21.2 Biosynthesis of Triacylglycerols
Most of the fatty acids synthesized or ingested by an organism have one of two fates, depending on the organism’s needs: incorporation into triacylglycerols for the storage of metabolic energy or incorporation into the phospholipid components of membranes. During rapid growth, synthesis of new membranes requires the production of membrane phospholipids; when an organism has a plentiful food supply but is not actively growing, it shunts most of its fatty acids into storage fats. Both pathways begin at the same point: the formation of fatty acyl esters of glycerol. In this section we examine the route to triacylglycerols and its regulation, and the production of glycerol 3-phosphate in the process of glyceroneogenesis.
Triacylglycerols and Glycerophospholipids Are Synthesized from the Same Precursors
Animals can synthesize and store large quantities of triacylglycerols, to be used later as fuel (see Box 17-1). Humans can store only a few hundred grams of glycogen in liver and muscle, barely enough to supply the body’s energy needs for 12 hours. However, a 70 kg human stores about 15 kg of triacylglycerol in its tissues, enough to support basal energy needs for as long as 12 weeks (see Table 23-5). Triacylglycerols have the highest energy content of all stored nutrients — more than 38 kJ/g. Whenever carbohydrate is ingested in excess of the organism’s capacity to store glycogen, the excess is converted to triacylglycerols and stored in adipose tissue. Plants also manufacture triacylglycerols as an energy-rich fuel, mainly stored in fruits, nuts, and seeds.
In animal tissues, triacylglycerols and glycerophospholipids such as phosphatidylethanolamine share two precursors, fatty acyl–CoA and l-glycerol 3-phosphate, and several biosynthetic steps. The vast majority of the glycerol 3-phosphate is derived from the glycolytic intermediate dihydroxyacetone phosphate (DHAP) by the action of the cytosolic NAD-linked glycerol 3-phosphate dehydrogenase; in liver and kidney, a small amount of glycerol 3-phosphate is also formed from glycerol by the action of glycerol kinase (Fig. 21-17). The other precursors of triacylglycerols are fatty acyl–CoAs, formed from fatty acids by acyl-CoA synthetases, the same enzymes responsible for the activation of fatty acids for β oxidation (see Fig. 17-5).
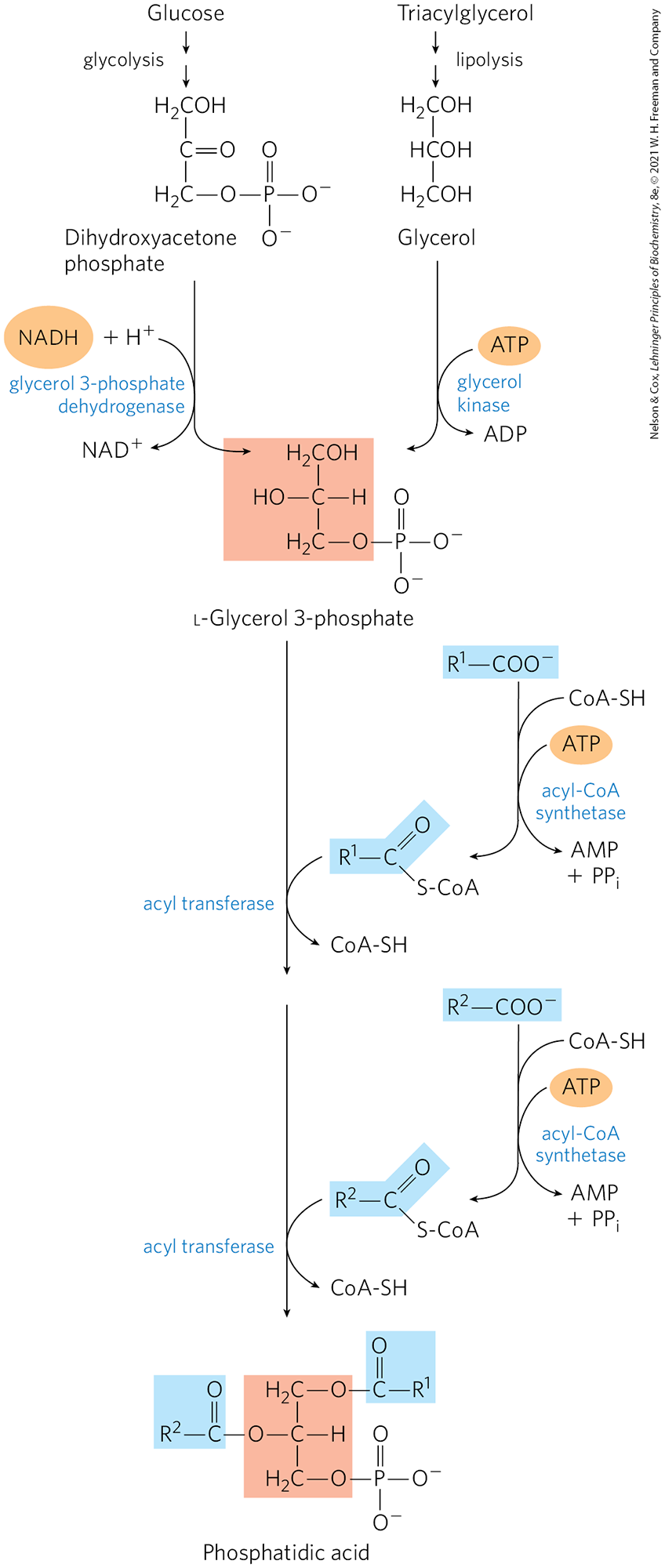
FIGURE 21-17 Biosynthesis of phosphatidic acid. A fatty acyl group is activated by formation of the fatty acyl–CoA, then transferred to ester linkage with l-glycerol 3-phosphate, formed in either of the two ways shown. Phosphatidic acid is shown here with the correct stereochemistry (l) at C-2 of the glycerol molecule. (The intermediate product with only one esterified fatty acyl group is lysophosphatidic acid.) To conserve space in subsequent figures (and in Fig. 21-14), both fatty acyl groups of glycerophospholipids, and all three acyl groups of triacylglycerols, are shown projecting to the right.
The first stage in the biosynthesis of triacylglycerols is acylation of the two free hydroxyl groups of l-glycerol 3-phosphate by two molecules of fatty acyl–CoA to yield diacylglycerol 3-phosphate, more commonly called phosphatidic acid, or phosphatidate (Fig. 21-17). Phosphatidic acid is present in only trace amounts in cells but is a central intermediate in lipid biosynthesis; it can be converted either to a triacylglycerol or to a glycerophospholipid. In the pathway to triacylglycerols, phosphatidic acid is hydrolyzed by phosphatidic acid phosphatase (also called lipin) to form a 1,2-diacylglycerol (Fig. 21-18). Diacylglycerols are then converted to triacylglycerols by transesterification with a third fatty acyl–CoA.
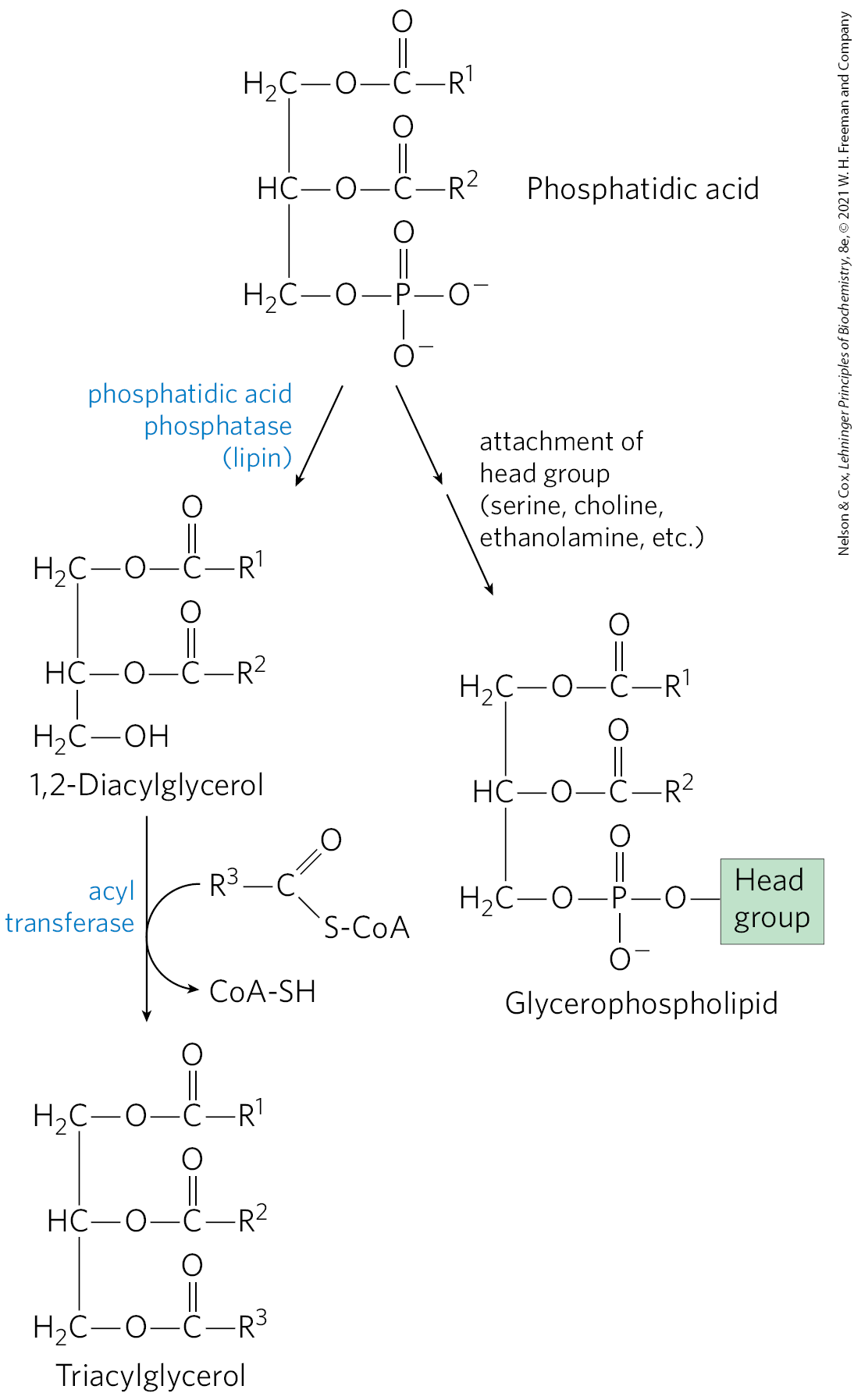
FIGURE 21-18 Phosphatidic acid in lipid biosynthesis. Phosphatidic acid is the precursor of both triacylglycerols and glycerophospholipids. The mechanisms for head-group attachment in phospholipid synthesis are described later in this section.
Triacylglycerol Biosynthesis in Animals Is Regulated by Hormones
In humans, the amount of body fat stays relatively constant over long periods, although there may be minor short-term changes as caloric intake fluctuates. Biosynthesis and degradation of triacylglycerols are regulated to meet the metabolic requirements of the moment. The rate of triacylglycerol biosynthesis is profoundly altered by the action of several hormones. Insulin, for example, promotes the conversion of carbohydrate to triacylglycerols (Fig. 21-19). People with severe diabetes mellitus, due to failure of insulin secretion or action, not only are unable to use glucose properly but also fail to synthesize fatty acids from carbohydrates or amino acids. If the diabetes is untreated, these individuals have increased rates of fat oxidation and ketone body formation (Chapter 17) and therefore lose weight.
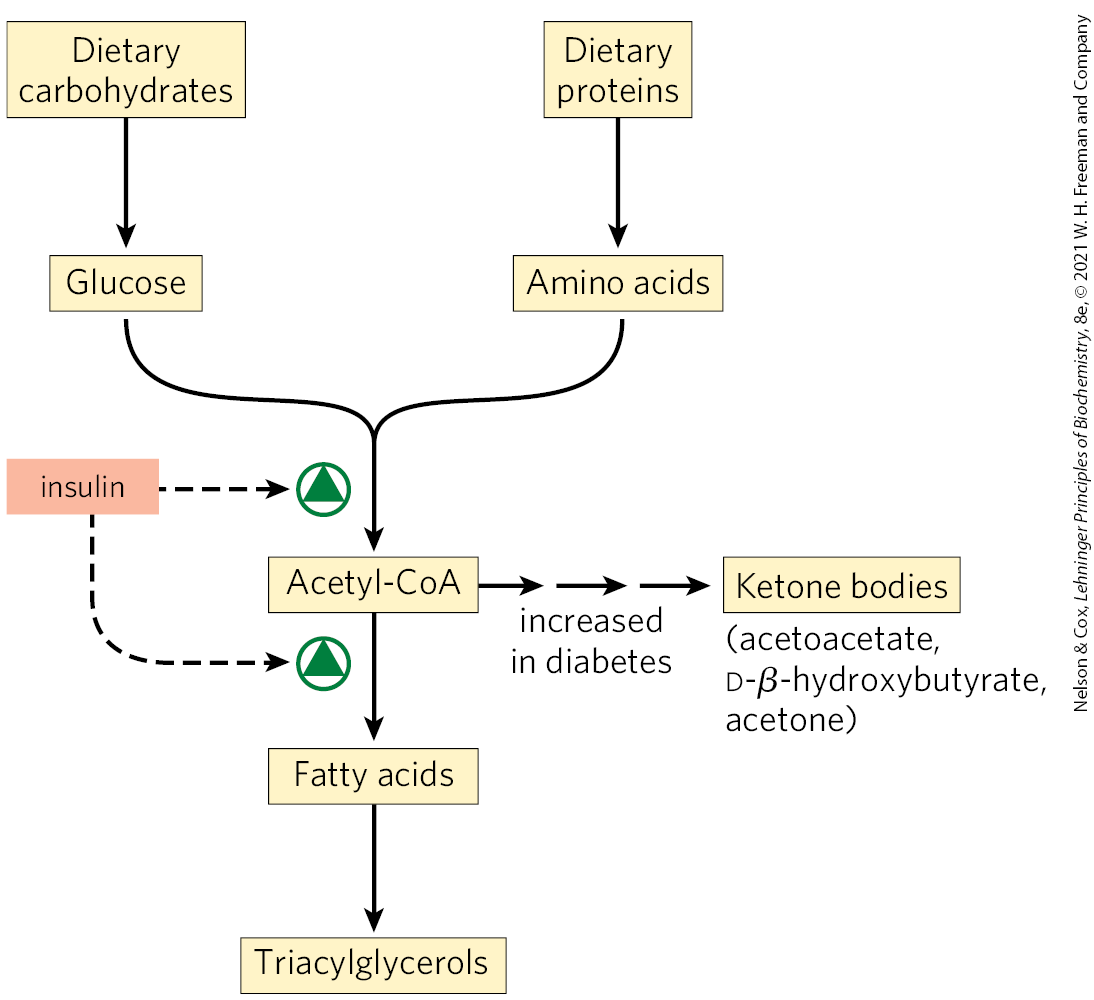
FIGURE 21-19 Regulation of triacylglycerol synthesis by insulin. Insulin stimulates conversion of dietary carbohydrates and proteins to fat. Individuals with diabetes mellitus either lack insulin or are insensitive to it. This results in diminished fatty acid synthesis, and the acetyl-CoA arising from catabolism of carbohydrates and proteins is shunted instead to ketone body production. People in severe ketosis smell of acetone, so the condition is sometimes mistaken for drunkenness.
Approximately 75% of all fatty acids released by triacylglycerol breakdown (lipolysis) are reesterified to form triacylglycerols rather than used for fuel. This ratio persists even under starvation conditions, when energy metabolism is shunted from the use of carbohydrate to the oxidation of fatty acids. Some of this fatty acid recycling takes place in adipose tissue, with the reesterification occurring before release into the bloodstream; some takes place via a systemic cycle in which free fatty acids are transported to the liver, recycled to triacylglycerol, exported again into the blood (transport of lipids in the blood is discussed in Section 21.4), and taken up again by adipose tissue, after release from triacylglycerol by extracellular lipoprotein lipase (Fig. 21-20; see also Fig. 17-1). Flux through this triacylglycerol cycle between adipose tissue and liver may be low when other fuels are available and the release of fatty acids from adipose tissue is limited, but, as noted above, the proportion of released fatty acids that are reesterified remains roughly constant at 75% under all metabolic conditions. The level of free fatty acids in the blood thus reflects both the rate of release of fatty acids and the balance between the synthesis and breakdown of triacylglycerols in adipose tissue and liver.
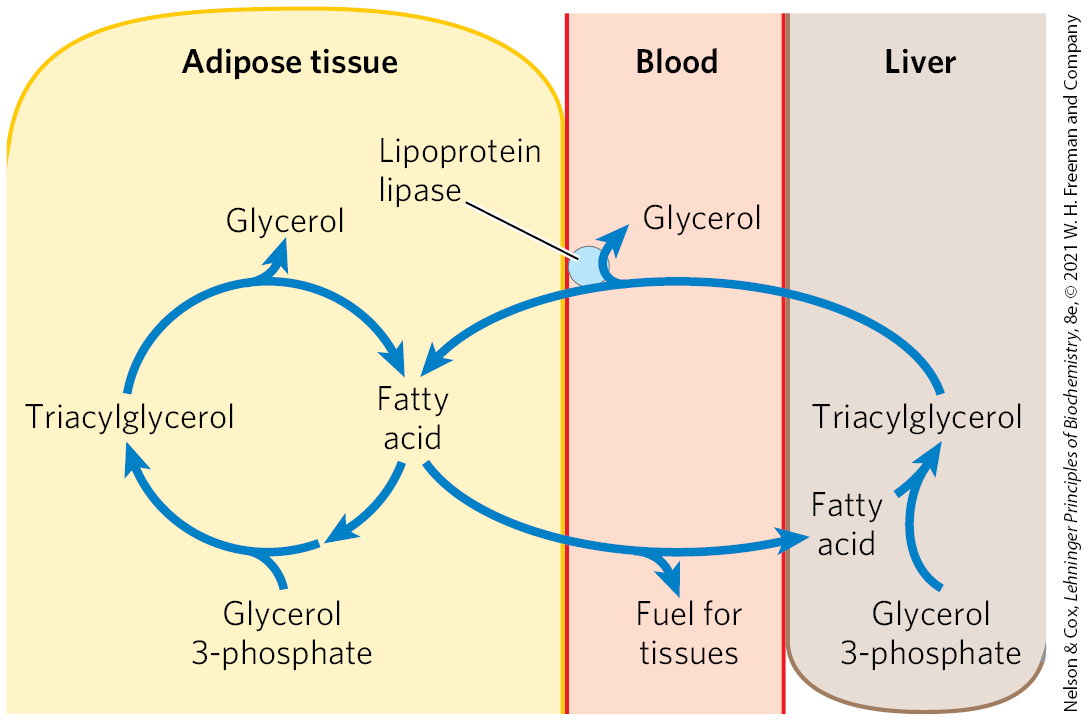
FIGURE 21-20 The triacylglycerol cycle. In mammals, triacylglycerol molecules are broken down and resynthesized in a triacylglycerol cycle during starvation. Some of the fatty acids released by lipolysis of triacylglycerol in adipose tissue pass into the bloodstream, and the remainder are used for resynthesis of triacylglycerol. Some of the fatty acids released into the blood are used for energy (in muscle, for example), and some are taken up by the liver and used in triacylglycerol synthesis. The triacylglycerol formed in the liver is transported in the blood back to adipose tissue, where the fatty acid is released by extracellular lipoprotein lipase, taken up by adipocytes, and reesterified into triacylglycerol.
When the mobilization of fatty acids is required to meet energy needs, release from adipose tissue is stimulated by the hormones glucagon and epinephrine (see Figs. 17-2, 17-13). Simultaneously, these hormonal signals decrease the rate of glycolysis and increase the rate of gluconeogenesis in the liver (providing glucose for the brain, as further elaborated in Chapter 23). The released fatty acid is taken up by several tissues, including muscle, where it is oxidized to provide energy. Much of the fatty acid taken up by liver is not oxidized but is recycled to triacylglycerol and returned to adipose tissue.
The function of the apparently futile triacylglycerol cycle is not well understood, but as we learn more about how the cycle is sustained via metabolism in two separate organs and is coordinately regulated, some possibilities emerge. For example, the excess capacity in the triacylglycerol cycle — the fatty acid that is eventually reconverted to triacylglycerol rather than oxidized as fuel — could represent an energy reserve in the bloodstream during fasting, one that could be more rapidly mobilized in a “fight or flight” emergency than stored triacylglycerol could be.
The constant recycling of triacylglycerols in adipose tissue even during starvation raises a second question: what is the source of the glycerol 3-phosphate required for this process? As noted above, glycolysis is suppressed under these conditions by the action of glucagon and epinephrine, so little DHAP is available. And glycerol released during lipolysis cannot be converted directly to glycerol 3-phosphate in adipose tissue, which lacks glycerol kinase (Fig. 21-17). So, how is sufficient glycerol 3-phosphate produced? The answer lies in the pathway of glyceroneogenesis, discovered in the 1960s by Lea Reshef, Richard Hanson, and John Ballard, and simultaneously by Eleazar Shafrir and his coworkers. The investigators were intrigued by the presence of two gluconeogenic enzymes, pyruvate carboxylase and phosphoenolpyruvate (PEP) carboxykinase, in adipose tissue, where glucose is not synthesized. Yet, the importance of this pathway was not appreciated until decades later. Glyceroneogenesis is intimately linked to the triacylglycerol cycle and, in a larger sense, to the balance between fatty acid and carbohydrate metabolism.
Adipose Tissue Generates Glycerol 3-Phosphate by Glyceroneogenesis
Glyceroneogenesis is a shortened version of gluconeogenesis, from pyruvate to DHAP (see Fig. 14-16), followed by conversion of the DHAP to glycerol 3-phosphate by cytosolic NAD-linked glycerol 3-phosphate dehydrogenase (Fig. 21-21). Glycerol 3-phosphate is subsequently used in triacylglycerol synthesis. There is a link between glyceroneogenesis and type 2 diabetes, as we shall see.
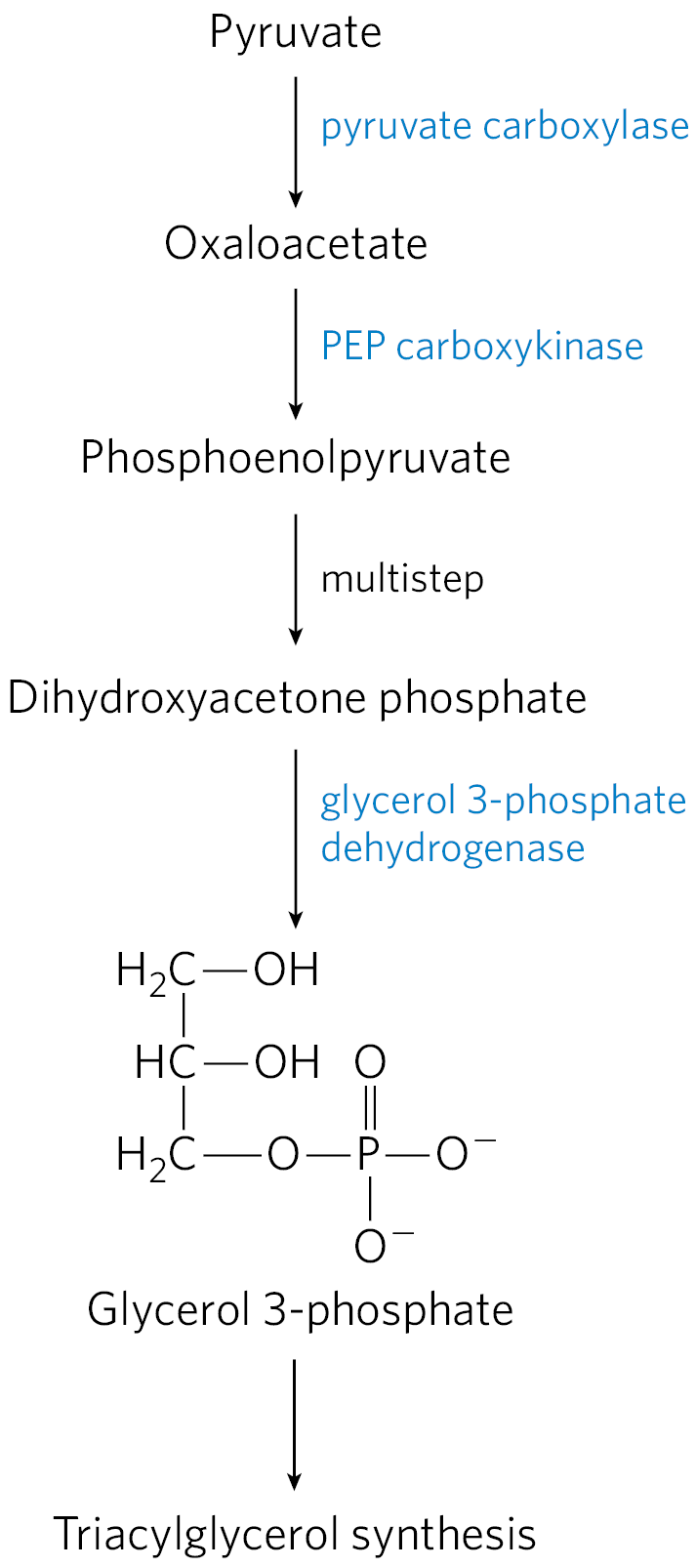
FIGURE 21-21 Glyceroneogenesis. The pathway is essentially an abbreviated version of gluconeogenesis, from pyruvate to dihydroxyacetone phosphate (DHAP), followed by conversion of DHAP to glycerol 3-phosphate, which is used for the synthesis of triacylglycerol.
Glyceroneogenesis has multiple roles. In adipose tissue, glyceroneogenesis coupled with reesterification of free fatty acids controls the rate of fatty acid release to the blood. In brown adipose tissue, the same pathway may control the rate at which free fatty acids are delivered to mitochondria for use in thermogenesis. And in fasting humans, glyceroneogenesis in the liver alone supports the synthesis of enough glycerol 3-phosphate to account for up to 65% of fatty acids reesterified to triacylglycerol.
Flux through the triacylglycerol cycle between liver and adipose tissue is controlled to a large degree by the activity of PEP carboxykinase, which limits the rate of both gluconeogenesis and glyceroneogenesis. Glucocorticoid hormones such as cortisol (a biological steroid derived from cholesterol; see Fig. 21-48) and dexamethasone (a synthetic glucocorticoid) regulate the levels of PEP carboxykinase reciprocally in the liver and adipose tissue. Acting through the glucocorticoid receptor, these steroid hormones increase the expression of the gene encoding PEP carboxykinase in the liver, thus increasing gluconeogenesis and glyceroneogenesis (Fig. 21-22).
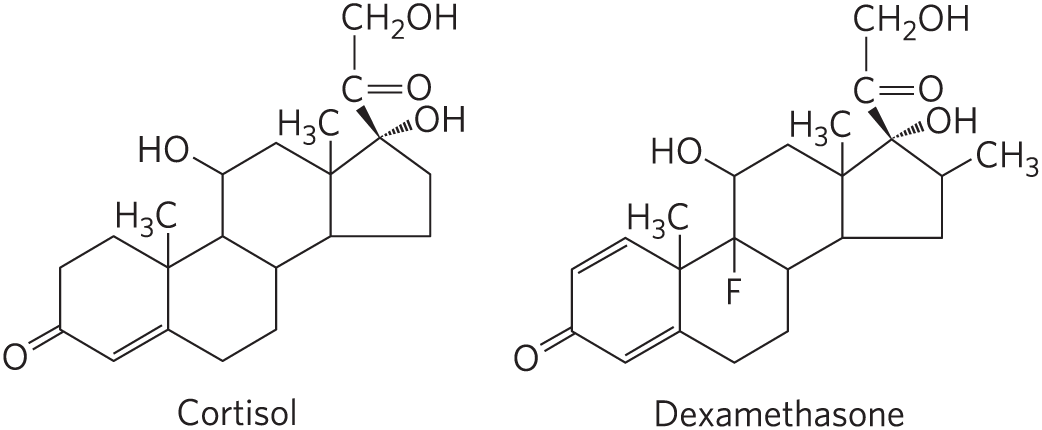
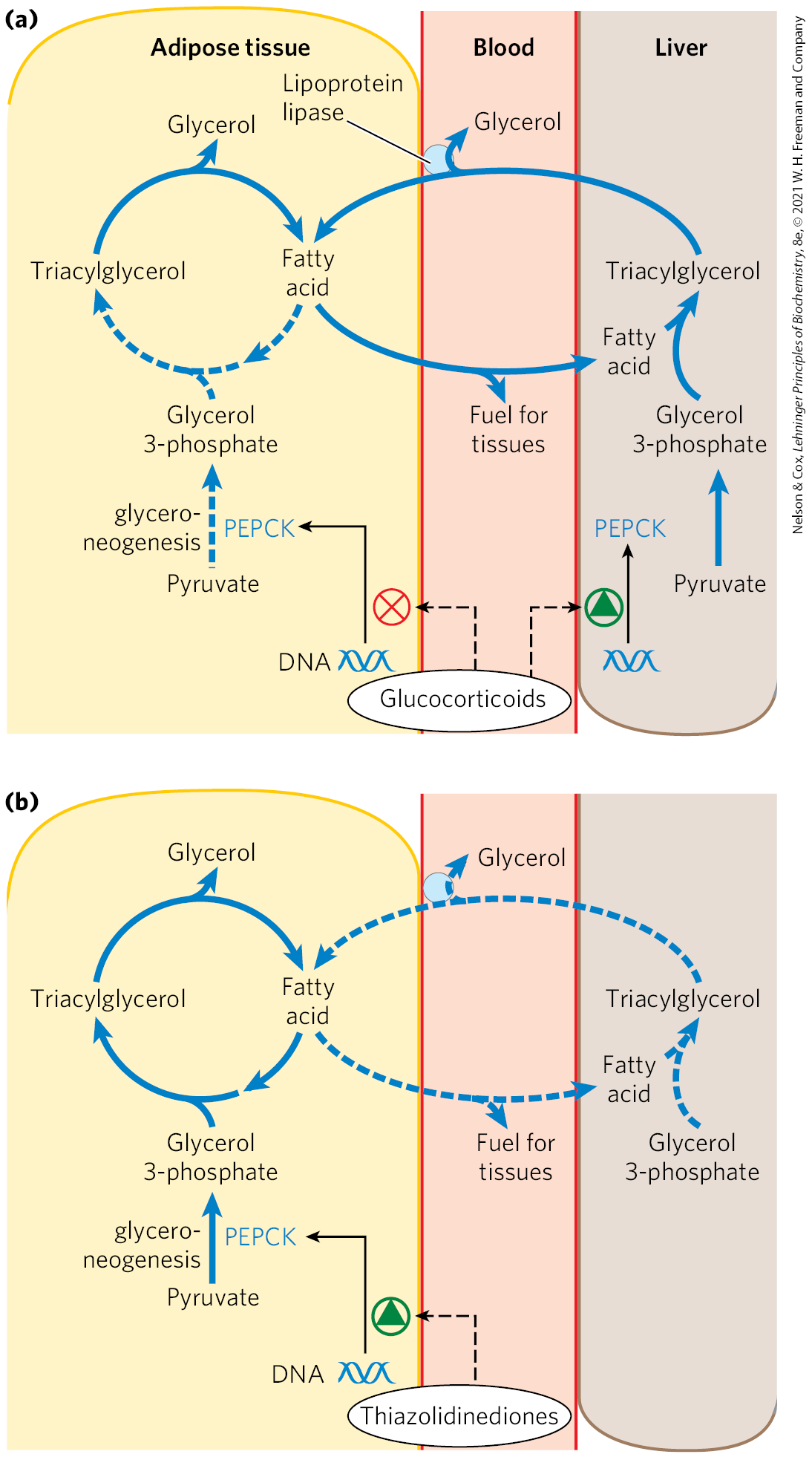
FIGURE 21-22 Regulation of glyceroneogenesis. (a) Glucocorticoid hormones stimulate glyceroneogenesis and gluconeogenesis in the liver, while suppressing glyceroneogenesis in adipose tissue (by reciprocal regulation of the gene expressing PEP carboxykinase (PEPCK) in the two tissues); this increases the flux through the triacylglycerol cycle. The glycerol freed by the breakdown of triacylglycerol in adipose tissue is released to the blood and transported to the liver, where it is primarily converted to glucose, although some is converted to glycerol 3-phosphate by glycerol kinase. (b) A class of drugs called thiazolidinediones is used to treat type 2 diabetes. In this disease, high levels of free fatty acids in the blood interfere with glucose utilization in muscle and promote insulin resistance. Thiazolidinediones activate a nuclear receptor called peroxisome proliferator-activated receptor (PPAR), which induces the activity of PEP carboxykinase. Therapeutically, thiazolidinediones increase the rate of glyceroneogenesis, thus increasing the resynthesis of triacylglycerol in adipose tissue and reducing the amount of free fatty acid in the blood. In both panels, dashed lines identify pathways in which flux declines under the conditions indicated.
Stimulation of glyceroneogenesis leads to an increase in the synthesis of triacylglycerol molecules in the liver and their release into the blood. At the same time, glucocorticoids suppress expression of the gene encoding PEP carboxykinase in adipose tissue. This results in a decrease in glyceroneogenesis in adipose tissue; recycling of fatty acids declines as a result, and more free fatty acids are released into the blood. Thus, regulation of glyceroneogenesis in the liver and adipose tissue affects lipid metabolism in opposite ways: a lower rate of glyceroneogenesis in adipose tissue leads to more fatty acid release (rather than recycling), whereas a higher rate in the liver leads to more synthesis and export of triacylglycerols. The net result is an increase in flux through the triacylglycerol cycle. When the glucocorticoids are no longer present, flux through the cycle declines as the expression of PEP carboxykinase increases in adipose tissue and decreases in the liver.
Thiazolidinediones Treat Type 2 Diabetes by Increasing Glyceroneogenesis
The connection between glyceroneogenesis and diabetes has stimulated new interest. High levels of free fatty acids in the blood interfere with glucose utilization in muscle and promote the insulin resistance that leads to type 2 diabetes. A class of drugs called thiazolidinediones reduces the levels of fatty acids circulating in the blood and increases sensitivity to insulin. Thiazolidinediones promote the increased expression of PEP carboxykinase in adipose tissue (Fig. 21-22), leading to increased synthesis of the precursors of glyceroneogenesis. The therapeutic effect of thiazolidinediones is thus due, at least in part, to the increase in glyceroneogenesis, which in turn increases the resynthesis of triacylglycerol in adipose tissue and reduces the release of free fatty acid from adipose tissue into the blood. Two thiazolidinediones have been available for treatment of type 2 diabetes: rosiglitazone (Avandia) and pioglitazone (Actos). Large-scale trials of rosiglitazone have indicated an increased risk of heart attack, so rosigliatazone has been withdrawn in the United Kingdom, India, South Africa, and many European countries. It remains available in the United States with limitations.
SUMMARY 21.2 Biosynthesis of Triacylglycerols
- Triacylglycerols are formed by reaction of two molecules of fatty acyl–CoA with glycerol 3-phosphate to form phosphatidic acid; this product is dephosphorylated to a diacylglycerol, then acylated by a third molecule of fatty acyl–CoA to yield a triacylglycerol.
- The synthesis and degradation of triacylglycerols are hormonally regulated.
- Mobilization and recycling of triacylglycerol molecules result in a triacylglycerol cycle. Triacylglycerols are resynthesized from free fatty acids and glycerol 3-phosphate even during starvation. The dihydroxyacetone phosphate precursor of glycerol 3-phosphate is derived from pyruvate via glyceroneogenesis.
- Thiazolidinediones stimulate glyceroneogenesis and can be used to treat type 2 diabetes.
 Most of the fatty acids synthesized or ingested by an organism have one of two fates, depending on the organism’s needs: incorporation into triacylglycerols for the storage of metabolic energy or incorporation into the phospholipid components of membranes. During rapid growth, synthesis of new membranes requires the production of membrane phospholipids; when an organism has a plentiful food supply but is not actively growing, it shunts most of its fatty acids into storage fats. Both pathways begin at the same point: the formation of fatty acyl esters of glycerol. In this section we examine the route to triacylglycerols and its regulation, and the production of glycerol 3-phosphate in the process of glyceroneogenesis.
Most of the fatty acids synthesized or ingested by an organism have one of two fates, depending on the organism’s needs: incorporation into triacylglycerols for the storage of metabolic energy or incorporation into the phospholipid components of membranes. During rapid growth, synthesis of new membranes requires the production of membrane phospholipids; when an organism has a plentiful food supply but is not actively growing, it shunts most of its fatty acids into storage fats. Both pathways begin at the same point: the formation of fatty acyl esters of glycerol. In this section we examine the route to triacylglycerols and its regulation, and the production of glycerol 3-phosphate in the process of glyceroneogenesis. In humans, the amount of body fat stays relatively constant over long periods, although there may be minor short-term changes as caloric intake fluctuates. Biosynthesis and degradation of triacylglycerols are regulated to meet the metabolic requirements of the moment. The rate of triacylglycerol biosynthesis is profoundly altered by the action of several hormones. Insulin, for example, promotes the conversion of carbohydrate to triacylglycerols (
In humans, the amount of body fat stays relatively constant over long periods, although there may be minor short-term changes as caloric intake fluctuates. Biosynthesis and degradation of triacylglycerols are regulated to meet the metabolic requirements of the moment. The rate of triacylglycerol biosynthesis is profoundly altered by the action of several hormones. Insulin, for example, promotes the conversion of carbohydrate to triacylglycerols (
 Triacylglycerols are formed by reaction of two molecules of fatty acyl–CoA with glycerol 3-phosphate to form phosphatidic acid; this product is dephosphorylated to a diacylglycerol, then acylated by a third molecule of fatty acyl–CoA to yield a triacylglycerol.
Triacylglycerols are formed by reaction of two molecules of fatty acyl–CoA with glycerol 3-phosphate to form phosphatidic acid; this product is dephosphorylated to a diacylglycerol, then acylated by a third molecule of fatty acyl–CoA to yield a triacylglycerol.