7.1 Monosaccharides and Disaccharides
The simplest of the carbohydrates, the monosaccharides, are either aldehydes or ketones with two or more hydroxyl groups; the six-carbon monosaccharides glucose and fructose have five hydroxyl groups. Many of the carbon atoms to which the hydroxyl groups are attached are chiral centers, which give rise to the many sugar stereoisomers found in nature. Stereoisomerism in sugars is biologically significant because the enzymes that act on sugars are strictly stereospecific, typically preferring one stereoisomer to another by three or more orders of magnitude, as reflected in values or binding constants. It is as difficult to fit the wrong sugar stereoisomer into an enzyme’s binding site as it is to put your left glove on your right hand.
We begin by describing the families of monosaccharides with backbones of three to seven carbons — their structure, their stereoisomeric forms, and the means of representing their three-dimensional structures on paper. We then discuss several chemical reactions of the carbonyl groups of monosaccharides. One such reaction, the addition of a hydroxyl group from within the same molecule, generates cyclic forms with four or more backbone carbons (the forms that predominate in aqueous solution). This ring closure creates a new chiral center, adding further stereochemical complexity to this class of compounds. The nomenclature for unambiguously specifying the configuration about each carbon atom in a cyclic form and the means of representing these structures on paper are described in some detail; this information will be useful as we discuss the metabolism of monosaccharides in Part II. We also introduce here some important monosaccharide derivatives that will be examined closely in later chapters.
The Two Families of Monosaccharides Are Aldoses and Ketoses
Monosaccharides are colorless, crystalline solids that are freely soluble in water but insoluble in nonpolar solvents. Most have a sweet taste (Box 7-1). The backbones of common monosaccharides are unbranched carbon chains in which all the carbon atoms are linked by single bonds. In this open-chain form, one of the carbon atoms is double-bonded to an oxygen atom to form a carbonyl group; each of the other carbon atoms has a hydroxyl group. If the carbonyl group is at an end of the carbon chain (that is, in an aldehyde group), the monosaccharide is an aldose; if the carbonyl group is at any other position (in a ketone group), the monosaccharide is a ketose. The simplest monosaccharides are the two three-carbon trioses: glyceraldehyde, an aldotriose, and dihydroxyacetone, a ketotriose (Fig. 7-1a).
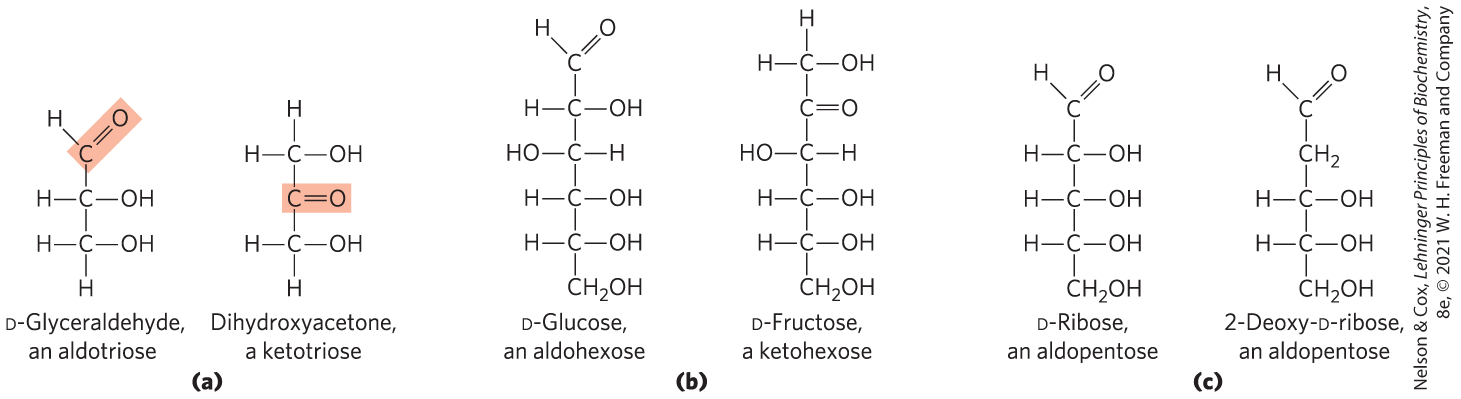
FIGURE 7-1 Representative monosaccharides. (a) Two trioses: an aldose and a ketose. The carbonyl group in each is shaded. (b) Two common hexoses. (c) The pentose components of nucleic acids. d-Ribose is a component of ribonucleic acid (RNA), and 2-deoxy-d-ribose is a component of deoxyribonucleic acid (DNA).
Part a shows D-glyceraldehyde, an aldotriose, and dihydroxyacetone, a ketotriose. D-glyceraldehyde is a vertical three carbon chain with highlighted C 2 double bonded to highlighted O and bonded to H, C 2 bonded to O H on the right and H on the left, and C 3 bonded to O H on the right and H below and to the left. Dihydroxyacetone is a vertical three-carbon chain with C 1 bonded to O H on the right and H above and to the left, highlighted C 2 bonded to highlighted O, and C 3 bonded to O H on the right and to H below and to the left. Part b shows D-glucose, an aldohexose, and D-fructose, a ketohexose. D-glucose is a vertical six-carbon chain with C 1 double bonded to O and bonded to H; C 2, C 4, and C 5 bonded to O H on the right and H on the left; C 3 bonded to H on the right and O H on the left; and C 6 bonded to 2 H and O H. D-fructose has a similar structure to D-glucose, except that C 1 is bonded to O H on the right and to H above and to the left and C 2 is double bonded to O. Part c shows D-ribose, an aldopentose, and 2-deoxy-D-ribose, an aldopentose. D-ribose is a five-carbon chain with C 1 double bonded to O and bonded to H; C 2, bonded to H 2; C 3 and C 4 each bonded to O H on the right and H on the left; and C 5 bonded to 2 H and O H. 2-deoxy-D-ribose has a similar structure, except that C 2 is bonded to 2 H instead of having an O H.
Monosaccharides with four, five, six, and seven carbon atoms in their backbones are called, respectively, tetroses, pentoses, hexoses, and heptoses. There are aldoses and ketoses of each of these chain lengths: aldotetroses and ketotetroses, aldopentoses and ketopentoses, and so on. The hexoses, which include the aldohexose d-glucose and the ketohexose d-fructose (Fig. 7-1b), are the most common monosaccharides in nature — the products of photosynthesis and key intermediates in the central energy-yielding reaction sequence in most organisms. The aldopentoses d-ribose and 2-deoxy-d-ribose (Fig. 7-1c) are components of nucleotides and nucleic acids (Chapter 8).
Monosaccharides Have Asymmetric Centers
All the monosaccharides except dihydroxyacetone contain one or more asymmetric (chiral) carbon atoms and thus occur in optically active isomeric forms (pp. 16–17). The simplest aldose, glyceraldehyde, contains one chiral center (the middle carbon atom) and therefore has two different optical isomers, or enantiomers (Fig. 7-2).

FIGURE 7-2 Three ways to represent the two enantiomers of glyceraldehyde. The enantiomers are mirror images of each other. Ball-and-stick models show the actual configuration of molecules. In Fischer projections, vertical lines point behind the plane of the page, and horizontal lines project above the page. Recall (see Fig. 1-17) that in perspective formulas, the wide end of a solid wedge projects out of the plane of the paper, toward the reader; a dashed wedge extends behind.
The three representations are ball-and-stick models, Fischer projection formulas, and perspective formulas. A ball-and-stick model is shown with C in the middle, O H to the front right, C H 2 O H to the rear below, H to the front left, and C H O to the rear behind. A mirror is shown to its right. The molecule in the mirror has C in the middle with O H to the front right, C H 2 O H to the bottom rear, C H O to the left side, and H to the right rear. Two Fischer projection formulas of glyceraldehyde are shown. D-glyceraldehyde has a central carbon with a yellow highlighted C H O above, a tan highlighted O H on the right, a blue highlighted C H 2 O H below, and H to the left. L-glyceraldehyde is similar, except that H is on the right and the tan highlighted O H is on the left. Two perspective formulas of glyceraldehyde are shown. D-glyceraldehyde has a central carbon with a hashed wedge to yellow highlighted C H O above, a solid wedge to tan highlighted O H on the right, a hashed wedge to blue highlighted C H 2 O H below, and a solid wedge to H on the left. L-glyceraldehyde is similar except that H is on the right and tan highlighted O H is on the left.
Key convention
One of the two enantiomers of glyceraldehyde is, by convention, designated the d isomer; the other is the l isomer. As for other biomolecules with chiral centers, the absolute configurations of sugars are known from x-ray crystallography. To represent three-dimensional sugar structures on paper, we often use Fischer projection formulas (Fig. 7-2). In these projections, bonds drawn horizontally indicate bonds that project out of the plane of the paper, toward the reader; bonds drawn vertically project behind the plane of the paper, away from the reader.
In general, a molecule with n chiral centers can have stereoisomers. Glyceraldehyde has ; the aldohexoses, with four chiral centers, have . The stereoisomers of monosaccharides of each carbon-chain length can be divided into two groups that differ in the configuration about the chiral center most distant from the carbonyl carbon. Those in which the configuration at this reference carbon is the same as that of d-glyceraldehyde are designated d isomers, and those with the same configuration as l-glyceraldehyde are l isomers. In other words, when the hydroxyl group on the reference carbon is on the right (dextro) in a projection formula that has the carbonyl carbon at the top, the sugar is the d isomer; when the hydroxyl group is on the left (levo), it is the l isomer. Of the 16 possible aldohexoses, eight are d forms and eight are l. Most of the hexoses of living organisms are d isomers. Why d isomers? An interesting and unanswered question. Recall that all of the amino acids found in proteins are exclusively one of two possible stereoisomers, l (p. 73). The basis for this initial preference for one isomer during evolution is unknown; however, once one isomer became prevalent, evolving enzymes able to use that isomer efficiently would have a selective advantage.
Figure 7-3 shows the structures of the d stereoisomers of all the aldoses and ketoses having three to six carbon atoms. The carbons of a sugar are numbered beginning at the end of the chain nearest the carbonyl group. Each of the eight d-aldohexoses, which differ in the stereochemistry at C-2, C-3, or C-4, has its own name: d-glucose, d-galactose, d-mannose, and so forth (Fig. 7-3a). The four- and five-carbon ketoses are designated by inserting “ul” into the name of a corresponding aldose; for example, d-ribulose is the ketopen-tose corresponding to the aldopentose d-ribose. (The importance of ribulose will become clear when we discuss the fixation of atmospheric by green plants, in Chapter 20.) The ketohexoses are named otherwise: for example, fructose (from the Latin fructus, “fruit”; fruits are one source of this sugar) and sorbose (from Sorbus, the genus of mountain ash, which has berries rich in the related sugar alcohol sorbitol). Two sugars that differ only in the configuration around one carbon atom are called epimers; d-glucose and d-mannose, which differ only in the stereochemistry at C-2, are epimers, as are d-glucose and d-galactose (which differ at C-4) (Fig. 7-4).
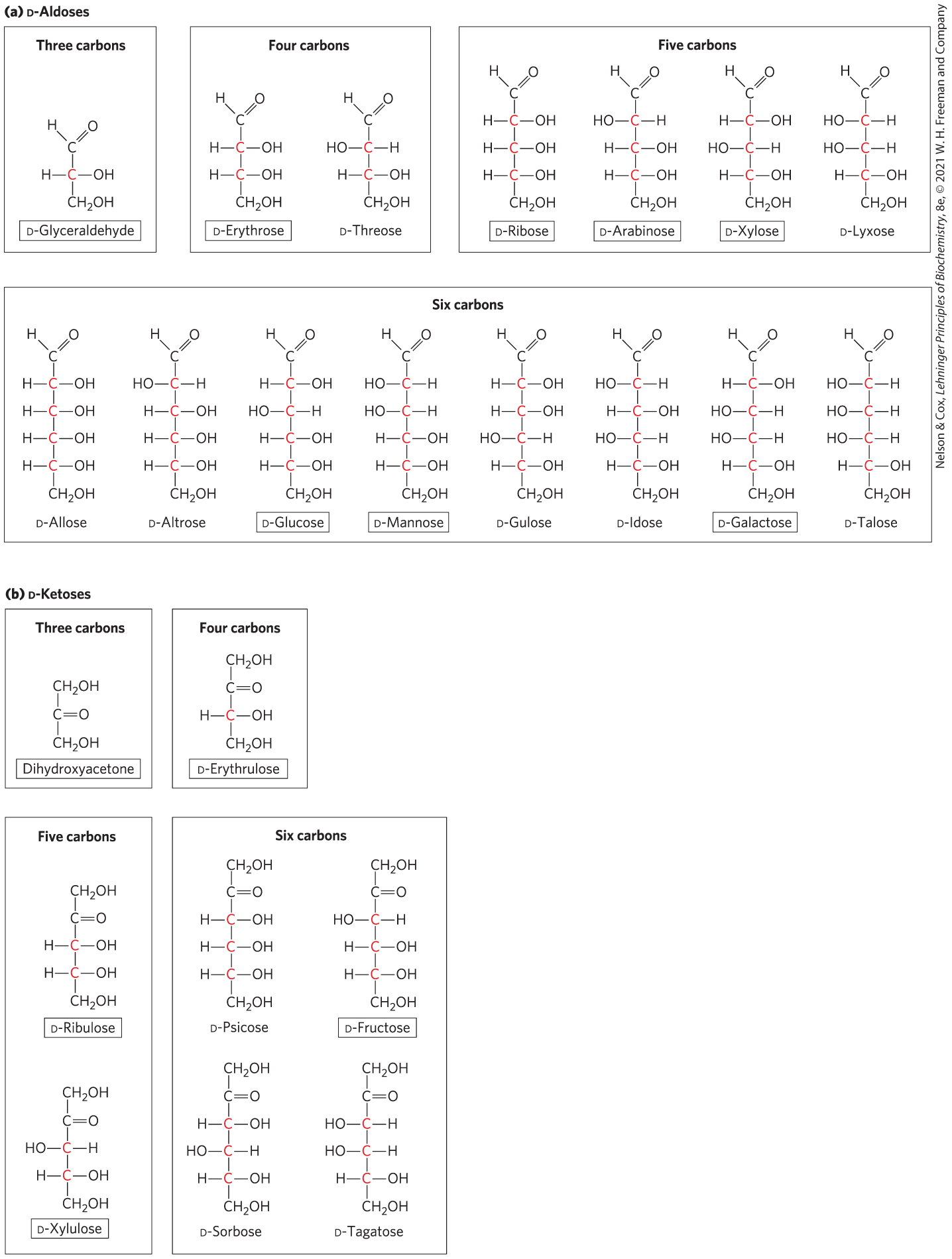
FIGURE 7-3 Aldoses and ketoses. The series of (a) d-aldoses and (b) d-ketoses having from three to six carbon atoms, shown as projection formulas. The carbon atoms in red are chiral centers. In all of these d isomers, the chiral carbon most distant from the carbonyl carbon has the same configuration as the chiral carbon in d-glyceraldehyde. Shown are the most common sugars in nature; you will encounter these again in this and later chapters.
Part a shows the D-aldoses. There is one three carbon D-aldose. D-glyceraldehyde, with its name in a box, has a vertical three carbon chain with C 1 double bonded to O and bonded to H, red highlighted C bonded to O H on the right and H on the left, and C 3 bonded to 2 H and O H. There are two four carbon D-aldoses. D-erythrose, with its name in a box, has a vertical four carbon chain with C 1 double bonded to O and bonded to H; red highlighted C 2 and C 3 both bonded to O H on the right and H on the left; and C 4 bonded to 2 H and O H. D-threose has a similar structure except that C 2 is bonded to H on the right and O H on the left. There are four five carbon D-aldoses. D-ribose, with its name in a box, is a five-carbon molecule with C 1 double bonded to O and bonded to H; red highlighted C 2, C 3, and C 4 all bonded to O H on the right and H on the left, and C 5 bonded to 2 H and O H. D-arabinose, with its name in a box, has a similar structure to D-ribose except that C 2 is bonded to H on the right and O H on the left. D-xylose, with its name in a box, has a similar structure to D-ribose except that C 3 is bonded to H on the right and O H on the left. D-lyxose has a similar structure to D-ribose except that C 2 and C 3 are both bonded to H on the right and to O H on the left. There are 8 six carbon D-aldoses. D-allose has a vertical six-carbon chain with C 1 double bonded to O and single bonded to H; red highlighted C 2, C 3, C 4, and C 5 are all bonded to O H on the right and H on the left, and C 6 is bonded to H 2 and O H. D-altrose has a similar structure to D-allose except that C 2 is bonded to H on the right and O H on the left. D-glucose, with its name in a box, has a similar structure to D-allose except that C-3 is bonded to H on the right and O H on the left. D-mannose, with its name in a box, has a similar structure to D-allose except that C 2 and C 3 both are bonded to H on the right and O H on the left. D-gulose has a similar structure to D-allose except that C 4 is bonded to H on the right and O H on the left. D-idose has a similar structure to D-allose except that C 2 and C 4 are bonded to H on the right and O H on the left. D-galactose, with its name in a box, is similar to D-allose except that C 3 and C 4 have H on the right and O H on the left. D-talose has a similar structure to D-allose except C 2, C 3, and C 4 all have H on the right and O H on the left. Part b shows the D-ketoses. There is one three carbon D-ketose, dihydroxyacetone, which has its name in a box and which has C 1 bonded to 2 H and O H, C 2 double bonded to O on the right, and C 3 bonded to 2 H and O H. There is one four carbon D-ketose, D-erythrulose, which has its name in a box and which has C 1 bonded to 2 H and O H, C 2 double bonded to O on the right, red highlighted C 3 bonded to O H on the right and H on the left, and C 4 bonded to 2 H and O H. There are two five carbon D-ketoses, D-ribulose and D-xylose. D-ribulose, which has its name in a box, has C 1 bonded to 2 H and O H; C 2 double bonded to O; red highlighted C 3 and C 4 both bonded to O H on the right and H on the left; and C 5 bonded to 2 H and O H. D-xylulose, which has its name in a box, has a similar structure to D-ribulose except that C 3 has H on the right and O H on the left. There are four six carbon D-ketoses. D-psicose has C 1 bonded to 2 H and O H; C 2 is double bonded to O; red highlighted C 3, C 4, and C 5 are all bonded to O H on the right and H on the left; and C 6 is bonded to 2 H and O H. D-fructose, which has its name in a box, has a similar structure to D-psicose except that C 3 is bonded to H on the right and O H on the left. D-sorbose has a similar structure to D-psicose, except that C 4 has H on the right and O H on the left. D-tagatose has a similar structure to D-psicose except that C 3 and C 4 both have H on the right and O H on the left.
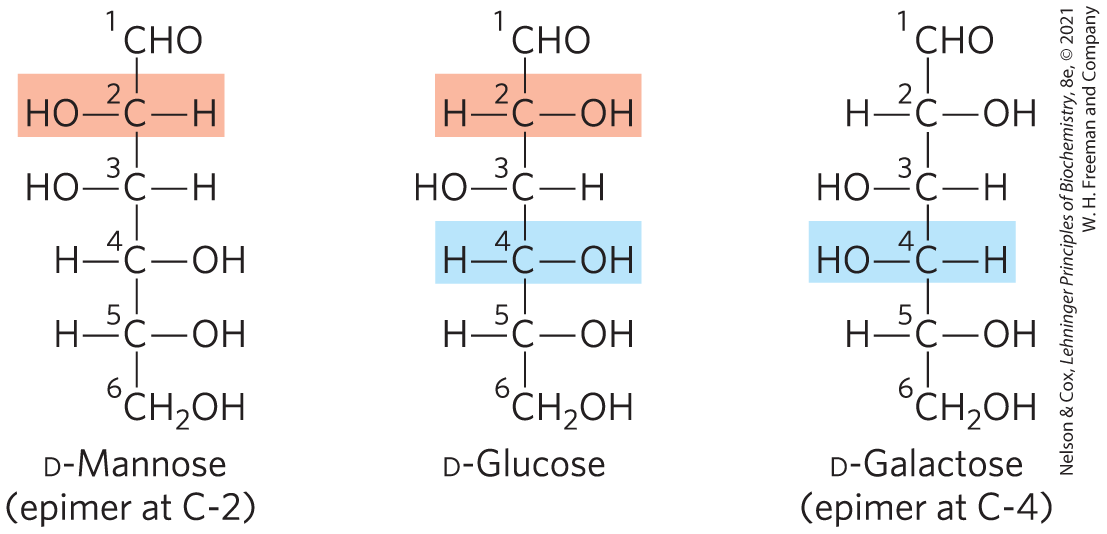
FIGURE 7-4 Epimers. d-Glucose and two of its epimers are shown as projection formulas. Each epimer differs from d-glucose in the configuration at one chiral center (shaded light red or blue).
D-mannose is an epimer at C-2. It is a vertical six-carbon chain with C 1 double bonded to O and bonded to H; C 2 bonded to H on the right and O H on the left with all of the atoms highlighted in a red band; C 3 bonded to H on the right and O H on the left, C 4 bonded to O H on the right and H on the left, C 5 bonded to O H on the right and H on the left; and C 6 bonded to 2 H and O H. D-glucose has a similar structure to D-mannose, except that C 2 is bonded to O H on the right and to H on the left, all highlighted with a red band. A blue band highlights C 4 and the O H and H bonded to it. D-galactose is an epimer at C-4. It has a similar structure to glucose, except that C 4 is bonded to H on the right and O H on the left, all highlighted with a blue band.
Some sugars occur naturally in their l form; examples are l-arabinose and the l isomers of some sugar derivatives that are common components of glycoconjugates (Section 7.3).
The Common Monosaccharides Have Cyclic Structures
For simplicity, we have thus far represented the structures of aldoses and ketoses as straight-chain molecules (Figs 7-3, 7-4). In fact, in aqueous solution, aldotetroses and all monosaccharides with five or more carbon atoms in the backbone occur predominantly as cyclic (ring) structures in which the carbonyl group has formed a covalent bond with the oxygen of a hydroxyl group in the same sugar molecule. The formation of these ring structures is the result of a general reaction between alcohols and aldehydes or ketones to form derivatives called hemiacetals or hemiketals. Two molecules of an alcohol can add to a carbonyl carbon; the product of the first addition is a hemiacetal (for addition to an aldose) or a hemiketal (for addition to a ketose). If the —OH and carbonyl groups are on the same molecule, a five- or six-membered ring results. Addition of the second molecule of alcohol produces the full acetal or ketal (Fig. 7-5), and the linkage formed is a glycosidic bond. When the two molecules that react are monosaccharides, the acetal or ketal formed is a disaccharide.
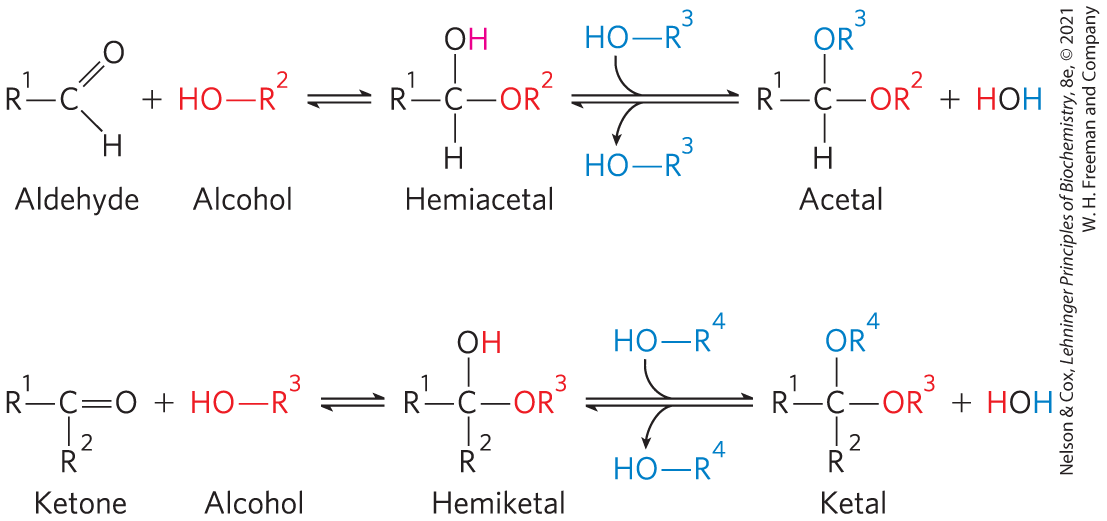
FIGURE 7-5 Formation of hemiacetals and hemiketals. An aldehyde or a ketone can react with an alcohol in a 1:1 ratio to yield a hemiacetal or a hemiketal, respectively, creating a new chiral center at the carbonyl carbon. Substitution of a second alcohol molecule produces an acetal or a ketal. When the second alcohol is part of another sugar molecule, the bond produced is a glycosidic bond.
The top series of reactions begins with an aldehyde that has a C that is double bonded to O on the upper right, single bonded to H on the lower right, and single bonded to R superscript 1 on the left. This is added to a red highlighted alcohol that has O H bonded to R superscript 2. This undergoes a reversible reaction to produce a hemiacetal that has a central C bonded to red highlighted O R superscript 2 on the right, H below, R superscript 1 on the left, and O bonded to red highlighted H above. This undergoes a reversible reaction to produce an acetal. During the forward reaction, blue highlighted H O bonded to R superscript 3 is added. This produces an acetal plus red highlighted H O blue highlighted H. During the reverse reaction, blue highlighted H O bonded to R superscript 3 is removed. The acetal has a central C bonded to red highlighted O R superscript 2 on the right, H below, R superscript 1 on the left, and blue highlighted O R superscript 3 above. The bottom series of reactions begins with a ketone that has a C that is double bonded to O on the right, single bonded to R superscript 2 below, and single bonded to R superscript 1 on the left. This is added to a red highlighted alcohol that has H O bonded to R superscript 3. This undergoes a reversible reaction to produce a hemiketal that has a central C bonded to red highlighted O R superscript 3 on the right, R superscript 2 below, R superscript 1 on the left, and O bonded to red highlighted H above. This undergoes a reversible reaction to produce an acetal. During the forward reaction, blue highlighted H O bonded to R superscript 4 is added. This produces a ketal plus red highlighted H O blue highlighted H. During the reverse reaction, blue highlighted H O bonded to R superscript 4 is removed. The ketal has a central C bonded to red highlighted O R superscript 3 on the right, R superscript 2 below, R superscript 1 on the left, and blue highlighted O R superscript 4 above.
The reaction with the first molecule of alcohol creates an additional chiral center (the carbonyl carbon). Because the alcohol can add in either of two ways, attacking either the “front” or the “back” of the carbonyl carbon, the reaction can produce either of two stereoisomeric configurations, denoted α and β. For example, d-glucose exists in solution as an intramolecular hemiacetal in which the free hydroxyl group at C-5 has reacted with the aldehydic C-1, rendering the latter carbon asymmetric and producing two possible stereoisomers, designated α and β (Fig. 7-6). Isomeric forms of monosaccharides that differ only in their configuration about the hemiacetal or hemiketal carbon atom are called anomers, and the carbonyl carbon atom is called the anomeric carbon.
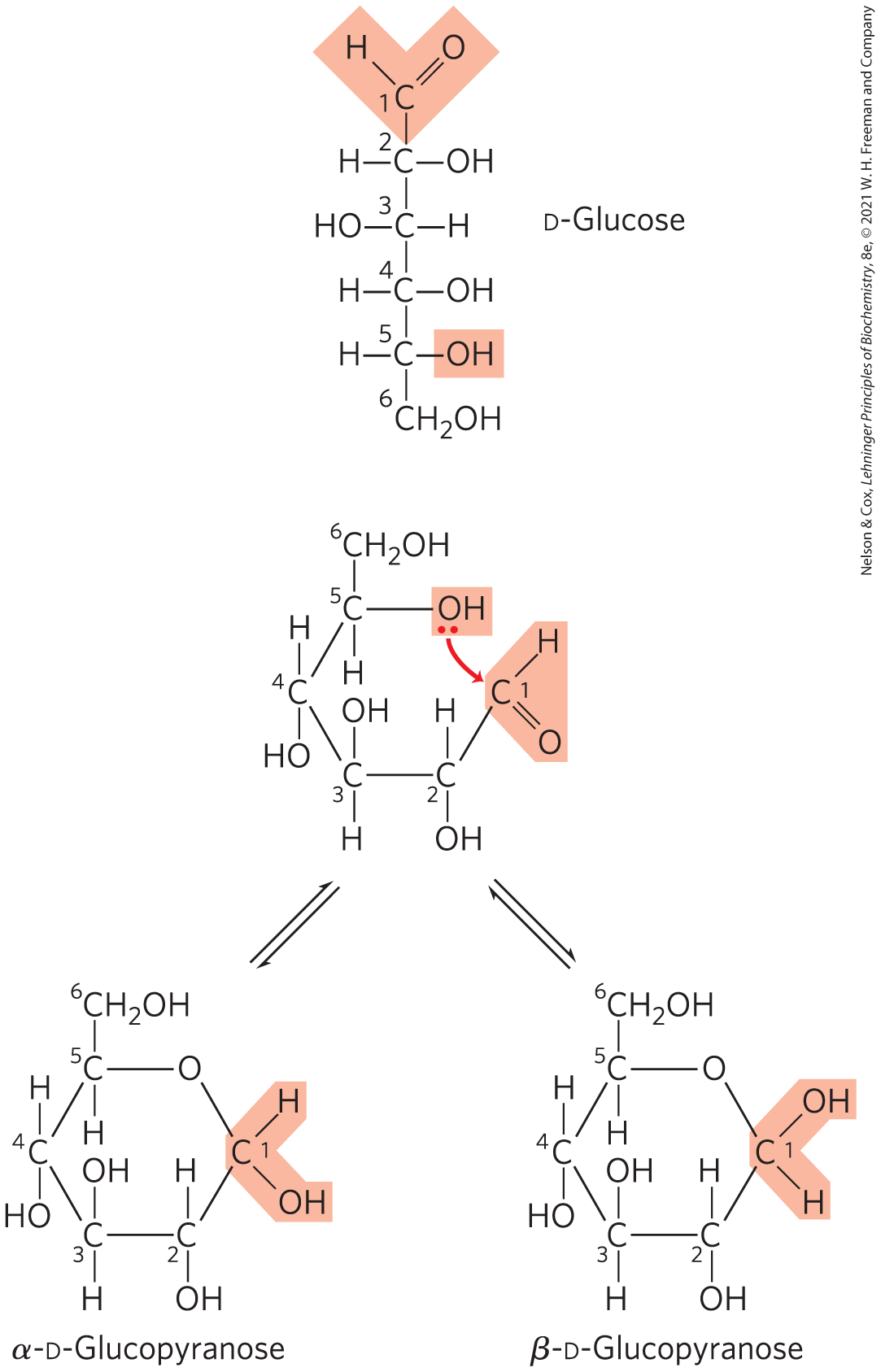
FIGURE 7-6 Formation of the two cyclic forms of d-glucose. Reaction between the aldehyde group at C-1 and the hydroxyl group at C-5 forms a hemiacetal linkage, producing either of two stereoisomers, the α and β anomers, which differ only in the stereochemistry around the hemiacetal carbon. This reaction is reversible. The interconversion of α and β anomers is called mutarotation.
D-glucose is shown as a vertical six-carbon chain. C 1 is double bonded to O and single bonded to H, and this entire group of atoms is highlighted in brown. C 2, C 4, and C 5 are all bonded to O H on the right and H on the left, but the O H on C 5 is highlighted in brown. C 3 is bonded to H on the right and O H on the left. C 6 is bonded to 2 H and O H. Below, the same molecule is bent into a hexagonal shape with C 1 at the right side vertex. C 2 is at the bottom right vertex with H above the ring and O H below the right; C 3 is at the bottom left vertex with O H above the ring and H below the ring; C 4 is at the left side vertex with H above the ring and O H below the ring, C 5 is at the top left vertex with C 6 above the ring and H below the ring; O H is at the top right vertex with a pair of highlighted electrons and an arrow pointing to C 1. C 6 is above the ring and is bonded to 2 H and O H. Two possible reversible reactions can occur. The first reaction produces Greek letter alpha D-glycopyranose. This has the ring structure already described, except that there is now a bond between O and C 1, the O in the ring is no longer bonded to H, and C 1 is bonded to H above the ring and O H below the right. C 1 and its associated H and O H are highlighted in brown. The other reversible reaction produces a similar structure, except that C 1 is bonded to O H above the ring and H below the ring. C 1 and its associated H and O H are highlighted in brown.
Six-membered ring compounds are called pyranoses because they resemble the six-membered ring compound pyran (shown in Fig. 7-7). The systematic names for the two ring forms of d-glucose are therefore α-d-glucopyranose and β-d-glucopyranose. Ketohexoses (such as fructose) also occur as cyclic compounds with α and β anomeric forms. In these compounds, the hydroxyl group at C-5 (or C-6) reacts with the keto group at C-2 to form a furanose (or pyranose) ring containing a hemiketal linkage (Fig. 7-5). d-Fructose readily forms the furanose ring (Fig. 7-7); the more common anomer of this sugar in combined forms or in derivatives is β-d-fructofuranose.
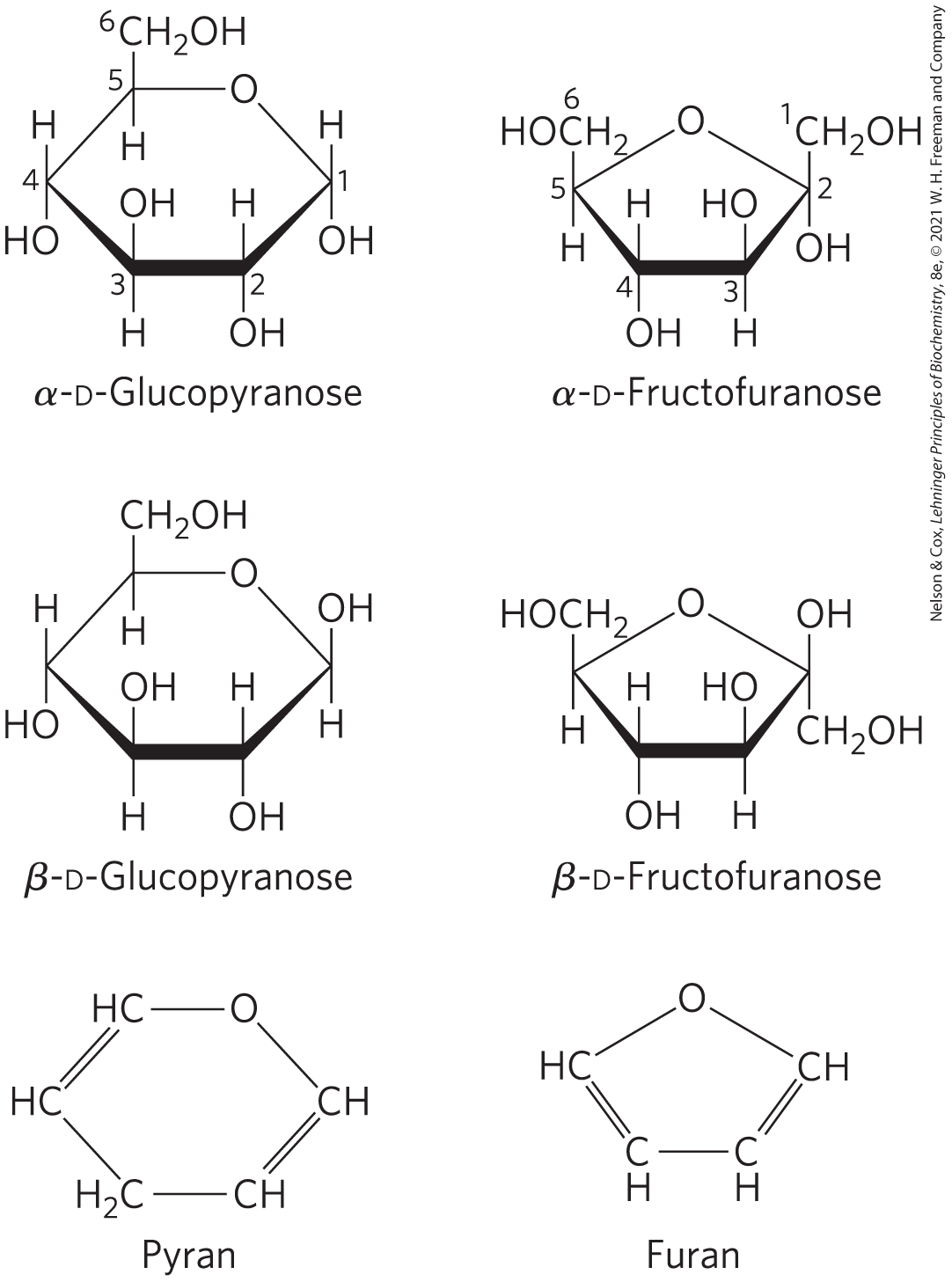
FIGURE 7-7 Pyranoses and furanoses. The pyranose forms of d-glucose and the furanose forms of d-fructose are shown here as Haworth perspective formulas. The edges of the ring nearest the reader are represented by bold lines. Hydroxyl groups below the plane of the ring in these Haworth perspectives would appear at the right side of a Fischer projection (compare with Fig. 7-6). Pyran and furan are shown for comparison.
Greek letter alpha D glucopyranose has a six-membered ring structure with a wedge showing on the front three bonds. There is a thick bond between C 2 and C 3 that meets similar thickness in the bonds on either side from C 3 to C 4 and from C 2 to C 1, which get narrower as they extend back to C 4 and C 1, respectively. C 1, C 2, and C 4 are bonded to H above the ring and O H below the ring; C 3 is bonded to O H above the ring and H below the right, and C 5 is bonded to C 6 above the ring and H below the ring. C 6 is bonded to 2 H and O H and O is between C 5 and C 1 at the top right vertex. Greek letter alpha D-fructofuranose has a five-membered ring with O at the top vertex. C 2 is at the right side vertex and is bonded to C 1 above and O H below. C 1 is bonded to 2 H and O H. C 3 is bonded to O H above and H below. F 4 is bonded to H above and O H below. C 5 is bonded to C 6 above and H below. C 6 is bonded to 2 H and O H. Greek letter beta D-glucopyranose is similar to Greek letter alpha D-glucopyranose, except that C 1 is bonded to O H above the right and H below the ring. Greek letter beta D-fructofuranose is similar to alpha D-fructofuranose, except that C 2 is bonded to O H above the ring and C 1 below the ring. Pyran is shown as a six-membered ring with O at the top right vertex; C H at the right, bottom right, left, and top left vertices; C H 2 at the bottom left vertex; and double bonds at the top left and bottom right sides. Furan is shown as a five-membered ring with O at the top vertex, with C H at the other vertices, and with double bonds on the left and right bottom side vertices.
Cyclic sugar structures are more accurately represented in Haworth perspective formulas than in the Fischer projections commonly used for linear sugar structures. In Haworth perspectives, the six-membered ring is tilted to make its plane almost perpendicular to that of the paper, with the bonds closest to the reader drawn thicker than those farther away, as in Figure 7-7.
Key convention
To convert the Fischer projection formula of any linear d-hexose to a Haworth perspective formula showing the molecule’s cyclic structure, draw the six-membered ring (five carbons, and one oxygen at the upper right), number the carbons in a clockwise direction beginning with the anomeric carbon, then place the hydroxyl groups. If a hydroxyl group is to the right in the Fischer projection, it is placed pointing down (i.e., below the plane of the ring) in the Haworth perspective; if it is to the left in the Fischer projection, it is placed pointing up (i.e., above the plane) in the Haworth perspective. The terminal group projects upward for the d enantiomer, downward for the l enantiomer. The hydroxyl on the anomeric carbon can point up or down. When the anomeric hydroxyl of a d-hexose is on the same side of the ring as C-6, the structure is by definition β; when it is on the opposite side from C-6, the structure is α.
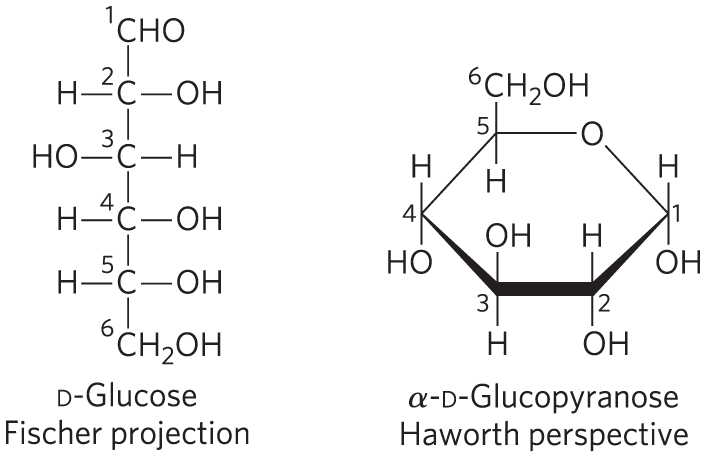
The D-glucose Fischer projection shows a vertical six-carbon chain with C 1 double bonded to O and bonded to H; C 2, C 4, and C 5 bonded to O H on the right and H on the left; C 3 bonded to H on the right and O H on the left; and C 6 bonded to 2 H and O H. Alpha D-glucopyranose is represented by Haworth perspective. It is a ring with O at the upper right vertex between C 5 and C 6. C 1, C 2, and C 4 all are bonded to H above and O H below; C 3 is bonded to O H above and H below; C 5 is bonded to C 6 above and H below; and C 6 is bonded to 2 H and O H. There is a thick bond between C 2 and C 3 that meets similar thickness in the bonds on either side from C 3 to C 4 and from C 2 to C 1, which get narrower as they extend back to C 4 and C 1, respectively.
WORKED EXAMPLE 7-1 Conversion of Fischer Projection to Haworth Perspective Formulas
Draw the Haworth perspective formulas for d-mannose and d-galactose.
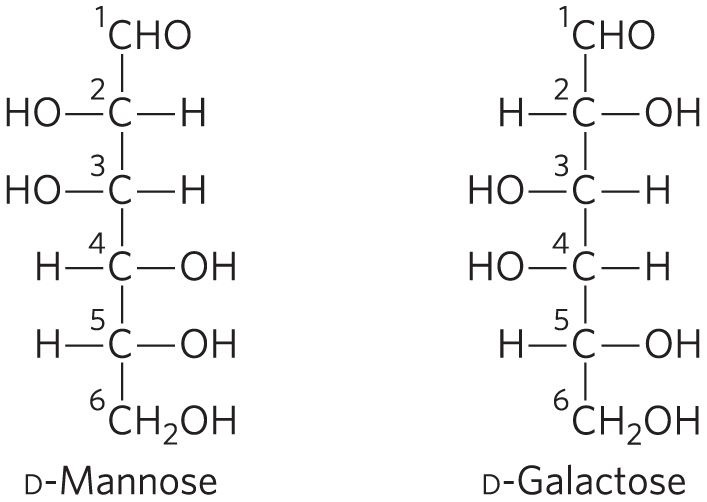
D-mannose is a vertical six-membered chain with C 1 double bonded to O and bonded to H; C 2 and C 3 bonded to H on the right and O H on the left; C 4 and C 5 bonded to O H on the right and H on the left; and C 6 bonded to H 2 and O H. D-galactose has a similar structure, except that C 2 and C 5 are bonded to O H on the right and H on the left and C 3 and C 4 are bonded to O H on the right and H on the left.
SOLUTION:
Pyranoses are six-membered rings, so start with six-membered Haworth structures with the oxygen atom at the top right. Number the carbon atoms clockwise, starting with the aldose carbon. For mannose, place the hydroxyls on C-2, C-3, and C-4 above, above, and below the ring, respectively (because in the Fischer projection they are on the left, left, and right sides of the mannose backbone). For d-galactose, the hydroxyls are oriented below, above, and above the ring for C-2, C-3, and C-4, respectively. The hydroxyl at C-1 can point either up or down; there are two possible configurations, α and β, at this carbon.
WORKED EXAMPLE 7-2 Drawing Haworth Perspective Formulas of Sugar Isomers
Draw the Haworth perspective formulas for α-d-mannose and β-l-galactose.
SOLUTION:
The Haworth perspective formula of d-mannose from Worked Example 7-1 can have the hydroxyl group at C-1 pointing either up or down. According to the Key Convention, for the α form, the C-1 hydroxyl is pointing down when C-6 is up, as it is in d-mannose.
For β-l-galactose, use the Fischer representation of d-galactose (see Worked Example 7-1) to draw the correct Fischer representation of l-galactose, which is its mirror image: the hydroxyls at C-2, C-3, C-4, and C-5 are on the left, right, right, and left sides, respectively. Now draw the Haworth perspective, a six-membered ring in which the —OH groups on C-2, C-3, and C-4 are oriented up, down, and down, respectively, because in the Fischer representation they are on the left, right, and right sides. Because it is the β form, the —OH on the anomeric carbon points down (same side as C-5).
The α and β anomers of d-glucose interconvert in aqueous solution by a process called mutarotation, in which one ring form (say, the α anomer) opens briefly into the linear form, then closes again to produce the β anomer (Fig. 7-6). Thus, a solution of β-d-glucose and a solution of α-d-glucose eventually form identical equilibrium mixtures having identical optical properties. This mixture consists of about one-third α-d-glucose, two-thirds β-d-glucose, and very small amounts of the linear form and the five-membered ring (glucofuranose) form.
Haworth perspective formulas like those in Figure 7-7 are commonly used to show the stereochemistry of ring forms of monosaccharides. However, the six-membered pyranose ring is not planar, as Haworth perspectives suggest, but tends to assume either of two “chair” conformations (Fig. 7-8). Recall from Chapter 1 that two conformations of a molecule are interconvertible without the breakage of covalent bonds, whereas two configurations can be interconverted only by breaking a covalent bond. To interconvert α and β configurations, the bond involving the ring oxygen atom has to be broken, but interconversion of the two chair forms (which are conformers) does not require bond breakage and does not change configurations at any of the ring carbons. The specific three-dimensional structures of the monosaccharide units are important in determining the biological properties and functions of some polysaccharides, as we shall see.

FIGURE 7-8 Conformational formulas of pyranoses. (a) Two chair forms of the pyranose ring of β-d-glucopyranose. Two conformers such as these are not readily interconvertible; an input of about 46 kJ of energy per mole of sugar is required to force the interconversion of chair forms. Another conformation, the “boat” (not shown), is seen only in derivatives with very bulky substituents. (b) The preferred chair conformation of α-d-glucopyranose.
Part a shows two possible chair forms of Greek letter beta D-glucopyranose. The first molecule, on the left, is a six-membered ring with C 4 positioned upward above C 3 and C 5, forming a triangle-shaped upward part. O and C 2 are higher than C 3 and C 5, creating an upward slanted central region. Then the bonds from O and C 2 angle downward to C 1, forming a downward pointing triangular part. A vertical red dotted line extends through the center of the slanted central region, separating C 5 from O and C 3 from C 2. The substituents are bonded by vertical lines or by angled bonds that extend away from the ring. The overall molecule has C 1 at the lower right with O H above the ring at an angle and H vertically beneath the ring; C 2 is higher and connected to C 1 by a bond that gets thicker as it approaches C 2; C 2 is bonded to H above by a vertical line and by O H below by a line that slants to the right; C 3 is lower and connected to C 2 by a thick bond; C 3 is bonded to O H above the ring by a slanted bond to the right and to H below the ring by a vertical line; C 4 is higher at the tip of a triangular shape and connected by a bond that narrows as it travels from C 3 to C 4; C 4 is bonded to H above by a vertical line and by O H below by a bond that angles to the left; C 5 is bonded to C H 2 O H above the ring by a bond that angles to the left and to H below the ring by a vertical line. The other chair form of Greek letter beta D-glucopyranose, on the right, has C 4 pointing down and C 1 pointing up. It is drawn with C 4 positioned lower than C 3 and C 5, forming a triangle-shaped downward part. O and C 2 are lower than C 3 and C 5, creating a downward slanted central region. Then the bonds from O and C 2 angle upward to C 1, forming an upward pointing triangular part. A vertical red dotted line extends through the center of the slanted central region, separating C 5 from O and C 3 from C 2. The overall molecule has C 1 at the upper right with O H bonded above by a vertical line and H bonded below the ring by a line slanting to the lower right; C 2 has H bonded above the ring at an angle and O H bonded below by a vertical line; C 3 has O H bonded above the ring by a vertical line and H bonded below the ring at an angle; C 4 has H bonded above the ring at an angle and O H bonded below the ring by a vertical line; C 5 has H bonded below the ring at an angle and C H 2 O H bonded above the ring by a vertical line. Part b shows Greek letter alpha D-glucopyranose. It has a similar structure to the left form of Greek letter beta D-glucopyranose with C 1 angled down, except that C 1 has H bonded above the ring at an angle and O H bonded below the ring by a vertical line.
Organisms Contain a Variety of Hexose Derivatives
In addition to simple hexoses such as glucose, galactose, and mannose, there are many sugar derivatives in which a hydroxyl group in the parent compound is replaced with another substituent, or a carbon atom is oxidized to a carboxyl group (Fig. 7-9). In glucosamine, galactosamine, and mannosamine, the hydroxyl at C-2 of the parent compound is replaced with an amino group. The amino group is commonly condensed with acetic acid, as in N-acetylglucosamine. This glucosamine derivative is part of many structural polymers, including those of the bacterial cell wall. Substitution of a hydrogen for the hydroxyl group at C-6 of l-galactose or l-mannose produces l-fucose or l-rhamnose, respectively. l-Fucose is found in the complex oligosaccharide components of glycoproteins and glycolipids; l-rhamnose is found in plant polysaccharides.
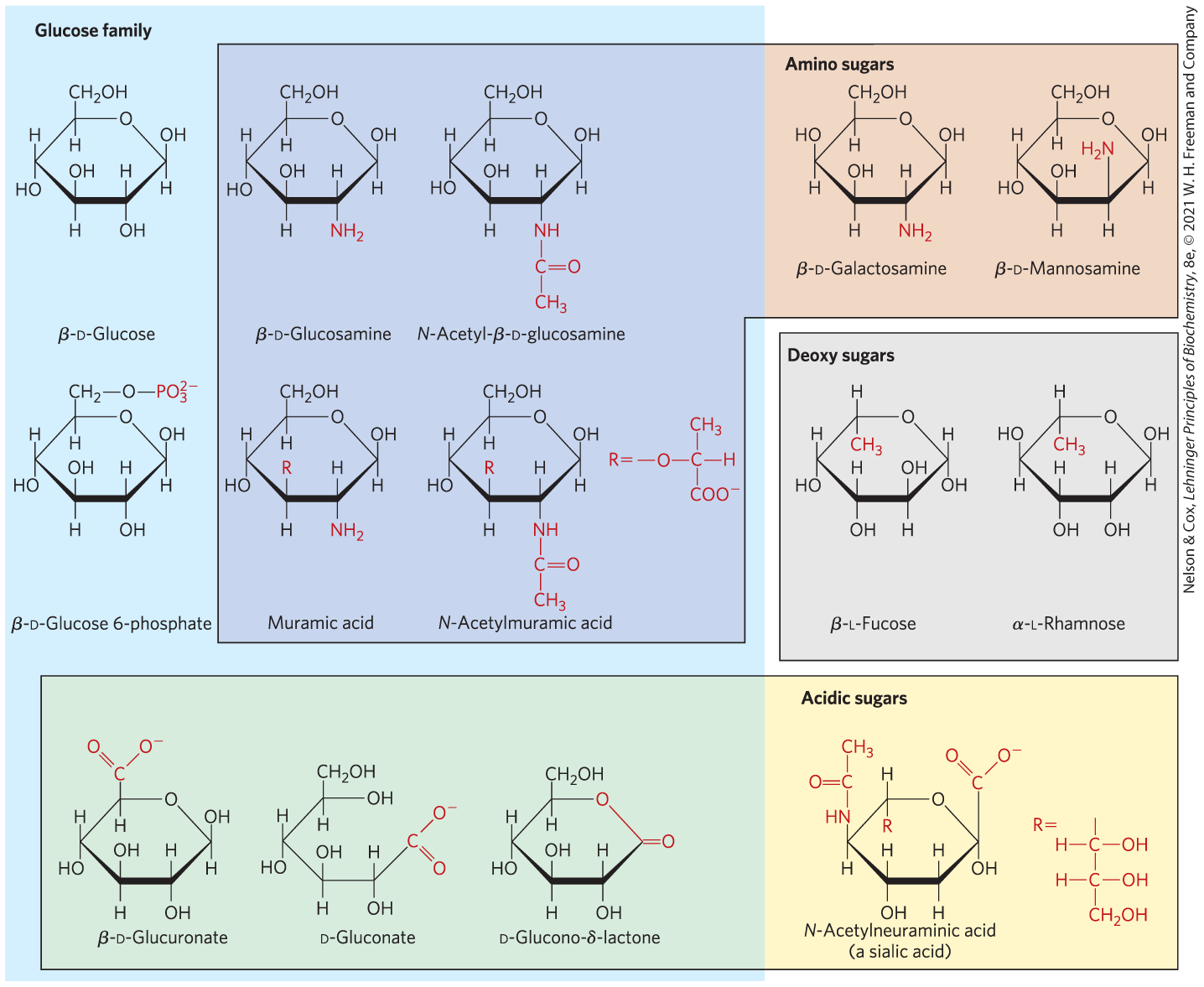
FIGURE 7-9 Some hexose derivatives important in biology. In amino sugars, an group replaces one of the —OH groups in the parent hexose. Substitution of OH for —OH produces a deoxy sugar; note that the deoxy sugars shown here occur in nature as the l isomers. The acidic sugars contain a carboxylate group, which confers a negative charge at neutral pH. d-Glucono-δ-lactone results from formation of an ester linkage between the C-1 carboxylate group and the C-5 (also known as the δ carbon) hydroxyl group of d-gluconate.
The sugars in each family are as follows. Some sugars are in more than one family. The glucose family includes Greek letter beta D-glucose, Greek letter beta D-glucose 6-phosphate, Greek letter beta D-glucosamine, N-acetyl Greek letter beta D-glucosamine, muramic acid, N-acetylmuramic acid, Greek letter beta D-glucuronate, D-gluconate, and D-glucono Greek letter delta lactone. The amino sugars include Greek letter beta D-galactosamine, Greek letter beta D-mannosamine, Greek letter beta D-glucosamine, N-acetyl Greek letter beta D-glucosamine, muramic acid, and N-acetylmuramic acid. The deoxysugars include Greek letter beta L-fucose and Greek letter alpha L-rhamnose. The acidic sugars include N-acetylneuraminic acid (a sialic acid), Greek letter beta D-glucuronate, D-gluconate, and D-glucono Greek letter delta lactone. Greek letter beta D-glucose is a ring with C 1 and C 3 bonded to O H above the ring and H below the ring; C 2 and C 3 bonded to H above the ring and O H below the ring, C 5 bonded to C 6 above the ring and H below the ring; C 6 bonded to 2 H and O H; and O at the top right vertex between C 5 and C 1. Greek letter beta D-glucose 6-phosphate has a similar structure to Greek letter beta D-glucose, except that C 6 is bonded to 2 H and to O that is further bonded to red highlighted P O subscript 3 superscript 3 minus. Greek letter beta D-glucosamine has a similar structure to beta D-glucose, except that C 2 is bonded below the ring to red highlighted N H 2. N-acetyl Greek letter beta D-glucosamine has a similar structure to Greek letter beta D-glucose, except that C 2 is bonded to a red highlighted substituent with N H further bonded to C that is double bonded to O and further bonded to C H 3. Muramic acid has a similar structure to Greek letter beta D-glucose, except that C 2 is bonded below the ring to red highlighted N H 2 and C 3 is bonded above the ring to red highlighted R. R is a red highlighted group with O that is further bonded to C that is bonded to C H 3 above, H on the right, and C O O minus below. N-acetylmuramic acid is similar to muramic acid, except that C 2 is bonded to N H that is further bonded to C that is double bonded to O and further bonded to C H 3. Greek letter beta D-galactosamine is similar to Greek letter beta D-glucose, except that it has C 2 bonded to red highlighted N H 2 below the ring. Greek letter beta D-mannosamine is similar to Greek letter beta D-glucose, except that it has C 2 bonded to red highlighted N H 2 above the ring. Greek letter beta L-fucose is similar to Greek letter beta D-glucose, except that C 1 is bonded to H above the ring and O H below the ring, C 2 is bonded to O H above the ring and H below the ring, C 3 is bonded to H above the ring and O H below the ring, and C 5 is bonded to H above the ring and red highlighted C H 3 below the ring. Greek letter alpha L-rhamnose is similar to Greek letter beta D-glucose, except that C 3 is bonded to H above the ring and O H below the ring, C 4 is bonded to O H above the ring and H below the ring, and C 5 is bonded to H above the right and red highlighted C H 3 below the ring. Greek letter beta D-glucuronate is similar to Greek letter beta D-glucose, except that it has C 5 bonded to a red highlighted group above the ring with C that is double bonded to O and single bonded to O minus. D-gluconate is similar to Greek letter beta D-glucose, except that O in the ring is bonded to H instead of to C 1, meaning the ring is missing its top right side, and red highlighted C 1 is single bonded to red highlighted O minus above the ring and double bonded to red highlighted O below the partial ring. D-glucono Greek letter delta lactone is similar to Greek letter beta D-glucose, except that O in the ring is red highlighted. This red O is connected to red highlighted C 1 by a red bond, and C 1 is connected by a red highlighted double bond to a red highlighted O. N-acetylneuraminic acid (a sialic acid) has a ring similar to Greek letter beta D-glucose, except that C 1 is bonded above to a red highlighted group of C double bonded to O and single bonded to O minus and below to O H, C 3 is bonded to H above the ring and O H below the ring, C 4 is bonded above the ring to a red highlighted group with N H bonded to C that is double bonded to O and bonded to C H 3 and to H below the ring, and C 5 is bonded to H above the ring and to red highlighted R below the ring. The red highlighted R is shown to be a red highlighted substituent with C bonded to O H on the right, H on the left, and C below that is bonded to O H on the right, H on the left, and C H 2 O H below.
Oxidation of the carbonyl (aldehyde) carbon of glucose to the carboxyl level produces gluconic acid, used in medicine as an innocuous counterion when administering positively charged drugs (such as quinine) or ions (such as ). Other aldoses yield other aldonic acids. Oxidation of the carbon at the other end of the carbon chain — C-6 of glucose, galactose, or mannose — forms the corresponding uronic acid: glucuronic, galacturonic, or mannuronic acid. Both aldonic acids and uronic acids form stable intramolecular esters called lactones (Fig. 7-9, lower left). The sialic acids are a family of sugars with the same nine-carbon backbone. One of them, N-acetylneuraminic acid (often referred to simply as “sialic acid”), is a derivative of N-acetylmannosamine that occurs in many glycoproteins and glycolipids on animal cell surfaces, providing sites of recognition by other cells or extracellular carbohydrate-binding proteins. The carboxylic acid groups of the acidic sugar derivatives are ionized at pH 7, and the compounds are therefore correctly named as the carboxylates — glucuronate, galacturonate, and so forth.
In the synthesis and metabolism of carbohydrates, the intermediates are very often not the sugars themselves but their phosphorylated derivatives. Condensation of phosphoric acid with one of the hydroxyl groups of a sugar forms a phosphate ester, as in glucose 6-phosphate (Fig. 7-9), the first metabolite in the pathway by which most organisms oxidize glucose for energy. Sugar phosphates are relatively stable at neutral pH and bear a negative charge. One effect of sugar phosphorylation within cells is to trap the sugar inside the cell; most cells do not have plasma membrane transporters for phosphorylated sugars. Phosphorylation also activates sugars for subsequent chemical transformation. Several important phosphorylated derivatives of sugars are components of nucleotides (discussed in the next chapter).
Sugars That Are, or Can Form, Aldehydes Are Reducing Sugars
Free aldehyde groups in sugars undergo a characteristic redox reaction with under alkaline conditions. As the sugar is oxidized from aldehyde to carboxylic acid, is reduced to , which forms a brick-red precipitate. This reaction defines reducing sugars, which include, for example, glucose, galactose, mannose, ribose, and glyceraldehyde. The reaction occurs only with a free aldose; but because cyclic aldoses are in equilibrium with their linear forms, which do have free aldehyde groups (Fig. 7-6), all aldose monosaccharides are reducing sugars. Ketoses that can rearrange (tautomerize) to form aldehydes are also reducing sugars; fructose and ribulose are reducing sugars, for example.
The metabolism of glucose in people with diabetes can be monitored by measuring urinary glucose with a simple qualitative assay for reducing sugar, or by measuring quantitatively the nonenzymatic reaction between glucose and the hemoglobin in blood (Box 7-2).
BOX 7-2 Medicine
Blood Glucose Measurements in the Diagnosis and Treatment of Diabetes
Glucose is the principal fuel for the brain. When the amount of glucose reaching the brain is too low, the consequences can be dire: lethargy, coma, permanent brain damage, and death. Complex hormonal mechanisms have evolved to ensure that the concentration of glucose in the blood remains high enough (about 5 mm) to satisfy the brain’s needs — but not too high, because elevated blood glucose can also have serious physiological consequences.
Individuals with insulin-dependent diabetes mellitus do not produce sufficient insulin, the hormone that normally serves to reduce blood glucose concentration. If the diabetes is untreated, blood glucose levels may rise to several-fold higher than normal. These high glucose levels are believed to be at least one cause of the serious long-term consequences of untreated diabetes — kidney failure, cardiovascular disease, blindness, and impaired wound healing — so one goal of therapy is to provide just enough insulin (by injection) to keep blood glucose levels near normal. To maintain the correct balance of exercise, diet, and insulin, individuals with diabetes must measure their blood glucose concentration several times a day, and adjust the amount of insulin injected appropriately.
The concentrations of glucose in blood can be determined by a simple assay for reducing sugar. A single drop of blood is added to a test strip containing the enzyme glucose oxidase, which catalyzes the reaction:
A second enzyme, a peroxidase, catalyzes reaction of the with a colorless compound to create a colored product, which is quantified with a simple photometer that reads out the blood glucose concentration.
Because blood glucose levels change with the timing of meals and exercise, single-time measurements do not reflect the average blood glucose over hours and days, so dangerous increases may go undetected. The average glucose concentration can be assessed by looking at its effect on hemoglobin, the oxygen-carrying protein in erythrocytes (p. 153). Transporters in the erythrocyte membrane equilibrate intracellular and plasma glucose concentrations, so hemoglobin is constantly exposed to glucose at whatever concentration is present in the blood. A series of relatively slow nonenzymatic reactions (Fig. 1) occurs between glucose and primary amino groups in hemoglobin (either the amino-terminal Val or the ε-amino groups of Lys residues) (Fig. 2). The rate of this process is proportional to the concentration of glucose, so the reaction can be used to estimate the average blood glucose level over weeks. The amount of glycated hemoglobin (GHB) present at any time reflects the average blood glucose concentration over the circulating lifetime of the erythrocyte (about 120 days), although the concentration in the two weeks before the test is the most important in setting the level of GHB.
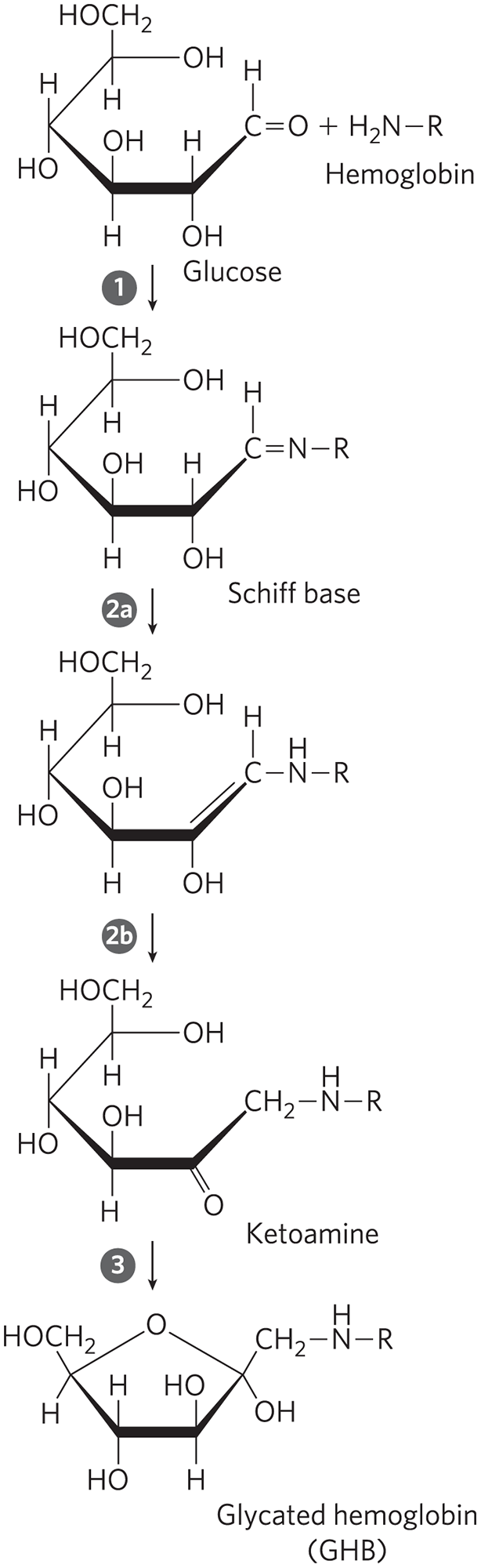
FIGURE 1 The nonenzymatic reaction of glucose with a primary amino group in hemoglobin begins with formation of a Schiff base, which undergoes a rearrangement to generate a stable product; this ketoamine can further cyclize to yield GHB.
The top molecule is a Haworth perspective of a six-membered ring labeled glucose. C 1 is bonded to H above and double bonded to O to the right; C 2 and C 4 are bonded to H above and O H below; C 3 is bonded to O H above and H below; C 5 is bonded to C H 2 O H above and to H below, and O at the top right vertex is bonded to H. There is no bond between O at the top right vertex and C 1. The molecule labeled H 2 N bonded to R is added and is labeled hemoglobin. Step 1 produces a Schiff base. This is similar to the starting ring, except that C 1 is bonded to H above and double bonded to N on the right that is further bonded to R. Step 2 a produces a similar ring in which there is a double bond on the lower right side between C 1 and C 2; C 1 is bonded to H above and to N H that is further bonded to R on the right; and C 2 is bonded to O H below the ring and nothing above the ring. Step 2 b produces a similar ring labeled a ketoamine in which C 1 is bonded to 2 H and further bonded to N H that is bonded to R, there is a single bond between C 1 and C 2, and C 2 is double bonded to O. Step 3 produces a five-membered ring labeled glycated hemoglobin (G H B). O is at the top vertex. The right side vertex is now the former C 2 and is bonded above the ring to the former C 1, which is bonded to 2 H and further bonded to N H that is bonded to R and below the ring to O H. The other atoms have changed position to fit the shape of the five-membered ring, with the former C 5 at the upper left vertex and bonded to O that is further bonded to the former C 2.
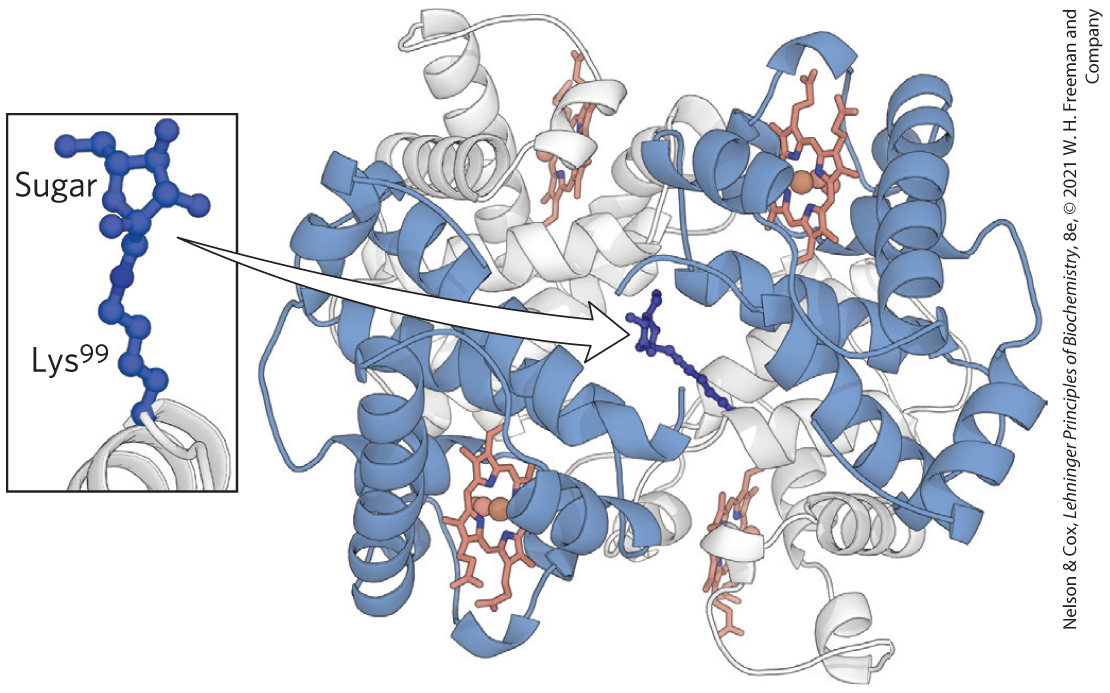
FIGURE 2 Glycated hemoglobin, showing the hemes (red) and the glycated residue (blue). [Data from PDB ID 3B75, N. T. Saraswathi et al., 2008.]
Hemoglobin has four subunits. Two large subunits made up of dark helices are at the lower to center left and middle to top right with light subunits of helices behind them. Each subunit contains a heme molecule represented by a ball-and-stick model. A chain is extending into the open region at the center with a ring at its end. A closeup shows that the ring is a sugar and it is attached to a chain labeled L y s superscript 9 9 that extends down to a thread extending from the end of a pale helix.
The extent of hemoglobin glycation (so named to distinguish it from glycosylation, the enzymatic transfer of glucose to a protein) is measured clinically by extracting hemoglobin from a small sample of blood and separating GHB from unmodified hemoglobin electrophoretically (Fig. 3), taking advantage of the charge difference resulting from modification of the amino group(s). Normal values of the monoglycated hemoglobin referred to as HbA1c are about 5% of total hemoglobin (corresponding to an average blood glucose level of 120 mg/100 mL). In people with untreated diabetes, however, this value may be as high as 13%, indicating an average blood glucose level of about 300 mg/100 mL — dangerously high. One criterion for success in an individual program of insulin therapy (the timing, frequency, and amount of insulin injected) is maintaining HbA1c values at about 7%.
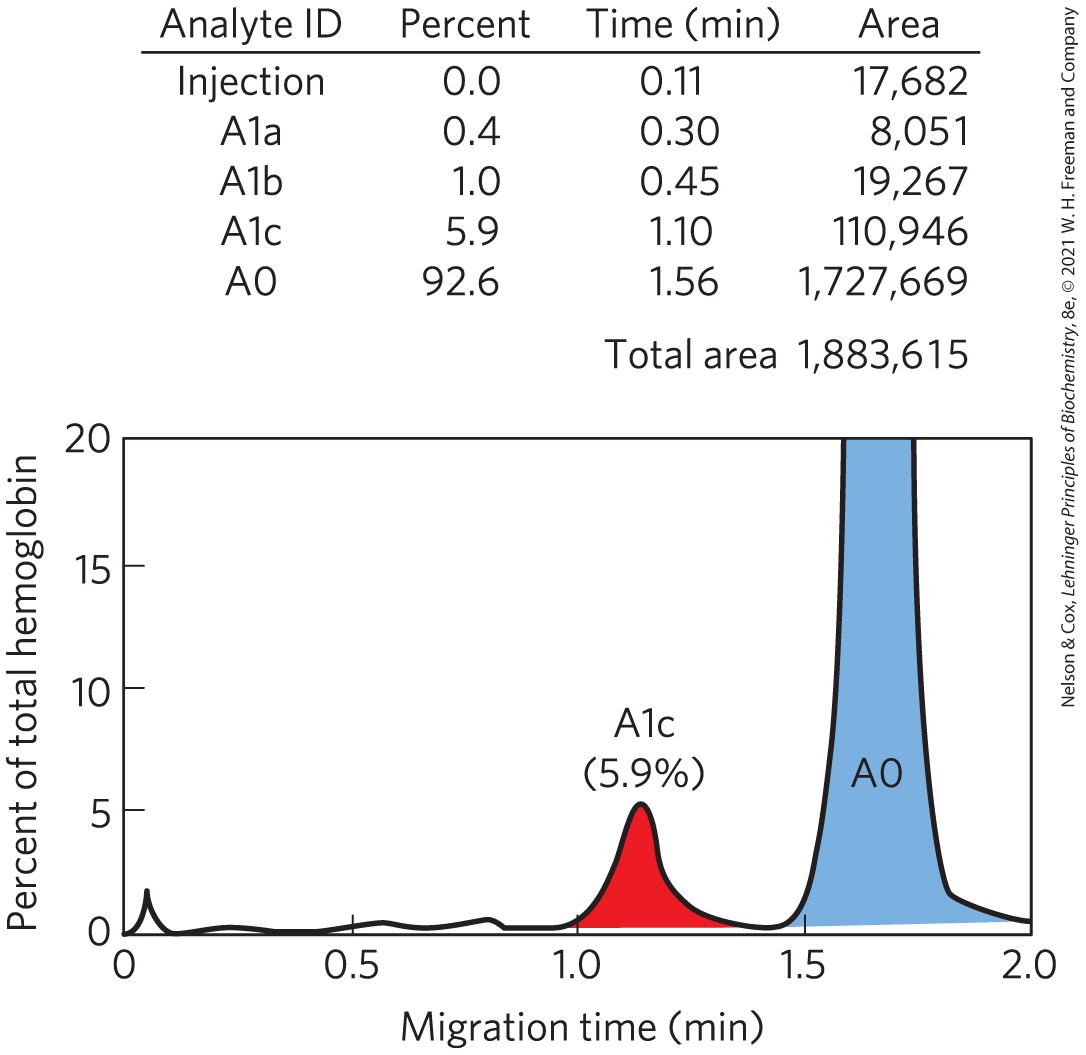
FIGURE 3 Pattern of hemoglobin (detected by its absorption at 415 nm) after electrophoretic separation of nonglycated (A0) and monoglycated (A1c) forms in a thin glass capillary. Integration of the area under the peaks allows calculation of the amount of GHB (HbA1c) as a percentage of total hemoglobin. Shown here is the profile of an individual with a normal level of HbA1c (5.9%).
A table is present above a graph. The table has four columns and five rows. The column headings are analyte I D, percent, time in minutes, and area. The data in the rows are as follows. Row 1: injection, 0.0 percent, 0.11 min, area 17,682; Row 2: A 1 lowercase a, 0.4 percent, 0.30 min, area 8,051; Row 3: A 1 lowercase b, 1.0 percent, 0.45 min, area 19,267; Row 4: A 1 lowercase c, 5.9 percent, 1.10 min, area 110,946; Row 5: A 0, 92.6 percent, 1.56 min, area 1,727,669. The total area is 1,883,615. The graph plots migration time in minutes on the horizontal axis, ranging from 0 to 2.0 and labeled in increments of 0.5, against percent of total hemoglobin on the vertical axis, ranging from 0 to 20 and labeled in increments of 5. The curve begins at the origin and has a small peak at (0.1, 2), then forms a wavy line along the horizontal axis until it begins to rise at (1.0, 0.1) to form a peak at (1.25, 5.9) under the heading A l lowercase c (5.9 percent). The curve moves down again with a slightly longer tail on the right to reach the horizontal axis at about (1.4, 0), before rising rapidly almost vertically to reach the top of the vertical axis at (1.6, 20). The curve appears again moving almost vertically downward from (1.8, 20) to (1.85, 1) and then slopes down to (2.0, 0), enclosing a region labeled A 0. The peak labeled A 1 lowercase c is shaded in red, and the peak labeled A 0 is shaded in blue. All data are approximate.
Glycated hemoglobin undergoes a series of rearrangements, oxidations, and dehydrations of the carbohydrate moiety to produce a heterogeneous mixture of advanced glycation end products (AGE), such as ε-N-carboxymethyllysine and methylglyoxal. AGE are believed to be responsible for at least some of the pathology of diabetes. AGE can leave the erythrocyte and form covalent cross-links between proteins, interfering with normal protein function (Fig. 4). The accumulation of relatively high concentrations of AGE in people with diabetes may, by cross-linking critical proteins, cause the damage to the kidneys, retinas, and cardiovascular system that characterizes the disease. Some AGE also can act through membrane receptors for AGE (RAGE), triggering intracellular responses that include activation of a transcription factor (NF-κB) and consequent changes in gene expression. This pathogenic process is a potential target for drug action.
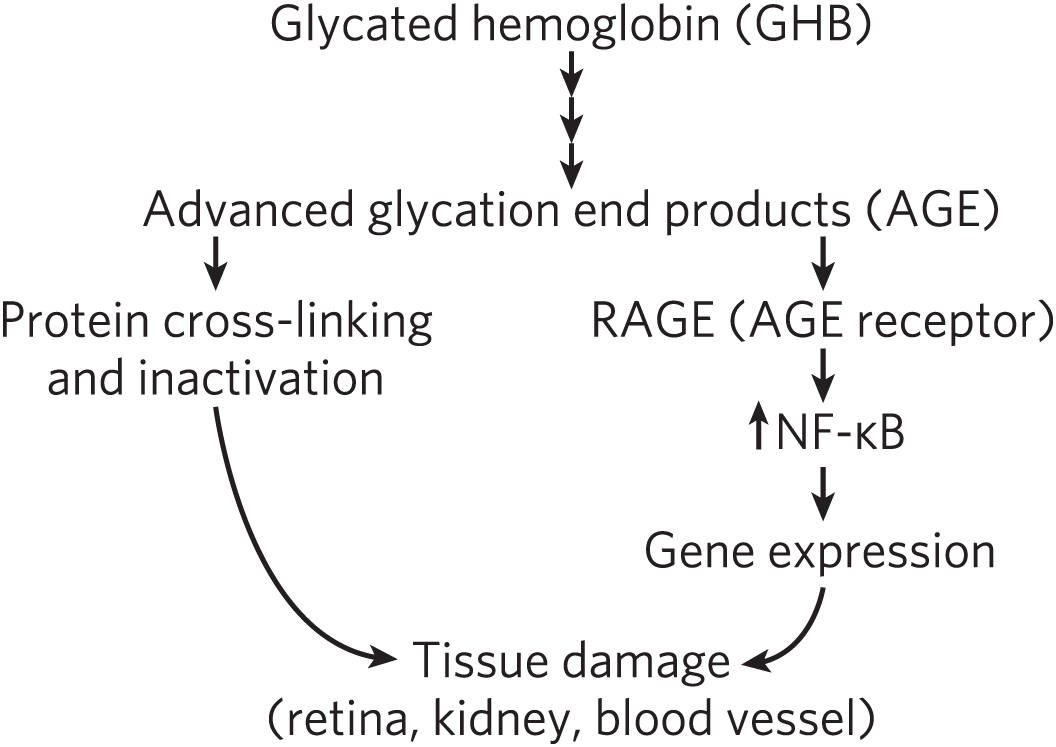
FIGURE 4 Pathways from glycated hemoglobin to the tissue damage associated with diabetes.
Glycated hemoglobin (G H B) is present at the top with three vertical arrows pointing down from it to advanced glycation end products (A G E). On the left, under advanced, a short arrow points to protein cross-linking and inactivation, from which a longer arrow curves down to tissue damage (retina, kidney, blood vessel). From the right of advanced glycation end products, under products, a short arrow points to R A G E (A G E receptor), from which a short arrow points to N F dash Greek letter kappa B. There is an arrow pointing upward next to N F dash Greek letter kappa B. A short arrow points down to gene expression and then a slightly longer arrow points down to tissue damage (retina, kidney, blood vessel).
Disaccharides (such as maltose, lactose, and sucrose) consist of two monosaccharides joined covalently by an O-glycosidic bond, which is formed when a hydroxyl group of one sugar molecule, typically in its cyclic form, reacts with the anomeric carbon of the other (Fig. 7-10). This reaction represents the formation of an acetal from a hemiacetal (such as glucopyranose) and an alcohol (a hydroxyl group of the second sugar molecule) (Fig. 7-5), and the resulting compound is called a glycoside. Glycosidic bonds are readily hydrolyzed by acid but resist cleavage by base. Thus disaccharides can be hydrolyzed to yield their free monosaccharide components by boiling with dilute acid.
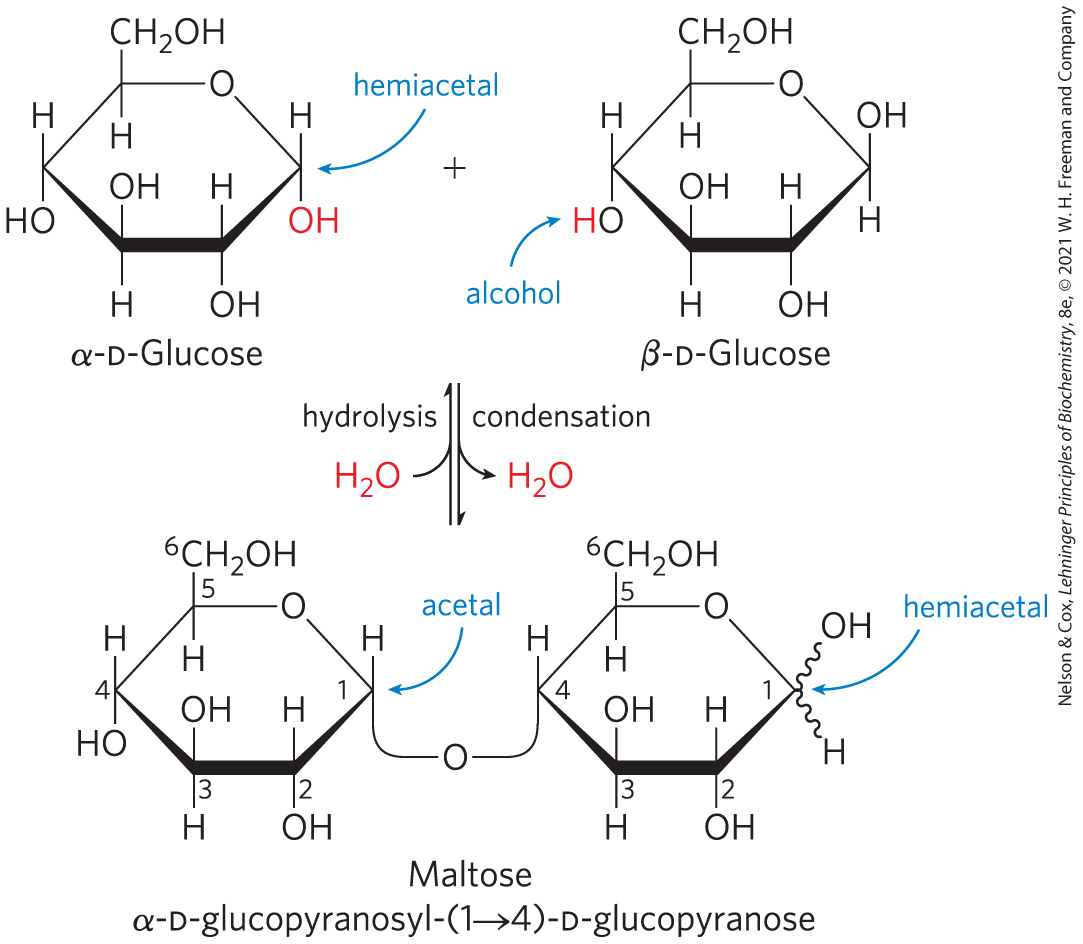
FIGURE 7-10 Formation of maltose. A disaccharide is formed from two monosaccharides (here, two molecules of d-glucose) when an —OH (alcohol) of one monosaccharide molecule (right) condenses with the intramolecular hemiacetal of the other (left), with elimination of and formation of a glycosidic bond. The reversal of this reaction is hydrolysis — attack by on the glycosidic bond. The maltose molecule, shown here, retains a reducing hemiacetal at the C-1 not involved in the glycosidic bond. Because mutarotation interconverts the α and β forms of the hemiacetal, the bonds at this position are sometimes depicted with wavy lines to indicate that the structure may be either α or β.
Alpha D-glucose is a six-membered ring with O at the top right vertex. C 1 is bonded to H above and red highlighted O H below, C 2 and C 3 are bonded to H above and O H below, C 3 is bonded to O H above and H below, and C 5 is bonded to C H 2 O H above and H below. The word hemiacetal in blue has an arrow pointing to C 1. Greek letter beta D-glucose is similar except that C 1 has O H above the ring and H below the ring and C 4 has O H below the ring with a red H. The word alcohol in blue has an arrow pointing to the red H. Greek letter alpha D glucose is added to Greek letter beta D-glucose. This produces a condensation reaction in which red highlighted H 2 O leaves. The reaction is reversible. The reverse reaction is a hydrolysis and requires the input of red highlighted H 2 O. The product is maltose or Greek letter alpha D-glucopyranosyl (1 right-hand arrow to 4)-D-glycopyranose. It has two rings that are connected by a U-shaped bond with O in the center. The left-hand ring is Greek letter alpha D-glucose but C 1 has a bond curving downward to the O in the center of the U-shaped bond. The blue word acetal has an arrow pointing to this C 1. The ring on the right is similar to Greek letter beta D-glucose, except that its C 1 has a wavy line up to O H above the ring and a wavy line down to H below the ring. The blue word hemiacetal has an arrow pointing to this C 1. Also, C 4 of Greek letter beta D-glucose has a bond extending down to the O in the center of the U before continuing to C 1 of the Greek letter alpha D-glucose.
When the anomeric carbon is involved in a glycosidic bond, the easy interconversion of linear and cyclic forms shown in Figure 7-6 is prevented. Formation of a glycosidic bond therefore renders a sugar nonreducing. In describing disaccharides or polysaccharides, the end of a chain with a free anomeric carbon (one not involved in a glycosidic bond) is called the reducing end.
The disaccharide maltose (Fig. 7-10) contains two d-glucose residues joined by a glycosidic linkage between C-1 (the anomeric carbon) of one glucose residue and C-4 of the other. Because the disaccharide retains a free anomeric carbon (C-1 of the glucose residue on the right in Fig. 7-10), maltose is a reducing sugar. The configuration of the anomeric carbon atom in the glycosidic linkage is α. The glucose residue with the free anomeric carbon is capable of existing in α- and β-pyranose forms.
Key convention
To name reducing disaccharides such as maltose unambiguously, and especially to name more complex oligosaccharides, several rules are followed. By convention, the name describes the compound written with its nonreducing end to the left, and we can “build up” the name in the following order. (1) Give the configuration (α or β) at the anomeric carbon joining the first monosaccharide unit (on the left) to the second. (2) Name the nonreducing residue; to distinguish five- and six-membered ring structures, insert “furano” or “pyrano” into the name. (3) Indicate in parentheses the two carbon atoms joined by the glycosidic bond, with an arrow connecting the two numbers; for example, shows that C-1 of the first-named sugar residue is joined to C-4 of the second. (4) Name the second residue. If there is a third residue, describe the second glycosidic bond by the same conventions. (To shorten the description of complex polysaccharides, three-letter abbreviations or colored symbols for the monosaccharides are often used, as given in Table 7-1.) Following this convention for naming oligosaccharides, maltose is α-d-glucopyranosyl--d-glucopyranose. Because most sugars encountered in this book are the d enantiomers and the pyranose form of hexoses predominates, we generally use a shortened version of the formal name of such compounds, giving the configuration of the anomeric carbon and naming the carbons joined by the glycosidic bond. In this abbreviated nomenclature, maltose is Glc .
| Abequose | Abe | Glucuronic acid | GlcA |
| Arabinose | Ara | Galactosamine | GalN |
| Fructose | Fru | Glucosamine | GlcN |
| Fucose | Fuc | N-Acetylgalactosamine | GalNAc |
| Galactose | Gal | N-Acetylglucosamine | GlcNAc |
| Glucose | Glc | Iduronic acid | IdoA |
| Mannose | Man | Muramic acid | Mur |
| Rhamnose | Rha | N-Acetylmuramic acid | Mur2Ac |
| Ribose | Rib | N-Acetylneuraminic acid (a sialic acid) | Neu5Ac |
| Xylose | Xyl | ||
| Note: In a commonly used convention, hexoses are represented as circles, N-acetylhexosamines as squares, and hexosamines as squares divided diagonally. All sugars with the “gluco” configuration are blue, those with the “galacto” configuration are yellow, and “manno” sugars are green. Other substituents can be added as needed: sulfate (S), phosphate (P), O-acetyl (OAc), or O-methyl (OMe). | |||
The disaccharide lactose (Fig. 7-11), which yields d-galactose and d-glucose on hydrolysis, occurs naturally in milk and gives milk its sweetness. The anomeric carbon of the glucose residue is available for oxidation, and thus lactose is a reducing disaccharide. The reducing end of this disaccharide, which by convention is drawn on the right, is glucose, and the disaccharide is named as a derivative of glucose. Its abbreviated name is Gal , with the linkage shown in parentheses; the anomeric carbon of galactose is β, and C-1 of Gal is linked to C-4 of Glc.
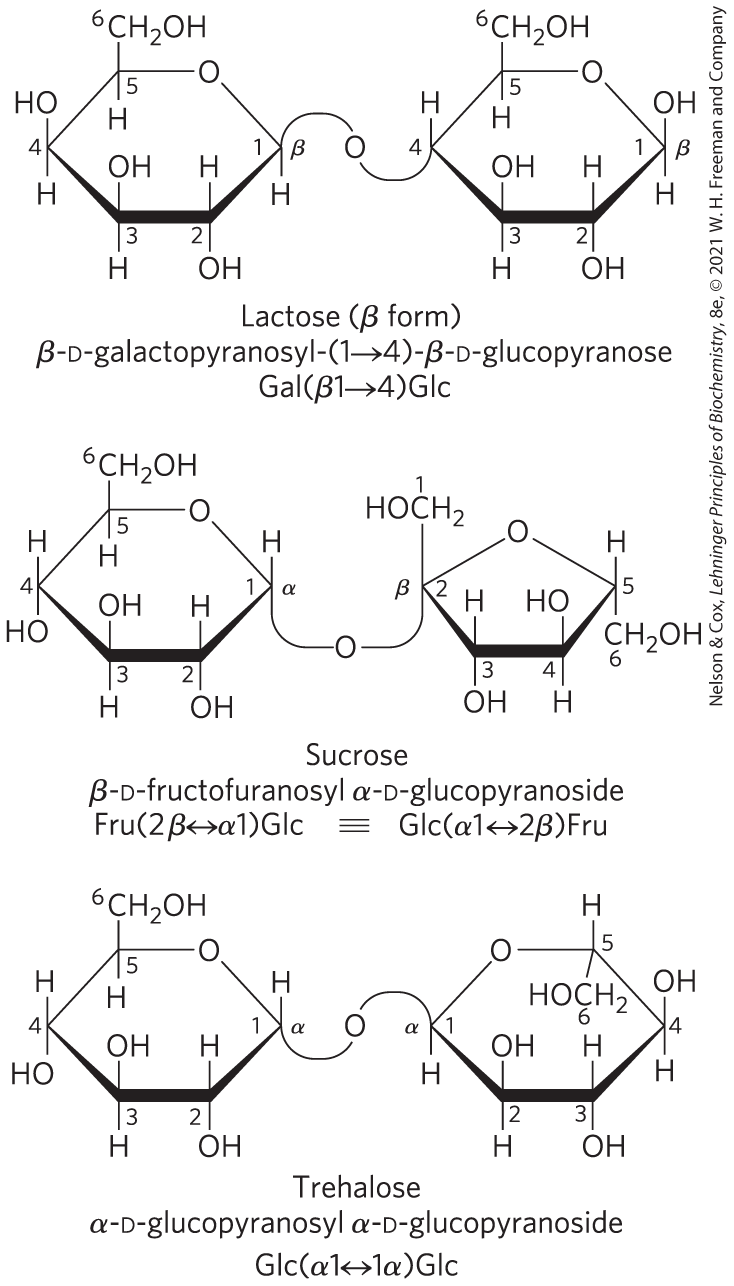
FIGURE 7-11 Three common disaccharides. Like maltose in Figure 7-10, these disaccharides are shown as Haworth perspectives. The common name, full systematic name, and abbreviation are given. Formal nomenclature for sucrose names glucose as the parent glycoside, although it is typically depicted as shown, with glucose on the left. The two abbreviated symbols shown for sucrose are equivalent .
Lactose (Greek letter beta form) has the full systematic name Greek letter beta D-galactopyranosal (1 arrow pointing to 4) Greek letter beta D-glucopyranose and the abbreviation G lowercase A L (Greek letter beta 1 arrow pointing to 4) G lowercase L C. Lactose has two rings. The left ring has C 1 with a Greek letter beta indicating a beta bond extending upward and bending down to O in the middle of the linkage with the ring to the right. C 1 has the beta bond above the ring and H below the ring; C 2 has H above the ring and O H below the ring; C 3 and C 4 have O H above the ring and H below the ring; C 5 has C 6 above the ring and H below the ring; and C 6 is bonded to 2 H and O H. O is between C 5 and C 1 at the top right vertex. The second ring has a Greek letter beta next to C 1 with O H above the ring and H below the ring. C 2 has H above the ring and O H below the ring; C 3 has O H above the ring and H below the ring; C 4 has H above the ring and a bond below the ring that curves up to O between the two rings, continues up, then curves down again in a sideways S shape to bond with C 1 of the first ring; C 5 has C 6 above the ring and H below the ring; and C 6 is bonded to 2 H and O H. O is between C 5 and C 6 at the top right vertex. Sucrose has the systematic name of Greek letter beta D-fructofuranosyl Greek letter alpha D-glucopyranoside and the abbreviation F lowercase R U (2 Greek letter beta double-headed arrow Greek letter alpha 1 ) G lowercase L C connected by three horizontal lines, an equivalency symbol, to G lowercase L C (Greek letter alpha 1 double-headed arrow 2 Greek letter beta) F lowercase R U. There is a six-membered ring on the left and a five-membered ring on the right. The two rings are connected by a U-shaped bond with O in the middle. The six-membered ring is on the left and has alpha next to C 1 with H bonded above the ring and a bond below the ring that curves down to the O between the rings; C 2 and C 4 have H above the ring and O H below the ring; C 3 has O H above the ring and H below the ring, C 5 has C 6 above the ring and H below the ring; and C 6 is bonded to 2 H and O H. O is between C 5 and C 1 at the top right vertex. The five-membered ring on the right has O at the top vertex with C 5 to its right and C 2 to its left. C 5 on the right is bonded to H above and to C 6 below, with C 6 bonded to 2 H and O H; C 4 is bonded to O H above the ring and H below the ring; C 3 is bonded to H above the ring and O H below the ring, C 2 has a Greek letter beta next to it and is bonded to C 1 above the ring that is further bonded to 2 H and O H and to O below the ring that is in the bond that joins with C 1 of the other ring. Trehalose has a systematic name of Greek letter alpha D-glucopyranosyl Greek letter alpha D-glucopyranoside and an abbreviation of G lowercase L C (Greek letter alpha 1 double-headed arrow 1 Greek letter alpha) G lowercase L C. It has two six-membered rings. There is a Greek letter alpha next to C 1 of each ring. C 1 of the left-hand ring is bonded to H above and has a bond extending below and then bending upward to join O midway between the rings before it bends down to join with C 1 of the right-hand ring in a sideways S shape; C 2 and C 4 are bonded to H above and O H below; C 3 is bonded to O H above and H below; C 5 is bonded to C 6 above and H below; and C 6 is bonded to 2 H and O H. O is between C 5 and C 1 at the upper right vertex. The second ring has C 4 on its right side and O at its upper left vertex. C 4 and C 2 are bonded to O H above the ring and H below the ring; C 3 is bonded to H above the ring and O H below the ring; C 1 is bonded above to the O that connects the rings and to H below; C 5 is bonded to H above the ring and C 6 below; and C 6 is bonded to 2 H and O H.
The enzyme lactase — absent in lactose-intolerant individuals — begins the digestive process in the small intestine by splitting the bond of lactose into monosaccharides, which can be absorbed from the small intestine. Lactose, like other disaccharides, is not absorbed from the small intestine, and in lactose-intolerant individuals the undigested lactose passes into the large intestine. Here, the increased osmolarity due to dissolved lactose opposes the absorption of water from the intestine into the bloodstream, causing watery, loose stools. In addition, fermentation of the lactose by intestinal bacteria produces large volumes of , which leads to the bloating, cramps, and gas associated with lactose intolerance.
Sucrose (table sugar) is a disaccharide of glucose and fructose. It is formed by plants but not by animals. In contrast to maltose and lactose, sucrose contains no free anomeric carbon atom; the anomeric carbons of both monosaccharide units are involved in the glycosidic bond (Fig. 7-11). Sucrose is therefore a nonreducing sugar, and its stability — its resistance to oxidation — makes it a suitable molecule for the storage and transport of energy in plants. In the abbreviated nomenclature, a double-headed arrow connects the symbols specifying the anomeric carbons and their configurations. Thus the abbreviated name of sucrose is either Fru or Fru. Sucrose is a major intermediate product of photosynthesis; in many plants it is the principal form in which sugar is transported from the leaves to other parts of the plant body.
Trehalose, (Fig. 7-11) — a disaccharide of d-glucose that, like sucrose, is a nonreducing sugar — is a major constituent of the circulating fluid (hemolymph) of insects. It serves as an energy-storage compound, and it serves as antifreeze in some invertebrate organisms.
SUMMARY 7.1 Monosaccharides and Disaccharides
- Sugars (saccharides) contain an aldehyde or ketone group and two or more hydroxyl groups.
- Monosaccharides generally contain several chiral carbons and therefore exist in a variety of stereochemical forms, which may be represented on paper as Fischer projections. Epimers are sugars that differ in configuration at only one carbon atom.
- Monosaccharides with at least six carbons commonly form cyclic structures, in which the aldehyde or ketone group is joined to a hydroxyl group of the same molecule. The cyclic structure can be represented as a Haworth perspective formula. The carbon atom originally found in the aldehyde or ketone group (the anomeric carbon) can assume either of two configurations, α and β, which are interconvertible by mutarotation. In the linear form of the monosaccharide, which is in equilibrium with the cyclic forms, the anomeric carbon is easily oxidized, making the compound a reducing sugar.
- Naturally occurring hexoses include some that have at C-2 (amino sugars), often with the amino group acetylated. Oxidation of the carbonyl carbon of glucose and other aldoses yields aldonic acids (gluconic acid); oxidation at C-6 produces uronic acids (glucuronate). Some sugar intermediates are phosphate esters (for example, glucose 6-phosphate).
- A hydroxyl group of one monosaccharide can add to the anomeric carbon of a second monosaccharide to form an acetal called a glycoside. Oligosaccharides are short polymers of several different monosaccharides joined by glycosidic bonds. At one end of the chain, the reducing end, is a monosaccharide residue with its anomeric carbon not involved in a glycosidic bond, and therefore available to be oxidized.
- Disaccharides and oligosaccharides are named as derivatives of the sugar at the reducing end. Their names provide the order of monosaccharide units, the configuration at each anomeric carbon, and the carbon atoms involved in the glycosidic linkage(s).
 This ring closure creates a new chiral center, adding further stereochemical complexity to this class of compounds. The nomenclature for unambiguously specifying the configuration about each carbon atom in a cyclic form and the means of representing these structures on paper are described in some detail; this information will be useful as we discuss the metabolism of monosaccharides in
This ring closure creates a new chiral center, adding further stereochemical complexity to this class of compounds. The nomenclature for unambiguously specifying the configuration about each carbon atom in a cyclic form and the means of representing these structures on paper are described in some detail; this information will be useful as we discuss the metabolism of monosaccharides in  All the monosaccharides except dihydroxyacetone contain one or more asymmetric (chiral) carbon atoms and thus occur in optically active isomeric forms (pp. 16–17). The simplest aldose, glyceraldehyde, contains one chiral center (the middle carbon atom) and therefore has two different optical isomers, or
All the monosaccharides except dihydroxyacetone contain one or more asymmetric (chiral) carbon atoms and thus occur in optically active isomeric forms (pp. 16–17). The simplest aldose, glyceraldehyde, contains one chiral center (the middle carbon atom) and therefore has two different optical isomers, or 
 two conformations of a molecule are interconvertible without the breakage of covalent bonds, whereas two configurations can be interconverted only by breaking a covalent bond. To interconvert α and β configurations, the bond involving the ring oxygen atom has to be broken, but interconversion of the two chair forms (which are conformers) does not require bond breakage and does not change configurations at any of the ring carbons. The specific three-dimensional structures of the monosaccharide units are important in determining the biological properties and functions of some polysaccharides, as we shall see.
two conformations of a molecule are interconvertible without the breakage of covalent bonds, whereas two configurations can be interconverted only by breaking a covalent bond. To interconvert α and β configurations, the bond involving the ring oxygen atom has to be broken, but interconversion of the two chair forms (which are conformers) does not require bond breakage and does not change configurations at any of the ring carbons. The specific three-dimensional structures of the monosaccharide units are important in determining the biological properties and functions of some polysaccharides, as we shall see. Medicine
Medicine formation of a Schiff base, which
formation of a Schiff base, which  undergoes a rearrangement to generate a stable product;
undergoes a rearrangement to generate a stable product;  this ketoamine can further cyclize to yield GHB.
this ketoamine can further cyclize to yield GHB. GlcA
GlcA GalN
GalN GlcN
GlcN Fuc
Fuc GalNAc
GalNAc Gal
Gal GlcNAc
GlcNAc Glc
Glc IdoA
IdoA Man
Man Neu5Ac
Neu5Ac Xyl
Xyl Sugars (saccharides) contain an aldehyde or ketone group and two or more hydroxyl groups.
Sugars (saccharides) contain an aldehyde or ketone group and two or more hydroxyl groups.