12.6 Gated Ion Channels
Certain cells in multicellular organisms are “excitable”: they can detect an external signal, convert it into an electrical signal (specifically, a change in plasma membrane potential), and pass it on. Changes in membrane potential are effected by gated ion channels. Excitable cells play central roles in nerve conduction, muscle contraction, hormone secretion, sensory processes, and learning and memory.
Ion Channels Underlie Rapid Electrical Signaling in Excitable Cells
The excitability of sensory cells, neurons, and myocytes depends on ion channels, signal transducers that provide a regulated path for the movement of inorganic ions such as , and across the plasma membrane in response to various stimuli. Recall from Chapter 11 that these ion channels are gated: they may be open or closed, depending on whether the associated receptor has been activated by the binding of its specific ligand (a neurotransmitter, for example) or by a change in the transmembrane electrical potential (voltage-gated ion channels). The ATPase is electrogenic; it creates a charge imbalance across the plasma membrane by carrying 3 out of the cell for every 2 carried in (Fig. 12-32a). The action of the ATPase makes the inside of the cell negative relative to the outside. Inside the cell, is much higher and is much lower than outside the cell (Fig. 12-32b). The direction of spontaneous ion flow across a polarized membrane is dictated by the electrochemical potential of that ion across the membrane, which has two components: the difference in concentration of the ion on the two sides of the membrane, and the difference in electrical potential , typically expressed in millivolts (see Eqn 11-4, p. 392). Given the ion concentration differences and a of about (inside negative), opening of a or channel will result in a spontaneous inward flow of or (and depolarization), whereas opening of a channel will result in a spontaneous outward flow of (and hyperpolarization) (Fig. 12-32b). In this case, moves out of the cell against the electrical gradient, because the large concentration difference exerts a stronger effect than the . For , the membrane potential predominates, so when a channel opens, flows outward.

FIGURE 12-32 Transmembrane electrical potential. (a) The electrogenic ATPase produces a transmembrane electrical potential of about −60 mV (inside negative). (b) Blue arrows show the direction in which ions tend to move spontaneously across the plasma membrane in an animal cell, driven by the combination of chemical and electrical gradients. The chemical gradient drives and inward (producing depolarization) and outward, against its electrical gradient (producing hyperpolarization). The electrical gradient drives outward, against its concentration gradient (producing depolarization).
The number of ions that must flow to produce a physiologically significant change in the membrane potential is negligible relative to the concentrations of , and in cells and extracellular fluid, so the ion fluxes that occur during signaling in excitable cells have essentially no effect on the concentrations of these ions. With , the situation is different; because the intracellular is generally very low , inward flow of can significantly alter the cytosolic , allowing it to serve as a second messenger.
The membrane potential of a cell at a given time is the result of the types and numbers of ion channels open at that instant. The precisely timed opening and closing of ion channels and the resulting transient changes in membrane potential underlie the electrical signaling by which the nervous system stimulates the skeletal muscles to contract, the heart to beat, or secretory cells to release their contents. Moreover, many hormones exert their effects by altering the membrane potential of their target cells. These mechanisms are not limited to animals; ion channels play important roles in the responses of bacteria, protists, and plants to environmental signals.
To illustrate the action of ion channels in cell-to-cell signaling, we describe the mechanisms by which a neuron passes a signal along its length and across a synapse to the next neuron (or to a myocyte) in a cellular circuit, using acetylcholine as the neurotransmitter.
Voltage-Gated Ion Channels Produce Neuronal Action Potentials
Signaling in the nervous system is accomplished by networks of neurons, specialized cells that carry an electrical impulse (action potential) from one end of the cell (the cell body) through an elongated cytoplasmic extension (the axon), to the synapse with the next neuron. The electrical signal triggers release of the neurotransmitter acetylcholine into the synaptic cleft, carrying the signal to the next cell (neuron) in the circuit. Initially, the plasma membrane of the presynaptic neuron is polarized (inside negative) through the action of the electrogenic ATPase, which pumps out three for every two pumped in (Fig. 12-32). A rapid sequence of opening and closing of several types of ion channels (Fig. 12-33) produces a wave of depolarization (an action potential) that sweeps the neuron from the cell body to the end of an axon. First, opening of a voltage-gated channel allows entry, and the resulting local depolarization causes the adjacent channel to open, and so on (Fig. 12-33, step ). The directionality of movement of the action potential is ensured by the brief refractory period that follows the opening of each voltage-gated channel. A split second after the action potential passes a point in the axon, voltage-gated channels open (step ), allowing exit, which brings about repolarization of the membrane to make it ready for the next action potential (step ).
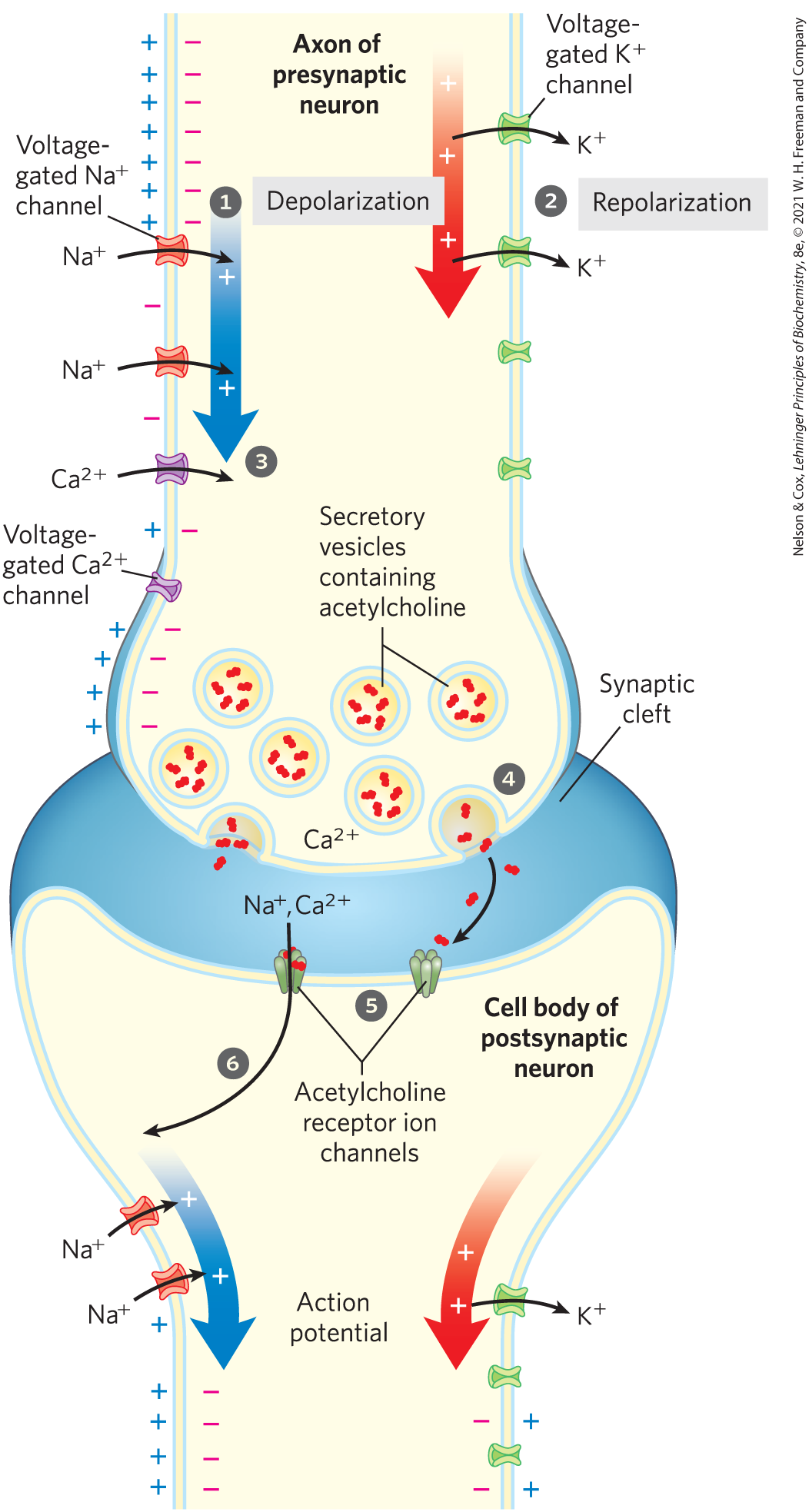
FIGURE 12-33 Role of voltage-gated and ligand-gated ion channels in neural transmission. The steps are described in the text. Stimulation of the presynaptic neuron (top) triggers a wave of depolarization (blue arrow) followed by repolarization (red arrow), producing an action potential that sweeps along the axon. At the synapse, depolarization opens channels, and entry triggers acetylcholine release into the synaptic cleft. Acetylcholine diffuses across the cleft, opening receptor channels, depolarizing the postsynaptic cell, and starting an action potential in the postsynaptic cell. Note that, for clarity, channels and channels are drawn on opposite sides of the axon, but both types are uniformly distributed in the axonal membrane. Also, positive and negative charges are shown only on the left, but as the wave of potential sweeps the axon, the membrane potential is the same at any given point along the axon.
When the wave of depolarization reaches the axon tip, voltage-gated channels open, allowing entry (step ). The resulting increase in internal triggers exocytotic release of the neurotransmitter acetylcholine into the synaptic cleft (step ). Acetylcholine binds to a receptor on the postsynaptic neuron or myocyte, causing its ligand-gated ion channel to open (step ). Extracellular and enter through this channel, depolarizing the postsynaptic cell. The electrical signal has thus passed to the cell body of the postsynaptic neuron and will move along its axon to a third neuron (or a myocyte) by this same sequence of events. We see, then, that gated ion channels convey signals in either of two ways: by changing the cytoplasmic concentration of an ion (such as ), which then serves as an intracellular second messenger, or by changing and affecting other membrane proteins that are sensitive to . The passage of an electrical signal through one neuron and on to the next illustrates both types of mechanism.
Neurons Have Receptor Channels That Respond to Different Neurotransmitters
Animal cells, especially those of the nervous system, contain a variety of ion channels gated by ligands, voltage, or both. Receptors that are themselves ion channels are classified as ionotropic, to distinguish them from receptors that generate a second messenger (metabotropic receptors). Acetylcholine acts on an ionotropic receptor in the postsynaptic cell. The acetylcholine receptor is a cation channel. When occupied by acetylcholine, the receptor opens to the passage of cations , triggering depolarization of the cell. The neurotransmitters serotonin, glutamate, and glycine all can act through ionotropic receptors that are structurally related to the acetylcholine receptor. Serotonin and glutamate trigger the opening of cation channels, whereas glycine opens -specific channels.
Depending on which ion passes through a channel, binding of the ligand (neurotransmitter) for that channel results in either depolarization or hyperpolarization of the target cell. A single neuron normally receives input from many other neurons, each releasing its own characteristic neurotransmitter with its characteristic depolarizing or hyperpolarizing effect. The target cell’s therefore reflects the integrated input (see Fig. 12-1f) from multiple neurons. The cell responds with an action potential only if the integrated input adds up to a net depolarization of sufficient magnitude.
The receptor channels for acetylcholine, glycine, glutamate, and γ-aminobutyric acid (GABA) are gated by extracellular ligands. Intracellular second messengers — such as cAMP, cGMP, , , and ATP — regulate ion channels of the type we saw in the sensory transductions of vision, olfaction, and gustation.
Toxins Target Ion Channels
Many of the most potent toxins found in nature act on ion channels. For example, dendrotoxin (from the black mamba snake) blocks the action of voltage-gated channels, tetrodotoxin (produced by puffer fish) acts on voltage-gated channels, and cobrotoxin (from cobras) disables acetylcholine receptor ion channels. Why, in the course of evolution, have ion channels become the preferred target of toxins, rather than some critical metabolic target such as an enzyme essential in energy metabolism?
Ion channels are extraordinary amplifiers; opening of a single channel can allow the flow of 10 million ions per second. Consequently, relatively few molecules of an ion-channel protein are needed per neuron for signaling functions. This means that a relatively small number of toxin molecules with high affinity for ion channels, acting from outside the cell, can have a pronounced effect on neurosignaling throughout the body. A comparable effect by way of a metabolic enzyme, typically present in cells at much higher concentrations than ion channels, would require far greater numbers of the toxin molecule.
SUMMARY 12.6 Gated Ion Channels
- Ion channels gated by membrane potential or ligands are central to signaling in neurons and other cells.
- The voltage-gated and channels of neuronal membranes carry the action potential along the axon as a wave of depolarization ( influx) followed by repolarization ( efflux). Arrival of an action potential at the distal end of a presynaptic neuron triggers neurotransmitter release.
- The neurotransmitter (acetylcholine, for example) diffuses to the postsynaptic neuron (or the myocyte, at a neuromuscular junction), binds to specific receptors in the plasma membrane, and triggers a change in . The cell body of the neuron has receptors for a variety of neurotransmitters or extracellular signals. The neuron’s is the sum of the effects of all ion-channel contributions.
- Neurotoxins, produced by many organisms, attack neuronal ion channels and are therefore fast-acting and deadly.
 The excitability of sensory cells, neurons, and myocytes depends on ion channels, signal transducers that provide a regulated path for the movement of inorganic ions such as , and across the plasma membrane in response to various stimuli. Recall from
The excitability of sensory cells, neurons, and myocytes depends on ion channels, signal transducers that provide a regulated path for the movement of inorganic ions such as , and across the plasma membrane in response to various stimuli. Recall from  ). The directionality of movement of the action potential is ensured by the brief refractory period that follows the opening of each voltage-gated channel. A split second after the action potential passes a point in the axon, voltage-gated channels open (step
). The directionality of movement of the action potential is ensured by the brief refractory period that follows the opening of each voltage-gated channel. A split second after the action potential passes a point in the axon, voltage-gated channels open (step  ), allowing exit, which brings about repolarization of the membrane to make it ready for the next action potential (step
), allowing exit, which brings about repolarization of the membrane to make it ready for the next action potential (step  ).
). ). The resulting increase in internal triggers exocytotic release of the neurotransmitter acetylcholine into the synaptic cleft (step
). The resulting increase in internal triggers exocytotic release of the neurotransmitter acetylcholine into the synaptic cleft (step  ). Acetylcholine binds to a receptor on the postsynaptic neuron or myocyte, causing its ligand-gated ion channel to open (step
). Acetylcholine binds to a receptor on the postsynaptic neuron or myocyte, causing its ligand-gated ion channel to open (step  ). Extracellular and enter through this channel, depolarizing the postsynaptic cell. The electrical signal has thus passed to the cell body of the postsynaptic neuron and will move along its axon to a third neuron (or a myocyte) by this same sequence of events. We see, then, that gated ion channels convey signals in either of two ways: by changing the cytoplasmic concentration of an ion (such as ), which then serves as an intracellular second messenger, or by changing and affecting other membrane proteins that are sensitive to . The passage of an electrical signal through one neuron and on to the next illustrates both types of mechanism.
). Extracellular and enter through this channel, depolarizing the postsynaptic cell. The electrical signal has thus passed to the cell body of the postsynaptic neuron and will move along its axon to a third neuron (or a myocyte) by this same sequence of events. We see, then, that gated ion channels convey signals in either of two ways: by changing the cytoplasmic concentration of an ion (such as ), which then serves as an intracellular second messenger, or by changing and affecting other membrane proteins that are sensitive to . The passage of an electrical signal through one neuron and on to the next illustrates both types of mechanism. Ion channels gated by membrane potential or ligands are central to signaling in neurons and other cells.
Ion channels gated by membrane potential or ligands are central to signaling in neurons and other cells.