12.1 General Features of Signal Transduction
Signal transductions are remarkably specific and exquisitely sensitive. Specificity is achieved by precise molecular complementarity between the signal and receptor molecules (Fig. 12-1a), mediated by the same kinds of weak (noncovalent) forces that mediate enzyme-substrate and antigen-antibody interactions. Multicellular organisms have an additional level of specificity, because the receptors for a given signal, or the intracellular targets of a given signal pathway, are present only in certain cell types. Thyrotropin-releasing hormone, for example, triggers responses in the cells of the anterior pituitary but not in hepatocytes, which lack receptors for this hormone. Epinephrine alters glycogen metabolism in hepatocytes but not in adipocytes; in this case, both cell types have receptors for the hormone, but whereas hepatocytes contain glycogen and the glycogen-metabolizing enzyme that is stimulated by epinephrine, adipocytes contain neither.
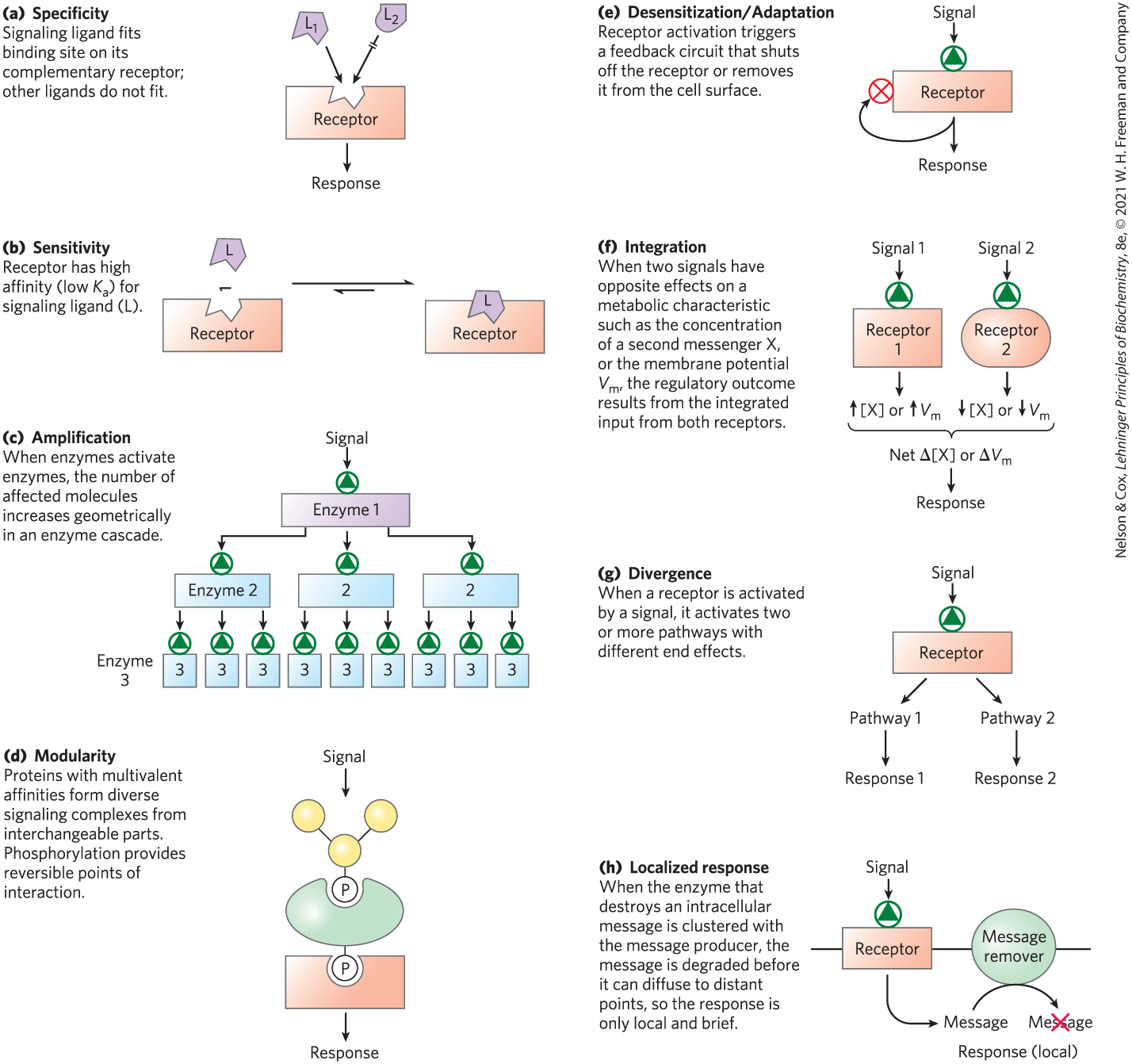
FIGURE 12-1 Eight features of signal-transducing systems.
Signal-Transducing Systems Share Common Features
The extraordinary sensitivity of signal transduction results from the high affinity of signal receptors for their ligands (Fig. 12-1b). This affinity can be expressed as the dissociation constant, , for the receptor-ligand interaction. is commonly or less, meaning that the receptor detects micromolar to nanomolar concentrations of a signaling ligand. In some cases, cooperativity in receptor-ligand interactions results in relatively large changes in receptor activation with small changes in ligand concentration, further increasing the sensitivity of signal detection.
Amplification results when an enzyme is activated by a signal receptor and, in turn, catalyzes the activation of many molecules of a second enzyme, each of which activates many molecules of a third enzyme and so on, in an enzyme cascade (Fig. 12-1c). Such cascades can produce amplifications of several orders of magnitude within milliseconds. The response to a signal must also be terminated, such that the downstream effects are in proportion to the strength of the original stimulus.
Interacting signaling proteins are modular. Many signaling proteins have multiple domains that recognize specific features in several other proteins, or in the cytoskeleton or plasma membrane. This modularity allows a cell to mix and match a set of signaling molecules to create a wide variety of multienzyme complexes with different functions or cellular locations. One common theme in these interactions is the binding of one modular signaling protein to phosphorylated residues in another protein; the resulting interaction can be regulated by phosphorylation or dephosphorylation of the protein partner (Fig. 12-1d). Nonenzymatic scaffold proteins with affinity for several enzymes that interact in cascades bring these enzymes together, ensuring that they interact at specific cellular locations and at specific times. Many of the regions involved in protein-protein interactions are intrinsically disordered (see Fig. 4-20), capable of folding differently depending on which protein they interact with. As a result, a single protein can have multiple functions in signaling pathways.
The sensitivity of receptor systems is subject to modification. When a signal is present continuously, the receptor system becomes desensitized (Fig. 12-1e), so that it no longer responds to the signal. When the stimulus falls below a certain threshold, the system again becomes sensitive. Think of what happens to your visual transduction system when you walk from bright sunlight into a darkened room or from darkness into the light.
Signal integration (Fig. 12-1f) is the ability of the system to receive multiple signals and produce a unified response appropriate to the combined needs of the cell or organism. Different signaling pathways converse with each other at several levels, generating complex cross talk that maintains homeostasis in the cell and the organism.
Signaling pathways are often divergent — branched rather than linear; one stimulus acts through one receptor, which activates two or more pathways with different downstream targets and responses (Fig. 12-1g).
A final noteworthy feature of signal-transducing systems is response localization within a cell (Fig. 12-1h). When the components of a signaling system are confined to a specific subcellular structure (a raft in the plasma membrane, for example), a cell can regulate a process locally, without affecting distant regions of the cell.
One of the revelations of research on signaling is the remarkable degree to which signaling mechanisms have been conserved during evolution. Although the number of different biological signals to which cells respond is probably in the thousands (Table 12-1 lists a few important types), and the kinds of responses elicited by these signals are comparably numerous, the machinery for transducing all of these signals is built from about 10 basic types of protein components (Table 12-2).
| Antigens | Mechanical touch |
| Cell surface glycoproteins, oligosaccharides | Microbial, insect pathogens |
| Developmental signals | Neurotransmitters |
| Extracellular matrix components | Nutrients |
| Growth factors | Odorants |
| Hormones | Pheromones |
| Hypoxia | Tastants |
| Light |
| Plasma membrane receptors with 7 transmembrane (7tm) helices |
| G proteins that bind GTP or GDP and interface with membrane receptors |
| Membrane enzymes with cyclic nucleotides as substrates or products |
| Protein kinases that phosphorylate GPCR receptors |
| Membrane protein tyrosine kinases |
| Cyclic nucleotide–dependent protein kinases |
| -binding proteins |
| -dependent protein kinases |
| Protein kinases that are activated during cell division |
| Nonenzymic protein scaffolds that bring modules together |
The General Process of Signal Transduction in Animals Is Universal
In this chapter we consider the molecular details of several representative signal-transduction systems, classified according to the type of receptor. The trigger for each system is different, but the general features of signal transduction are common to all: a signal (ligand) interacts with a receptor; the activated receptor interacts with cellular machinery, producing a second signal or a change in the activity of a cellular protein; the metabolic activity of the target cell undergoes a change; and finally, the transduction event ends. To illustrate these general features of signaling systems, we will look at examples of four basic receptor types (Fig. 12-2).
- G protein–coupled receptors that indirectly activate (through GTP-binding proteins, or G proteins) enzymes that generate intracellular second messengers. Examples include the β-adrenergic receptor system that responds to epinephrine (Section 12.2) and the vision, olfaction, and gustation systems (Section 12.3).
- Receptor enzymes in the plasma membrane that have an enzymatic activity on the cytoplasmic side, triggered by ligand binding on the extracellular side. Receptors with tyrosine kinase activity, for example, catalyze the phosphorylation of Tyr residues in specific intracellular target proteins. Examples include the insulin receptor (Section 12.4) and receptor guanylyl cyclases (see Box 12-2).
- Gated ion channels of the plasma membrane that open and close (hence the term “gated”) in response to the binding of chemical ligands or changes in transmembrane potential. These are the simplest signal transducers (Section 12.6).
- Nuclear receptors that bind specific ligands (such as the hormone estrogen) and alter the rate at which specific genes are transcribed and translated into cellular proteins. Because steroid hormones function through mechanisms intimately related to the regulation of gene expression, we consider them briefly in Section 12.7 and defer a detailed discussion of their action until Chapters 23 and 28.
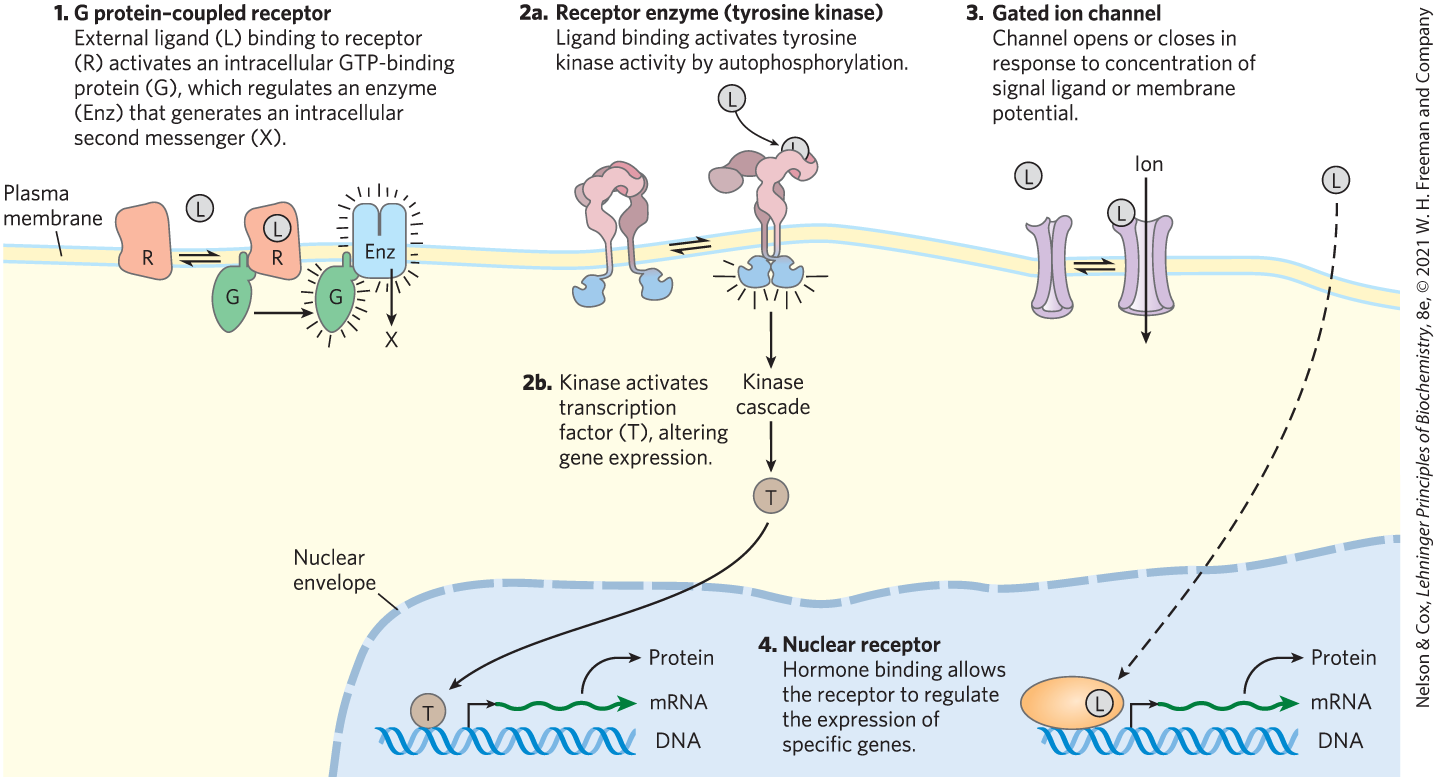
FIGURE 12-2 Four general types of signal transducers.
As we begin this discussion of biological signaling, a word about the nomenclature of signaling proteins is in order. These proteins are typically discovered in one context and named accordingly, then prove to be involved in a broader range of biological functions for which the original name is not helpful. For example, the retinoblastoma protein, pRb, was initially identified as the product of a mutation that contributes to cancer of the retina (retinoblastoma), but it is now known to function in many pathways essential to cell division in all cells, not just those of the retina. Some genes and proteins are given noncommittal names: the tumor suppressor protein p53, for example, is a protein of 53 kDa, but its name gives no clue to its great importance in the regulation of cell division and the development of cancer. In this chapter we generally define these protein names as we encounter them, introducing the names that are commonly used by researchers in the field.
SUMMARY 12.1 General Features of Signal Transduction
- All cells have specific and highly sensitive signal-transducing mechanisms, which have been conserved during evolution.
- A wide variety of stimuli act through specific protein receptors in the plasma membrane.
- In all types of signal transduction, receptors bind the signal ligand and initiate a process that amplifies the signal, integrates it with input from other receptors, and transmits the information throughout the cell. If the signal persists, receptor desensitization reduces or ends the response.
- Multicellular organisms have four general types of signaling mechanisms: plasma membrane proteins that act through G proteins, receptors with internal enzyme activity (such as tyrosine kinase), gated ion channels, and nuclear receptors that bind steroids and alter gene expression.
 Amplification results when an enzyme is activated by a signal receptor and, in turn, catalyzes the activation of many molecules of a second enzyme, each of which activates many molecules of a third enzyme and so on, in an
Amplification results when an enzyme is activated by a signal receptor and, in turn, catalyzes the activation of many molecules of a second enzyme, each of which activates many molecules of a third enzyme and so on, in an  This modularity allows a cell to mix and match a set of signaling molecules to create a wide variety of multienzyme complexes with different functions or cellular locations. One common theme in these interactions is the binding of one modular signaling protein to phosphorylated residues in another protein; the resulting interaction can be regulated by phosphorylation or dephosphorylation of the protein partner (
This modularity allows a cell to mix and match a set of signaling molecules to create a wide variety of multienzyme complexes with different functions or cellular locations. One common theme in these interactions is the binding of one modular signaling protein to phosphorylated residues in another protein; the resulting interaction can be regulated by phosphorylation or dephosphorylation of the protein partner ( All cells have specific and highly sensitive signal-transducing mechanisms, which have been conserved during evolution.
All cells have specific and highly sensitive signal-transducing mechanisms, which have been conserved during evolution.