23.1 Hormone Structure and Action
Hormones are small molecules or proteins that are produced in one tissue, released into the bloodstream, and carried to other tissues, where they act through specific receptors to bring about changes in cellular activities. Hormones serve to coordinate the metabolic activities of several tissues or organs. Virtually every process in a complex organism is regulated by one or more hormones: maintenance of blood pressure, blood volume, and electrolyte balance; embryogenesis; sexual differentiation, development, and reproduction; hunger, eating behavior, digestion, and fuel allocation — to name but a few.
The coordination of metabolism in mammals is achieved by the neuroendocrine system. Individual cells in one tissue sense a change in the organism’s circumstances and respond by secreting a chemical messenger that passes to another cell in the same or different tissue, where the messenger binds to a specific receptor molecule and triggers a change in this target cell. These chemical messengers may relay information over very short or very long distances. In neuronal signaling (Fig. 23-1a), the chemical messenger is a neurotransmitter (acetylcholine, for example) that may travel only a fraction of a micrometer across a synaptic cleft to the next neuron in a network. In endocrine signaling (Fig. 23-1b), the messenger is a hormone that is carried in the bloodstream to neighboring cells or to distant organs and tissues; it may travel a meter or more before encountering its target cell. Except for this anatomic difference, the neuronal and endocrine signaling mechanisms are remarkably similar, and the same molecule can sometimes act as both neurotransmitter and hormone. Epinephrine and norepinephrine, for example, serve as neurotransmitters at certain synapses of the brain and at neuromuscular junctions of smooth muscle and as hormones that regulate fuel metabolism in liver and muscle. The following discussion of cellular signaling emphasizes hormone action, drawing on discussions of fuel metabolism in earlier chapters, but most of the fundamental mechanisms described here also occur in neurotransmitter action.
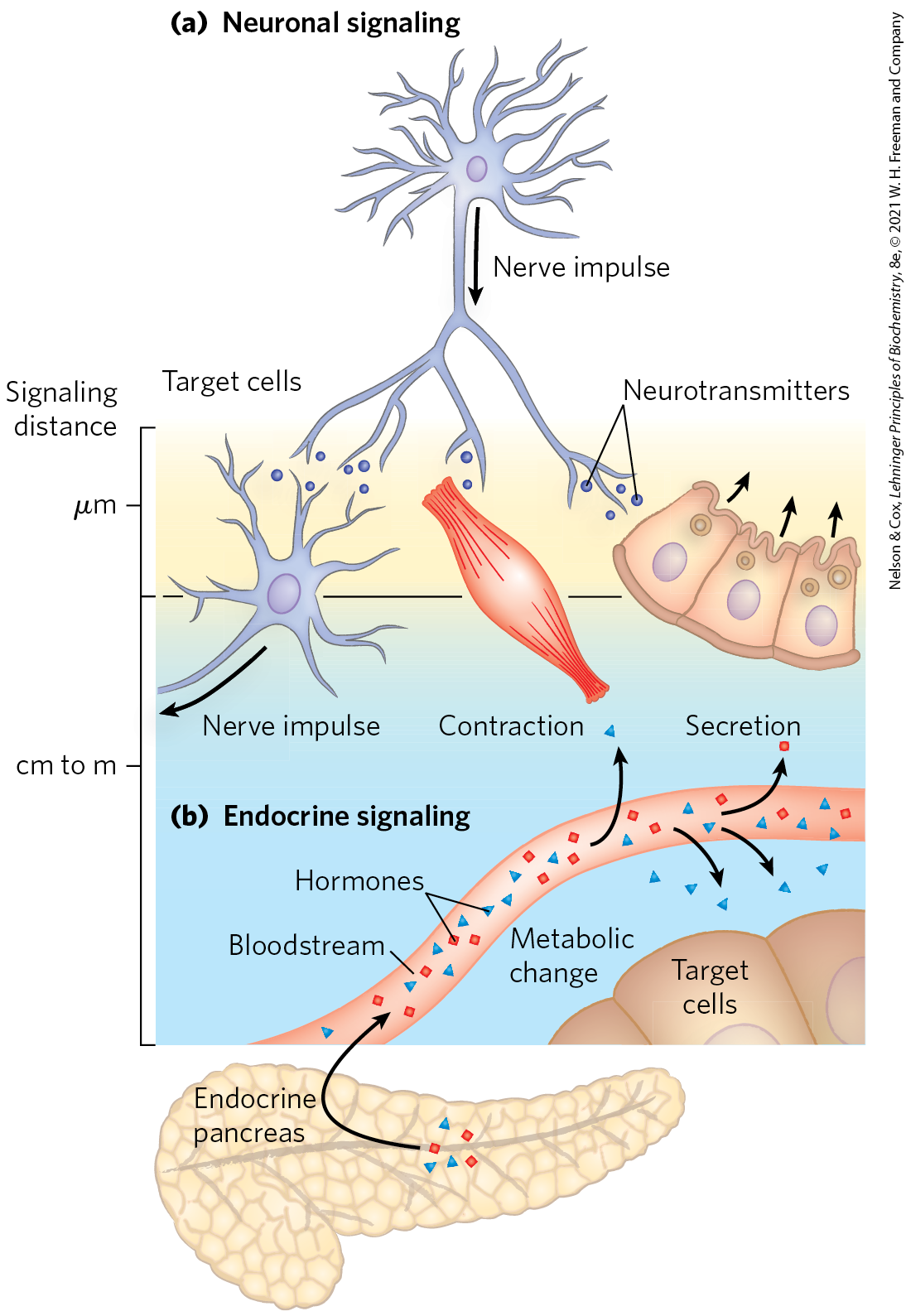
FIGURE 23-1 Signaling by the neuroendocrine system. (a) In neuronal signaling, electrical signals (nerve impulses) originate in the cell body of a neuron and travel very rapidly over long distances to the axon tip, where neurotransmitters are released and diffuse to the target cell. The target cell (another neuron, a myocyte, or a secretory cell) is only a fraction of a micrometer or a few micrometers away from the site of neurotransmitter release. (b) In endocrine signaling, hormones (such as insulin produced in pancreatic β cells) are secreted into the bloodstream, which carries them throughout the body to target tissues that may be a meter or more away from the secreting cell. Both neurotransmitters and hormones interact with specific receptors on or in their target cells, triggering responses.
Hormones Act through Specific High-Affinity Cellular Receptors
Hormones exert their effects through specific receptors in target cells. The high affinity of the hormone-receptor interaction allows cells to respond to very low concentrations of hormone. Recall from Chapter 12 (Fig. 12-2) that there are four general types of intracellular consequences of ligand-receptor interaction: (1) a second messenger, such as cAMP, cGMP, or inositol trisphosphate, generated inside the cell, acts as an allosteric regulator of one or more enzymes; (2) a receptor tyrosine kinase is activated by the extracellular hormone; (3) a change in membrane potential results from the opening or closing of a hormone-gated ion channel; and (4) a steroid or steroidlike molecule causes a change in the level of expression (transcription of DNA into mRNA) of one or more genes, mediated by a nuclear hormone receptor protein. It can be helpful to characterize cell surface hormone receptors as metabotropic, those that activate or inhibit an enzyme downstream from the receptor, or ionotropic, those that open or close an ion channel in the plasma membrane, resulting in a change in membrane potential or in the concentration of an ion such as (Fig. 23-2).
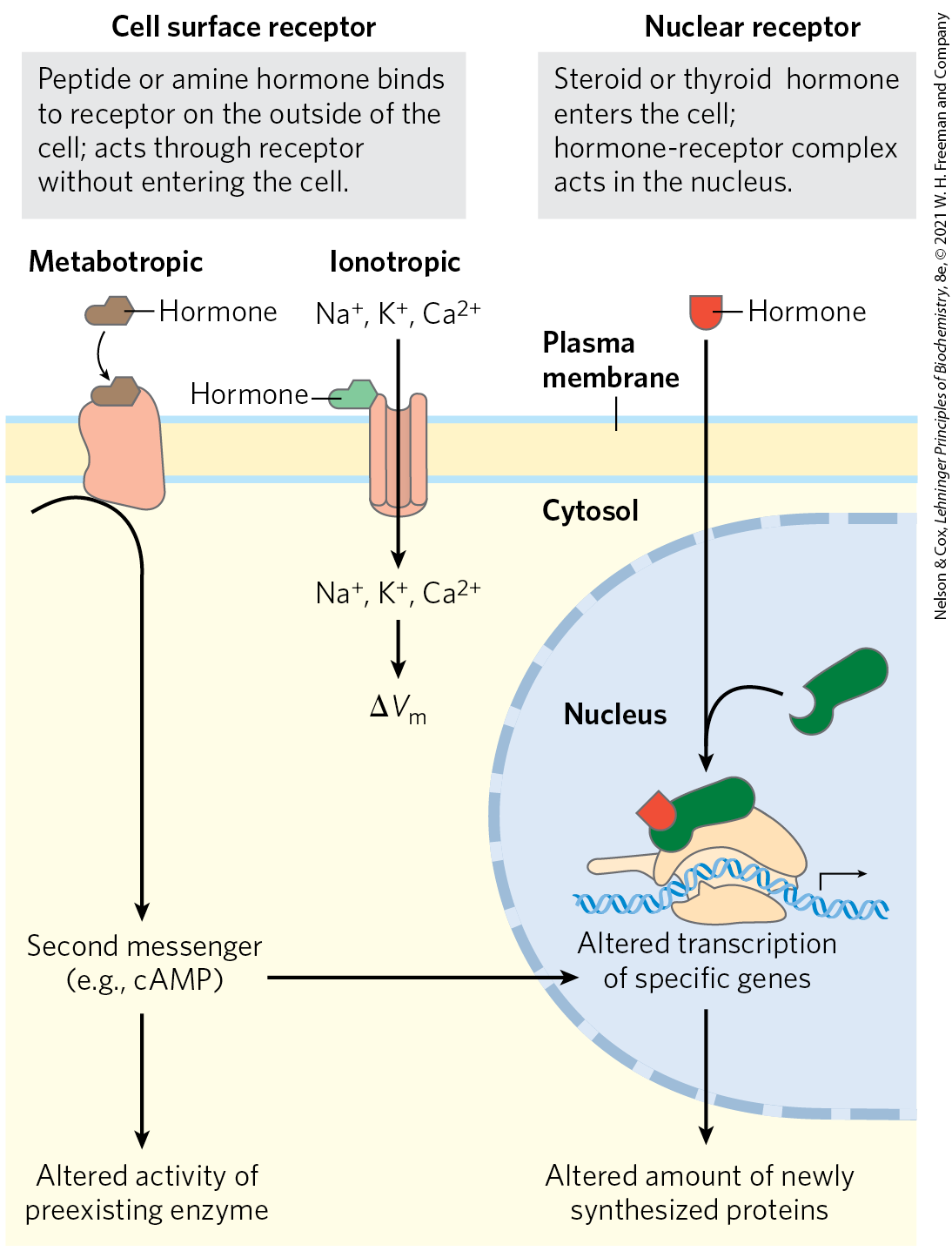
FIGURE 23-2 Two general mechanisms of hormone action. The peptide and amine hormones are faster-acting than steroid and thyroid hormones. Peptide hormones act from outside the cell, binding a plasma membrane hormone receptor. Steroid and thyroid hormones pass through the plasma membrane and enter the nucleus, where they regulate the expression of specific genes.
A single hormone molecule, in forming a hormone-receptor complex, activates a catalyst that produces many molecules of second messenger, so the receptor serves as both signal transducer and signal amplifier. The signal may be further amplified by a signaling cascade, such as we saw in the regulation of glycogen synthesis and breakdown by epinephrine (see Fig. 12-7). Signal amplification allows one epinephrine molecule to result in the production of many thousands or millions of molecules of glucose 1-phosphate from glycogen.
Water-insoluble hormones, including the steroid, retinoid, and thyroid hormones, readily pass through the plasma membrane of their target cells to reach their receptor proteins in the nucleus (Fig. 23-2). The hormone-receptor complex itself carries the message: it interacts with DNA to alter the expression of specific genes, changing the enzyme content of the cell and thereby changing cellular metabolism (see Fig. 12-34).
Hormones that act through plasma membrane receptors generally trigger very rapid physiological or biochemical responses. Just seconds after the adrenal medulla secretes epinephrine into the bloodstream, skeletal muscle responds by accelerating the breakdown of glycogen. By contrast, the thyroid hormones and the sex (steroid) hormones promote maximal responses in their target tissues only after hours or even days. These differences in response time correspond to different modes of action. In general, the fast-acting hormones lead to a change in the activity of one or more preexisting enzymes in the cell, by allosteric mechanisms or covalent modification. The slower-acting hormones generally alter gene expression, resulting in the synthesis of more (upregulation) or less (downregulation) of the regulated protein(s).
Hormones Are Chemically Diverse
Mammals have several classes of hormones, distinguishable by their diverse chemical structures and their modes of action (Table 23-1). Peptide, catecholamine, eicosanoid, and endocannabinoid hormones act from outside the target cell via cell surface receptors. Steroid, vitamin D, retinoid, and thyroid hormones enter the cell and act through nuclear receptors. Nitric oxide (a gas) also enters the cell, but activates a cytosolic enzyme, guanylyl cyclase.
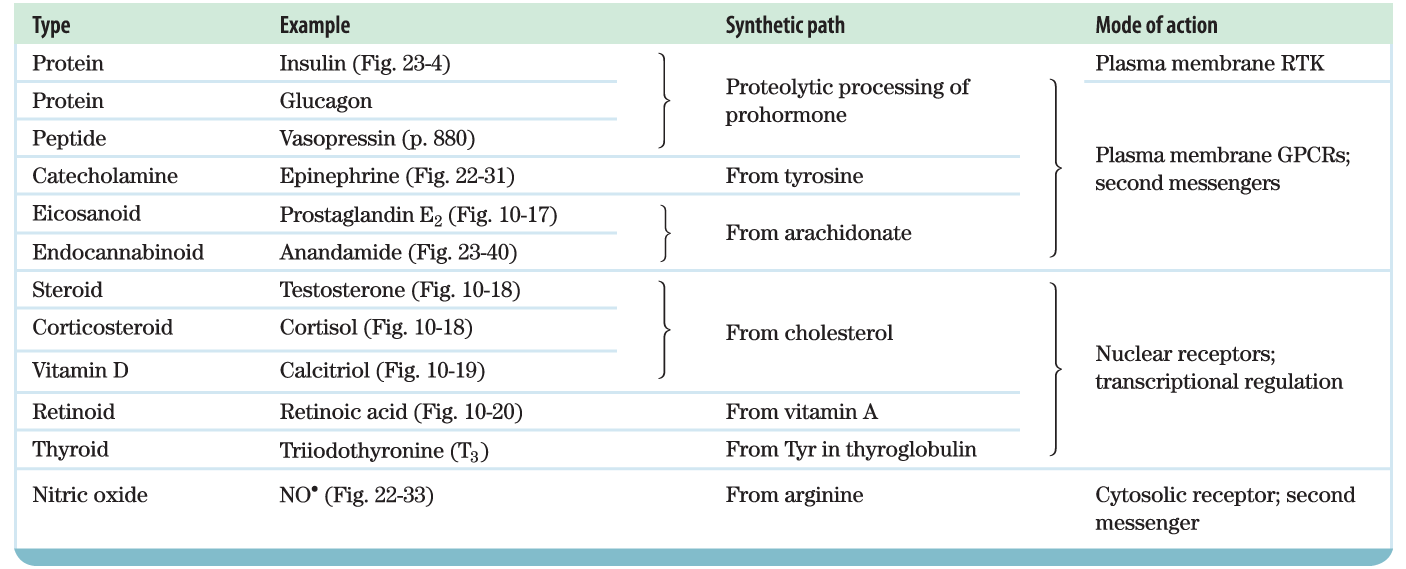 |
Hormones can also be classified by the way they get from their point of release to their target tissue. Endocrine (from the Greek endon, “within,” and krinein, “to release”) hormones are released into the blood and carried to target cells throughout the body (insulin and glucagon are examples). Paracrine hormones are released into the extracellular space and diffuse to neighboring target cells (the eicosanoid hormones are of this type). Autocrine hormones affect the same cell that releases them, binding to receptors on the cell surface.
The peptide hormones vary in size from 3 (thyrotropin-releasing hormone; Fig. 23-3) to more than 200 (human chorionic gonadotropin) amino acid residues. They include the pancreatic hormones insulin, glucagon, and somatostatin; the parathyroid hormone calcitonin; and all the hormones of the hypothalamus and pituitary. Peptide hormones (some of which are actually small proteins) are synthesized as proproteins (prohormones) that are activated upon release by proteolytic cleavage, much as we saw with zymogen activation of pancreatic enzymes (Fig. 6-42) and the blood-clotting cascade (Fig. 6-44). In some cases, a preproprotein is synthesized and processed to create more than one active peptide product.
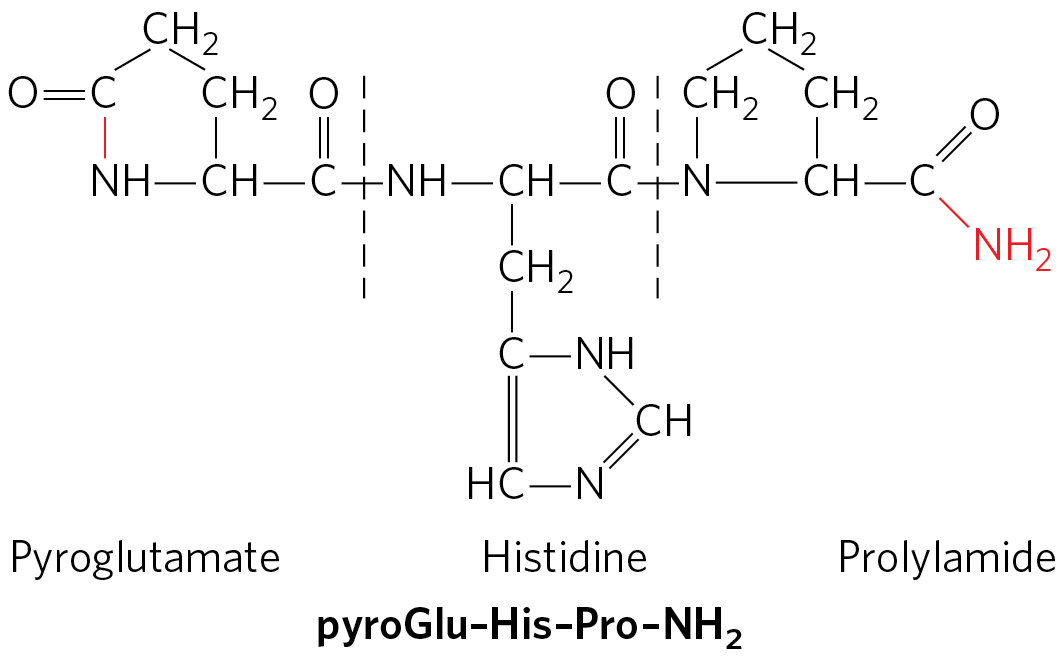
FIGURE 23-3 The structure of thyrotropin-releasing hormone (TRH). Purified (through heroic efforts) from extracts of hypothalamus, TRH proved to be a derivative of the tripeptide Glu–His–Pro. The side-chain carboxyl group of the amino-terminal Glu forms an amide (red bond) with the residue’s α-amino group, creating pyroglutamate, and the carboxyl group of the carboxyl-terminal Pro is converted to an amide (red ). Such modifications are common among the small peptide hormones. In a typical protein of , the charges on the amino- and carboxyl-terminal groups contribute relatively little to the overall charge on the molecule, but in a tripeptide these two charges dominate the properties of the molecule. Formation of the amide derivatives removes these charges.
Insulin is a small protein ( 5,800) with two polypeptide chains, A and B, joined by two disulfide bonds. (The amino acid sequence of bovine insulin is shown in Figure 3-24.) It is synthesized in the pancreas as an inactive single-chain precursor, preproinsulin (Fig. 23-4), with an amino-terminal “signal sequence” that directs its passage into secretory vesicles. (Signal sequences are discussed in Chapter 27; see Fig. 27-38.) Proteolytic removal of the signal sequence and formation of three disulfide bonds produces proinsulin, which is stored in secretory granules in pancreatic β cells. When blood glucose is elevated sufficiently to trigger insulin secretion, proinsulin is converted to active insulin by specific proteases, which cleave two peptide bonds to form the mature insulin molecule and C peptide, which are released into the blood by exocytosis. The capillaries that serve peptide-producing endocrine glands are fenestrated (punctuated with tiny holes or “windows”), so the hormone molecules readily enter the bloodstream for transport to target cells elsewhere. Insulin, acting through its receptor tyrosine kinase (Fig. 12-21) has profound effects on both anabolic and catabolic processes in many tissues, which we explore in detail below.
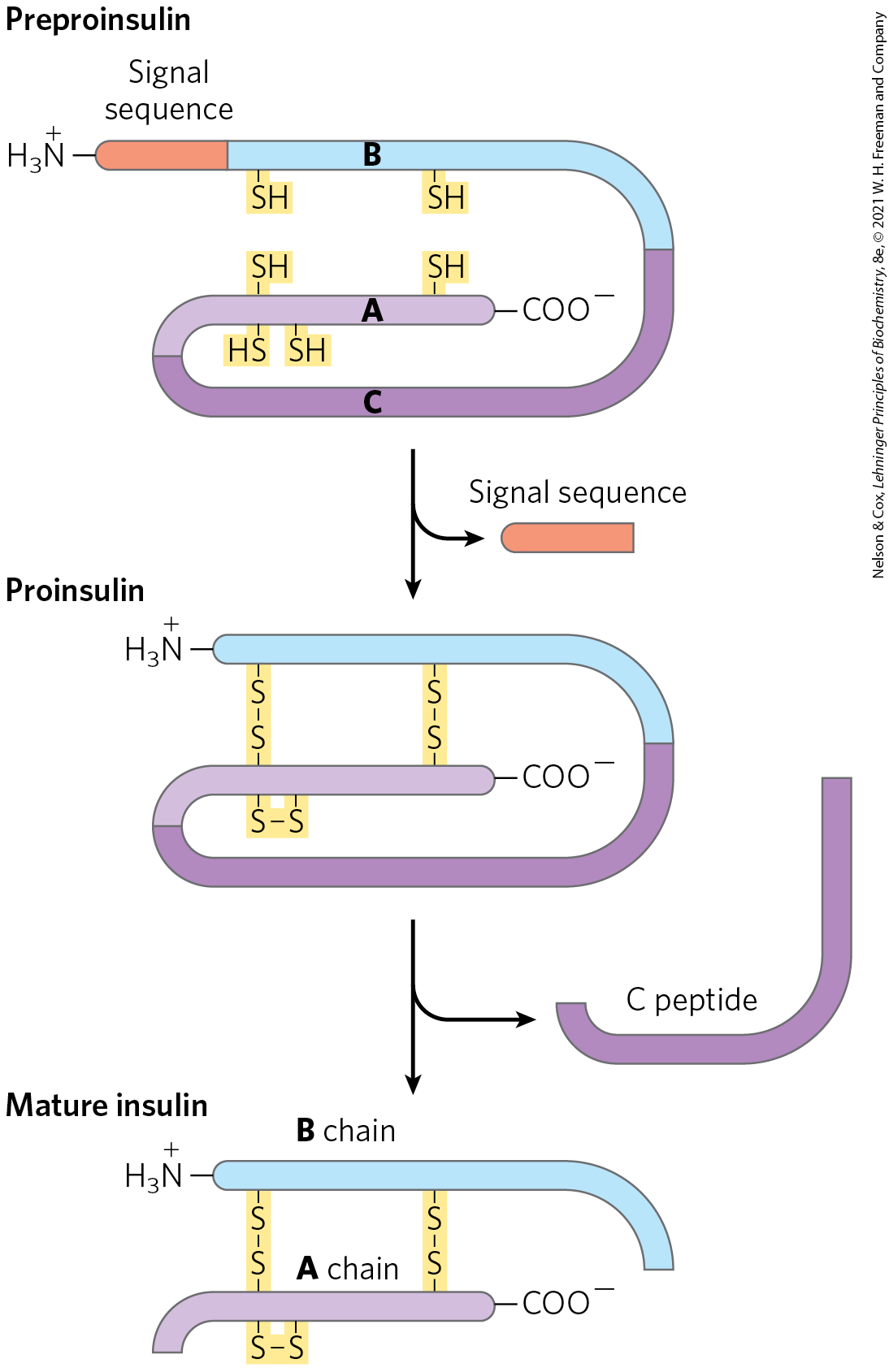
FIGURE 23-4 Insulin. Mature insulin is formed from its larger precursor preproinsulin by proteolytic processing. Removal of a 23 amino acid segment (the signal sequence) at the amino terminus of preproinsulin and formation of three disulfide bonds produce proinsulin. Further proteolytic cuts remove the C peptide from proinsulin to produce mature insulin, composed of A and B chains.
Pro-opiomelanocortin (POMC) is a spectacular example of a proprotein that undergoes specific cleavage to produce several active hormones. The POMC gene encodes a large polypeptide that contains within it at least nine biologically active peptides (Fig. 23-5). The proprotein is processed differently in different tissues, depending on which proteases the cells express. The active products influence an astonishing number of physiological systems.
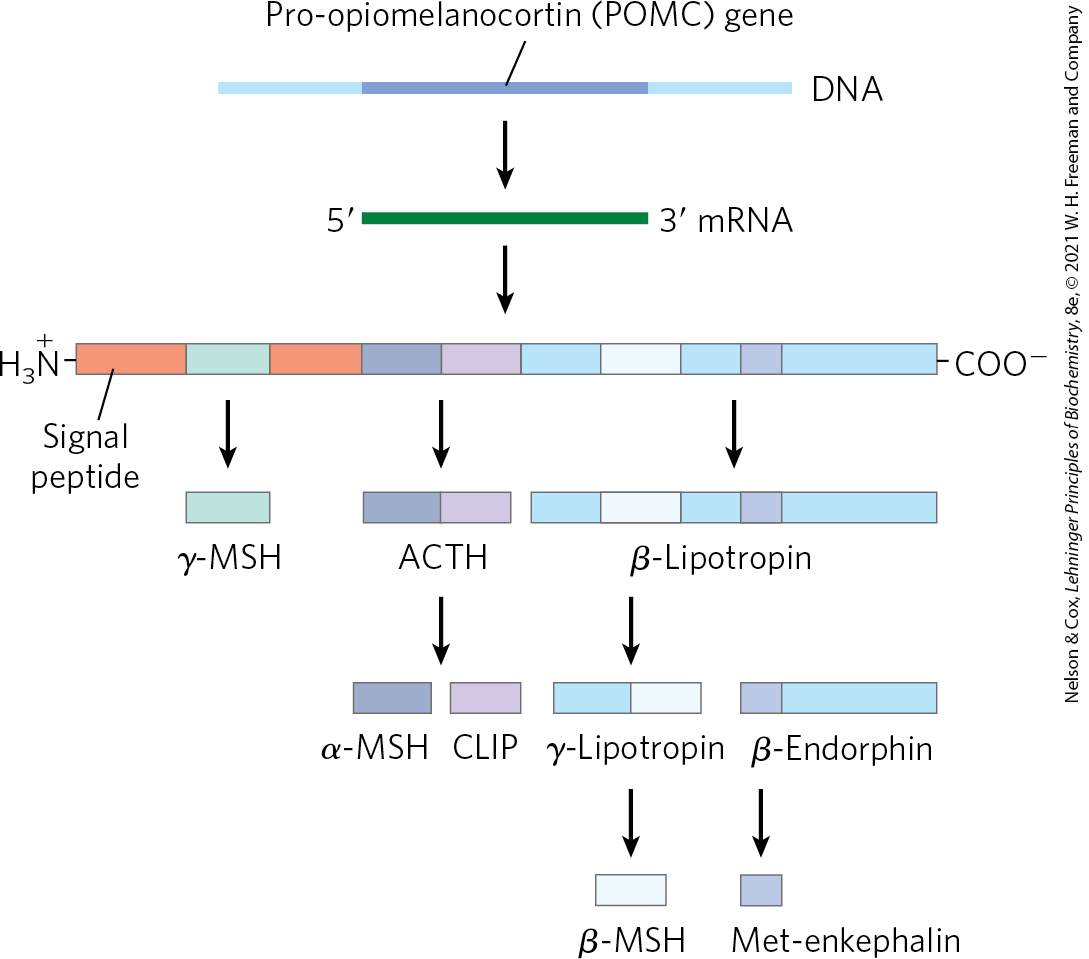
FIGURE 23-5 Proteolytic processing of the pro-opiomelanocortin (POMC) precursor. The initial gene product of the POMC gene is a long polypeptide that undergoes cleavage by a series of specific proteases to produce ACTH (corticotropin), β- and γ-lipotropin, α-, β-, and γ-MSH (melanocyte-stimulating hormone, or melanocortin), CLIP (corticotropin-like intermediary peptide), β-endorphin, and Met-enkephalin. The points of cleavage are pairs of basic residues, Arg–Lys, Lys–Arg, or Lys–Lys.
Some Hormones Are Released by a “Top-Down” Hierarchy of Neuronal and Hormonal Signals
The changing levels of specific hormones regulate specific cellular processes, but what regulates the regulators — what sets the level of each hormone? The brief answer is that the central nervous system receives input from many internal and external sensors — signals about danger, hunger, dietary intake, blood composition, for example — and orchestrates the production of appropriate hormonal signals by the endocrine tissues. For a more complete answer, consider the path of cortisol release by the adrenal gland, triggered by a stress detected by the central nervous system. Figure 23-6 illustrates the “chain of command” in this top-down hormonal signaling hierarchy. The hypothalamus, a pea-size region of the brain (Fig. 23-7), is the coordination center of the endocrine system; it receives and integrates messages from the central nervous system. In response, the hypothalamus produces releasing factors, including corticotropin-releasing hormone (CRH), that pass directly to the nearby pituitary gland through blood vessels and neurons that connect the two glands. The pituitary gland secretes adrenocorticotropic hormone (also called corticotropin or ACTH), which travels through the blood to the adrenal cortex and triggers the release of cortisol. Cortisol, the ultimate hormone in this cascade, acts through its receptor in many types of target cells to alter their metabolism. In hepatocytes, one effect of cortisol is to increase the rate of gluconeogenesis.
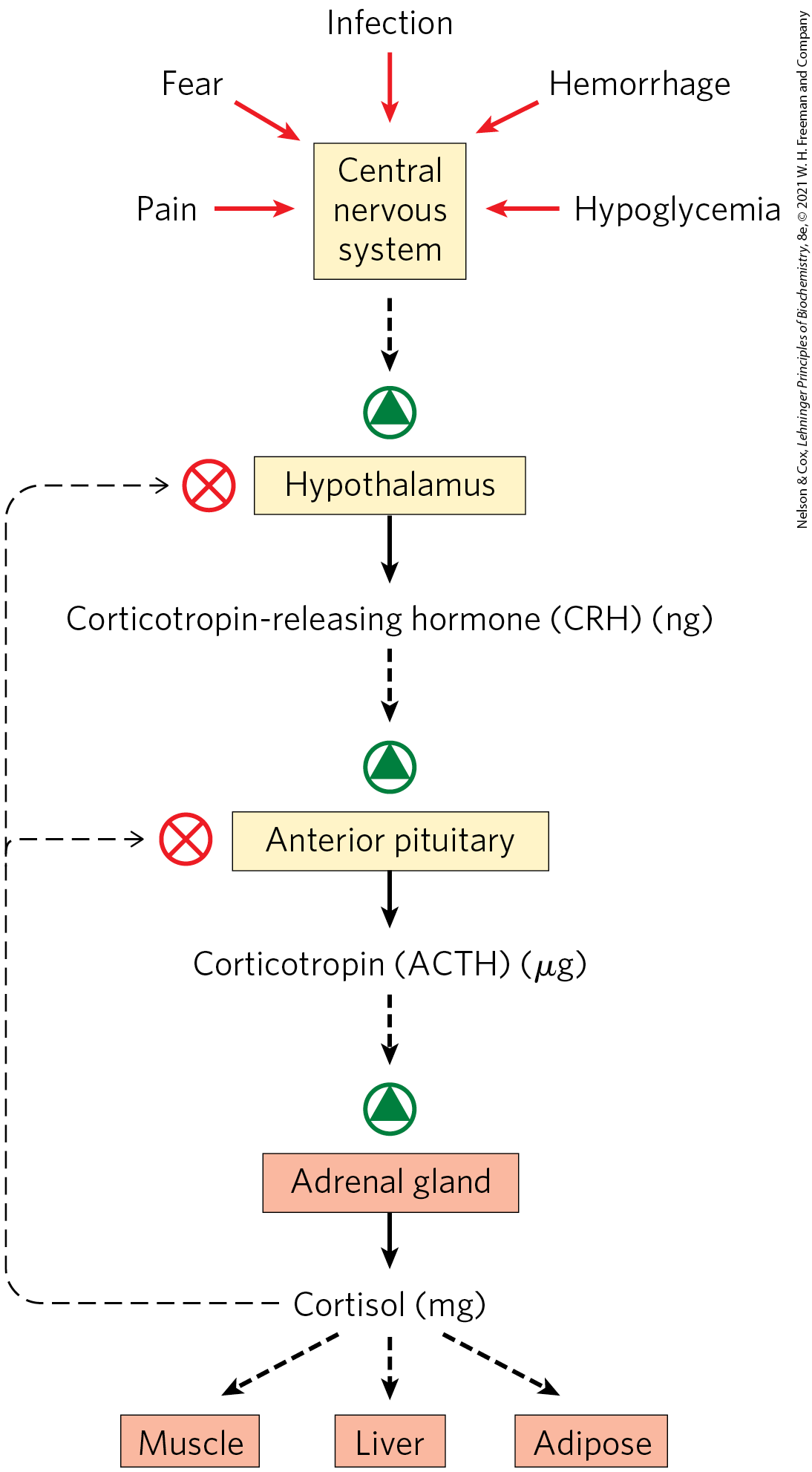
FIGURE 23-6 Cascade of top-down hormone release following central nervous system input to the hypothalamus. Solid black arrows indicate hormone production and release; broken black arrows indicate the action of hormones on target tissues. In each endocrine tissue along the pathway, a stimulus from the level above is received, amplified, and transduced into release of the next hormone in the cascade. The cascade is sensitive to regulation at several levels through feedback inhibition (thin, dashed arrows) by the ultimate hormone (in this case, cortisol). The product therefore regulates its own production, as in feedback inhibition of biosynthetic pathways within a single cell.
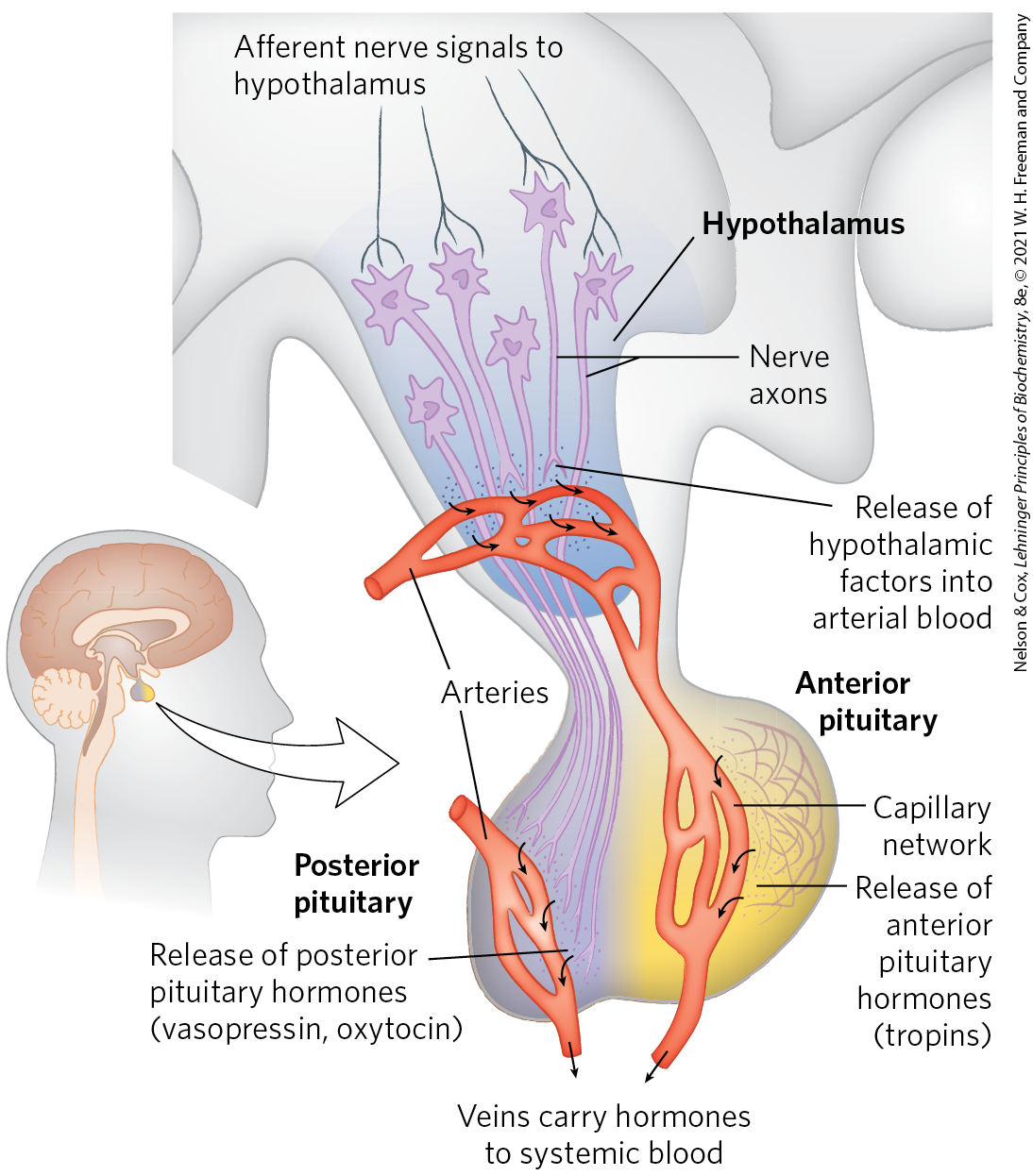
FIGURE 23-7 Neuroendocrine origins of hormone signals. Location of the hypothalamus and pituitary gland and details of the hypothalamus-pituitary system. Signals from connecting neurons stimulate the hypothalamus to secrete releasing factors into a blood vessel that carries the hormones directly to a capillary network in the anterior pituitary. In response to each releasing factor, the anterior pituitary releases the appropriate hormone into the general circulation. Posterior pituitary hormones are synthesized in neurons arising in the hypothalamus; the hormones are transported along axons to nerve endings in the posterior pituitary and stored there until released into the blood in response to a neuronal signal.
Hormonal cascades such as those responsible for the release of cortisol result in large amplifications of the initial signal and allow exquisite fine-tuning of the output of the ultimate hormone (Fig. 23-6). At each level in the cascade, a small signal elicits a larger response. For example, the initial electrical signal to the hypothalamus results in the release of a few nanograms of corticotropin-releasing hormone, which elicits the release of a few micrograms of corticotropin. Corticotropin acts on the adrenal cortex to cause the release of milligrams of cortisol, for an overall amplification of at least a millionfold.
At each level of a hormonal cascade, feedback inhibition of earlier steps in the cascade is possible; an unnecessarily elevated level of the ultimate hormone or of an intermediate hormone inhibits the release of earlier hormones in the cascade. These feedback mechanisms accomplish the same end as those that limit the output of a biosynthetic pathway (compare Fig. 23-6 with Fig. 22-37): a product is synthesized (or released) only until the necessary concentration is reached. In the blood-clotting cascade (Fig. 6-44) we saw a similar pattern: a signal stimulates a cascade of protein activations; feedback mechanisms limit their action and the duration of the response.
“Bottom-Up” Hormonal Systems Send Signals Back to the Brain and to Other Tissues
In addition to the top-down hierarchy of hormonal signaling shown in Figure 23-6, some hormones are produced in the digestive tract, muscle, and adipose tissue and communicate the current metabolic state to the hypothalamus (Fig. 23-8). These signals are integrated in the hypothalamus, and an appropriate neuronal or hormonal response is elicited.
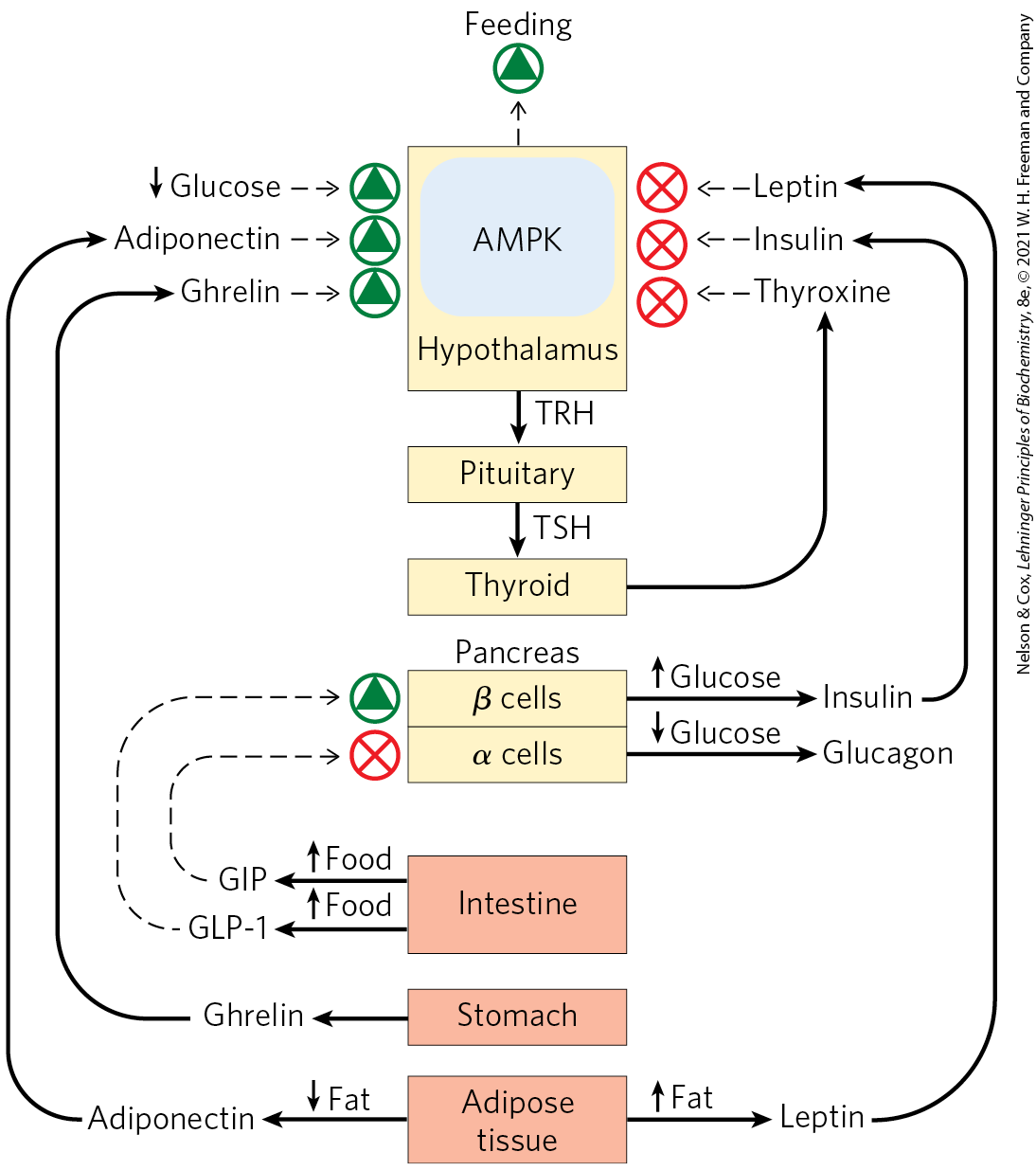
FIGURE 23-8 Regulation of feeding behavior by two-way information flow between tissues and the hypothalamus. When food intake and energy production are adequate, peptide hormones released by the stomach, intestine, and adipose tissue feed back on the hypothalamus to signal satiety and reduce feeding behavior. Other tissue-specific peptide hormones signal inadequate supplies of stored triacylglycerols or low blood glucose levels. All of these signals impinge, directly or indirectly, on AMP-activated protein kinase (AMPK) in the hypothalamus, which integrates these signals and influences feeding behavior and energy-yielding metabolism in the tissues. Nerves carry electrical signals from the brain to the other tissues to complete the information circuit and achieve homeostasis (not shown). TRH, thyrotropin-releasing hormone; GLP-1, glucagon-like peptide-1; GIP, gastric inhibitory polypeptide.
Adipokines, for example, are peptide hormones, produced in adipose tissue, that signal the adequacy of fat reserves. The adipokine leptin, released when adipose tissue is well-filled with triacylglycerols, acts in the brain to inhibit feeding behavior, whereas adiponectin signals depletion of fat reserves and stimulates feeding. Other tissues produce and release other hormones. Ghrelin is produced in the gastrointestinal tract when the stomach is empty and acts in the hypothalamus to stimulate feeding behavior; when the stomach fills, ghrelin release ceases. Incretins are peptide hormones produced in the gut after ingestion of a meal; they increase secretion of insulin and decrease secretion of glucagon from the pancreas. Neuropeptide Y (NPY) is a hormone produced in the hypothalamus and in the adrenal glands. Its release promotes feeding and reduces energy expenditure for nonessential activities. Peptide YY , produced in the intestine, signals satiety in the brain. Irisin is a peptide hormone produced in muscle as a result of exercise; it acts to convert white adipose tissue into beige adipose tissue, which dissipates energy as heat (see Section 23.2).
We return to these hormones (summarized in Table 23-2) when we discuss the regulation of body mass in humans (Section 23.4).
Hormone |
Production site(s) |
Target tissue(s) |
Action(s) |
|---|---|---|---|
Insulin |
Pancreatic β cells |
Muscle, adipose, liver |
Stimulates glucose uptake and synthesis of glycogen and fat |
Glucagon |
Pancreatic α cells |
Liver, adipose |
Stimulates gluconeogenesis and glucose release to blood |
Leptin |
Adipose tissue |
Hypothalamus |
Reduces hunger |
Adiponectin |
Adipose tissue |
Muscle, liver, others |
Stimulates catabolism and feeding behavior |
Ghrelin |
Stomach, intestine |
Brain |
Signals hunger |
Incretins: GLP-1, GIP |
Intestine |
Pancreas |
Stimulate insulin release |
NPY |
Hypothalamus, adrenals |
Brain, autonomic nervous system |
Stimulates feeding behavior |
Intestine |
Brain |
Signals satiety |
|
Irisin |
Muscle (after exercise) |
Adipose |
Turns white adipose tissue to beige |
SUMMARY 23.1 Hormone Structure and Action
- Hormones connect all of the organs in the body, carrying information and signals between the central nervous system and all of the tissues.
- Hormones are chemically diverse with a wide range of biological roles. Peptide hormones are synthesized as proteins on ribosomes, then cleaved proteolytically to form the active peptide(s).
- Hormones are regulated by a top-down hierarchy of interactions between the brain and endocrine glands: nerve impulses stimulate the hypothalamus to send specific hormones to the pituitary gland, thus stimulating (or inhibiting) the release of a second rank of hormones. The anterior pituitary hormones in turn stimulate other endocrine glands (adrenals, for example) to secrete their characteristic hormones, which in turn stimulate specific target tissues.
- Some hormones act in bottom-up signaling: adipose tissue, muscle, and the gastrointestinal tract release peptide hormones that act on other tissues or in the central nervous system.
 Mammals have several classes of hormones, distinguishable by their diverse chemical structures and their modes of action (
Mammals have several classes of hormones, distinguishable by their diverse chemical structures and their modes of action ( Hormones connect all of the organs in the body, carrying information and signals between the central nervous system and all of the tissues.
Hormones connect all of the organs in the body, carrying information and signals between the central nervous system and all of the tissues.