ketones.)
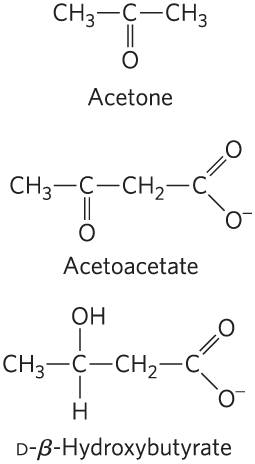
Acetone, produced in smaller quantities than the other ketone bodies, is exhaled. Acetoacetate and d-β-hydroxybutyrate are transported by the blood to tissues other than the liver (extrahepatic tissues), where they are converted to acetyl-CoA and oxidized in the citric acid cycle, providing much of the energy required by tissues such as skeletal and heart muscle and the renal cortex. The brain, which preferentially uses glucose as fuel, can adapt to the use of acetoacetate and d-β-hydroxybutyrate under starvation conditions, when glucose is unavailable. The brain cannot use fatty acids as fuel, because they do not cross the blood-brain barrier. The production and export of ketone bodies from the liver to extrahepatic tissues allows continued oxidation of fatty acids in the liver when acetyl-CoA is not being oxidized in the citric acid cycle.
Ketone Bodies, Formed in the Liver, Are Exported to Other Organs as Fuel
The first step in the formation of acetoacetate, occurring in the liver (Fig. 17-16), is the enzymatic condensation of two molecules of acetyl-CoA, catalyzed by thiolase; this is simply the reversal of the last step of β oxidation. The acetoacetyl-CoA then condenses with another molecule of acetyl-CoA to form β-hydroxy-β-methylglutaryl-CoA (HMG-CoA), which is cleaved to free acetoacetate and acetyl-CoA. The acetoacetate is reversibly reduced by d-β-hydroxybutyrate dehydrogenase, a mitochondrial enzyme, to d-β-hydroxybutyrate. This enzyme is specific for the d stereoisomer; it does not act on l-β-hydroxyacyl-CoAs and is distinct from l-β-hydroxyacyl-CoA dehydrogenase of the β-oxidation pathway. This difference in stereospecificity of the two enzymes that use β-hydroxyacyl-CoAs as substrates in fatty acid breakdown and fatty acid synthesis means that the cell can maintain separate pools of β-hydroxyacyl-CoAs, earmarked for either breakdown or synthesis.
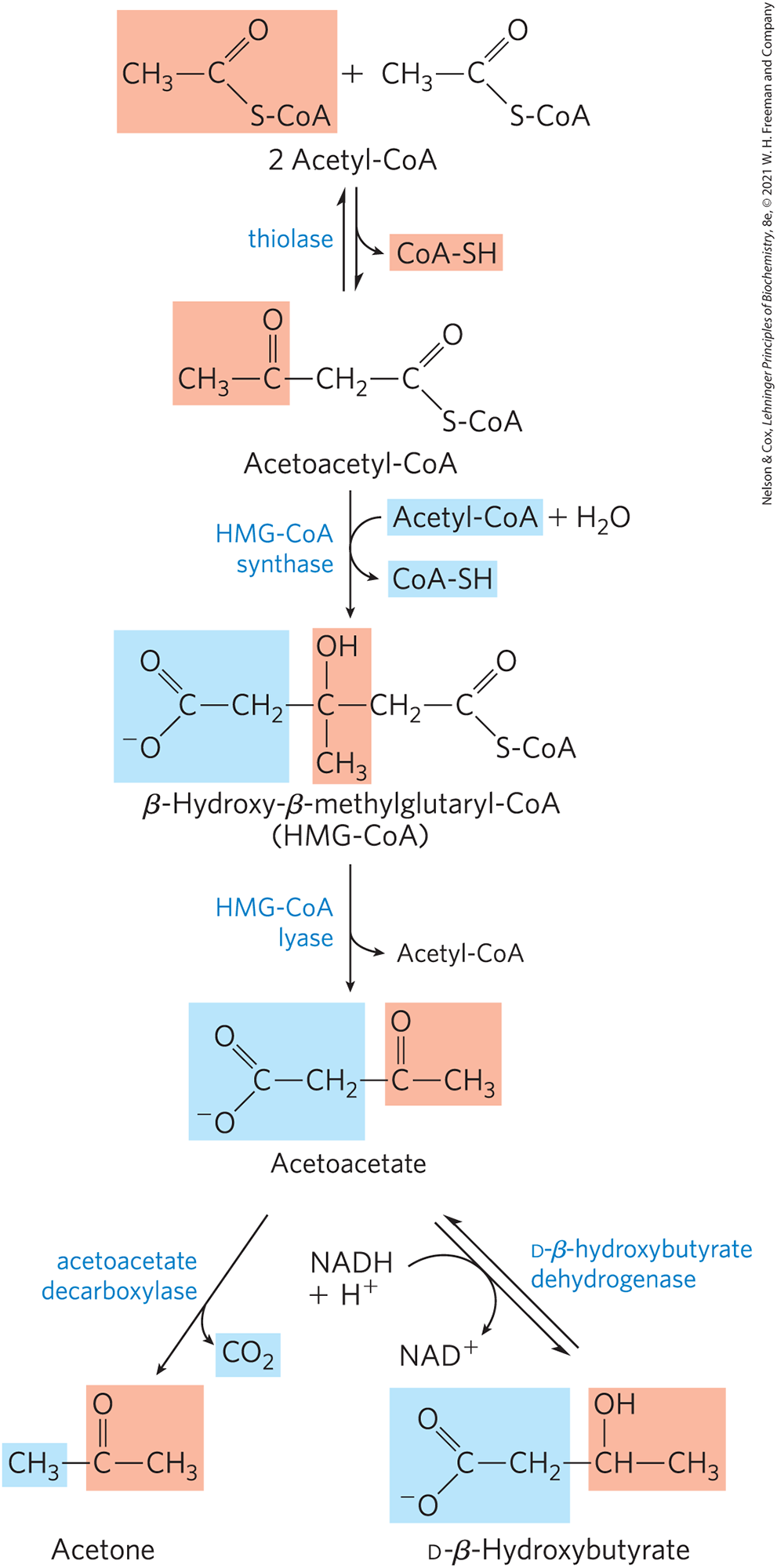
FIGURE 17-16 Formation of ketone bodies from acetyl-CoA. Healthy, well-nourished individuals produce ketone bodies at a relatively low rate. When acetyl-CoA accumulates (as in starvation or untreated diabetes, for example), thiolase catalyzes the condensation of two acetyl-CoA molecules to acetoacetyl-CoA, the parent compound of the three ketone bodies. The reactions of ketone body formation occur in the matrix of liver mitochondria. The six-carbon compound β-hydroxy-β-methylglutaryl-CoA (HMG-CoA) is also an intermediate of sterol biosynthesis, but the enzyme that forms HMG-CoA in that pathway is cytosolic. HMG-CoA lyase is present only in the mitochondrial matrix.
In extrahepatic tissues, d-β-hydroxybutyrate is oxidized to acetoacetate by d-β-hydroxybutyrate dehydrogenase (Fig. 17-17). The acetoacetate is activated to its coenzyme A ester by transfer of CoA from succinyl-CoA, an intermediate of the citric acid cycle (see Fig. 16-7), in a reaction catalyzed by β-ketoacyl-CoA transferase, also called thiophorase. The acetoacetyl-CoA is then cleaved by thiolase to yield two molecules of acetyl-CoA, which enter the citric acid cycle. Thus the ketone bodies are used as fuels in all tissues except liver, which lacks β-ketoacyl-CoA transferase. The liver is therefore a producer of ketone bodies for other tissues, but not a consumer.
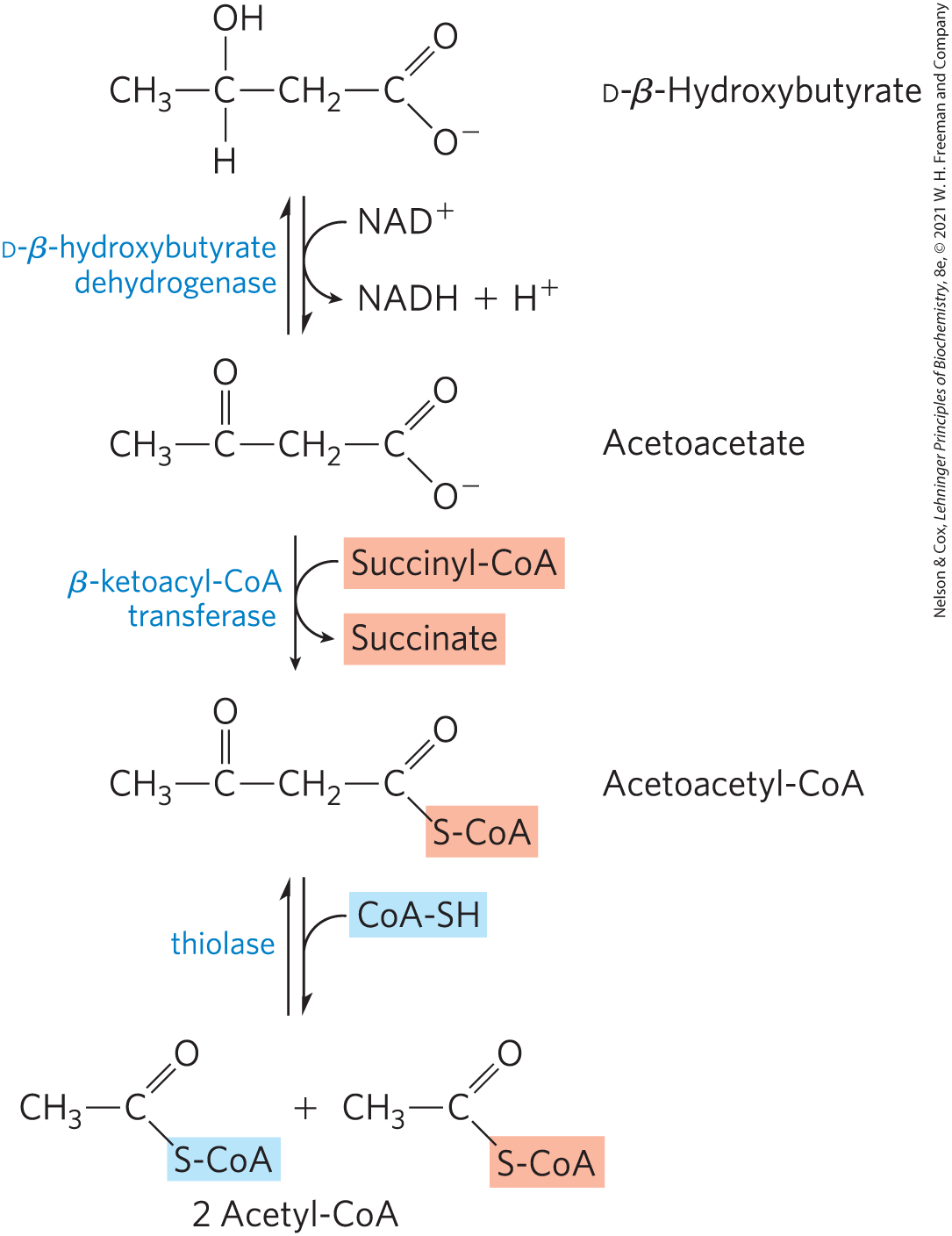
FIGURE 17-17 D-β-Hydroxybutyrate as a fuel. d-β-Hydroxybutyrate, synthesized in the liver, passes into the blood and thus to other tissues, where it is converted in three steps to acetyl-CoA. It is first oxidized to acetoacetate, which is activated with coenzyme A donated from succinyl-CoA, then split by thiolase. The acetyl-CoA thus formed enters the citric acid cycle.
The production and export of ketone bodies by the liver allows continued oxidation of fatty acids with only minimal oxidation of acetyl-CoA. When intermediates of the citric acid cycle are being siphoned off for glucose synthesis by gluconeogenesis, for example, oxidation of cycle intermediates slows — and so does acetyl-CoA oxidation. Moreover, the liver contains only a limited amount of coenzyme A, and when most of it is tied up in acetyl-CoA, β oxidation slows for want of the free coenzyme. The production and export of ketone bodies frees coenzyme A, allowing continued fatty acid oxidation.
Ketone Bodies Are Overproduced in Diabetes and during Starvation
Starvation (including very-low-calorie diets) and untreated diabetes mellitus lead to overproduction of ketone bodies in the liver, with several adverse effects on health. During starvation, gluconeogenesis depletes citric acid cycle intermediates, diverting acetyl-CoA from oxidation of mobilized stored fats to ketone body production (Fig. 17-18). In untreated diabetes, when the insulin level is insufficient, extrahepatic tissues cannot take up glucose efficiently from the blood, either for fuel or for conversion to fat. Under these conditions, levels of malonyl-CoA fall, inhibition of carnitine acyltransferase 1 is relieved, and fatty acids enter mitochondria to be degraded to acetyl-CoA (see Fig. 17-13). But this acetyl-CoA cannot pass through the citric acid cycle because cycle intermediates have been drawn off for use as substrates in gluconeogenesis. The resulting accumulation of acetyl-CoA accelerates the formation of ketone bodies and their release into the blood beyond the capacity of extrahepatic tissues to oxidize them. The increased blood levels of acetoacetate and d-β-hydroxybutyrate lower the blood pH, causing the condition known as acidosis. Extreme acidosis can lead to coma and in some cases death. Ketone bodies in the blood and urine of individuals with untreated diabetes can reach extraordinary levels — a blood concentration of 90 mg/100 mL (compared with a normal level of ) and urinary excretion of 5,000 mg/24 h (compared with a normal rate of ). This condition is called ketosis or, when combined with acidosis, ketoacidosis.
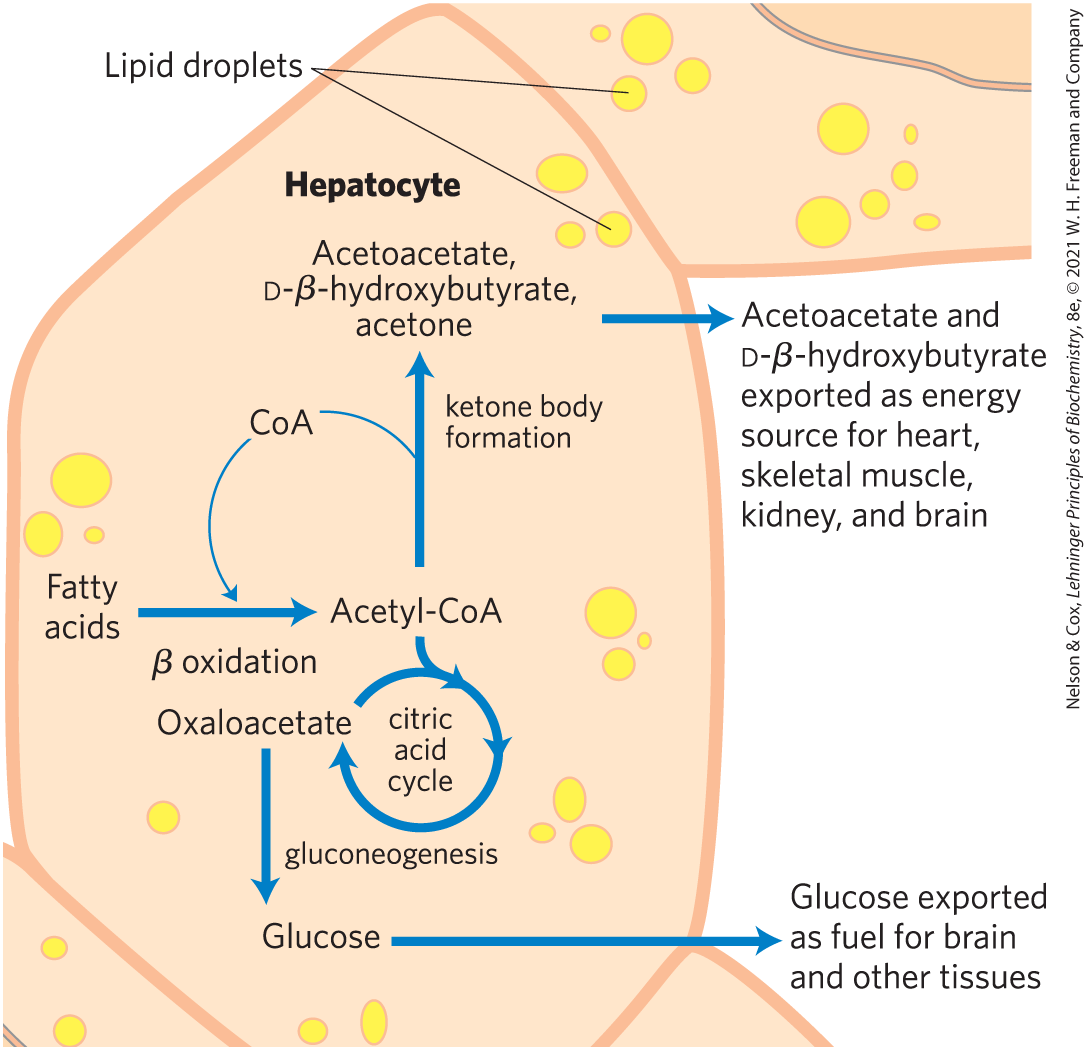
FIGURE 17-18 Ketone body formation and export from the liver. Conditions that promote gluconeogenesis (untreated diabetes, severely reduced food intake) slow the citric acid cycle (by drawing off oxaloacetate) and enhance the conversion of acetyl-CoA to acetoacetate. The released coenzyme A allows continued β oxidation of fatty acids.
In healthy people, acetone is formed in very small amounts from acetoacetate, which is easily decarboxylated, either spontaneously or by the action of acetoacetate decarboxylase (Fig. 17-16). Because individuals with untreated diabetes produce large quantities of acetoacetate, their blood contains significant amounts of acetone, which is toxic. Acetone is volatile and imparts a characteristic odor to the breath, which is sometimes useful in diagnosing diabetes.
SUMMARY 17.3 Ketone Bodies
- The ketone bodies — acetone, acetoacetate, and d-β-hydroxybutyrate — are formed in the liver when fatty acids are the principal fuel supporting whole-body metabolism. Acetoacetate and β-hydroxybutyrate serve as fuel molecules in extrahepatic tissues, including the brain, through oxidation to acetyl-CoA and entry into the citric acid cycle.
- Overproduction of ketone bodies in uncontrolled diabetes or severely reduced calorie intake can lead to life-threatening ketoacidosis, characterized by high concentrations of ketones in blood and urine and lower blood pH.
 Acetoacetate and
Acetoacetate and  Starvation (including very-low-calorie diets) and untreated diabetes mellitus lead to overproduction of ketone bodies in the liver, with several adverse effects on health. During starvation, gluconeogenesis depletes citric acid cycle intermediates, diverting acetyl-CoA from oxidation of mobilized stored fats to ketone body production (
Starvation (including very-low-calorie diets) and untreated diabetes mellitus lead to overproduction of ketone bodies in the liver, with several adverse effects on health. During starvation, gluconeogenesis depletes citric acid cycle intermediates, diverting acetyl-CoA from oxidation of mobilized stored fats to ketone body production (

 The ketone bodies — acetone, acetoacetate, and
The ketone bodies — acetone, acetoacetate, and