23.5 Diabetes Mellitus
Diabetes mellitus is a relatively common disease: about 9% of the U.S. population, and nearly 25% of the U.S. population over the age of 65, show some degree of abnormality in glucose metabolism that is indicative of diabetes or a tendency toward the condition. There are two major clinical classes of diabetes mellitus: type 1 diabetes, sometimes referred to as insulin-dependent diabetes mellitus (IDDM), and type 2 diabetes, or non-insulin-dependent diabetes mellitus (NIDDM), also called insulin-resistant diabetes. The discovery of insulin and its role in diabetes led to its development as a pharmaceutical, saving millions of lives (Box 23-2).
Diabetes Mellitus Arises from Defects in Insulin Production or Action
Type 1 diabetes usually begins early in life, and symptoms quickly become severe. This disease responds to insulin injection, because the metabolic defect stems from an autoimmune destruction of pancreatic β cells and a consequent inability to produce sufficient insulin. Type 1 diabetes requires both insulin therapy and careful, lifelong control of the balance between dietary intake, activity, and insulin dose. Characteristic symptoms of untreated type 1 (and type 2) diabetes are excessive thirst and frequent urination, leading to the intake of large volumes of water. These symptoms are due to excretion of large amounts of glucose in the urine. (“Diabetes mellitus” means “excessive excretion of sweet urine.”)
Type 2 diabetes is slower to develop (typically but not always in obese adults), and the symptoms are milder and often go unrecognized at first. This is really a group of diseases in which the regulatory activity of insulin is disordered: insulin is produced, but some feature of the insulin-response system is defective. Many individuals with this disorder are insulin-resistant. The connection between type 2 diabetes and obesity (discussed below) is an active area of research.
The pathology of diabetes includes cardiovascular disease, renal failure, blindness, and neuropathy. In 2019, the global mortality from diabetes was estimated at 4.2 million and rising. It is essential to understand diabetes and its relationship to obesity and to find countermeasures that prevent or reverse the damage done by this disease.
Individuals with either type of diabetes are unable to take up glucose efficiently from the blood. Recall that insulin triggers the movement of GLUT4 glucose transporters to the plasma membrane in muscle and adipose tissue (see Fig. 12-23 and Box 11-1). Biochemical measurements on blood and urine samples are essential in the diagnosis and treatment of diabetes. A sensitive diagnostic criterion is the level of HbA1c, a glucose derivative of hemoglobin, which forms in the blood and reflects the average blood glucose level (see Box 7-2). Another measurement to confirm the diagnosis of diabetes is the glucose-tolerance test. The individual fasts overnight, then drinks a test dose of 100 g of glucose dissolved in a glass of water. The blood glucose concentration is measured before the test dose and at 30 min intervals for several hours thereafter. A healthy individual assimilates the glucose readily, the blood glucose rising to no more than about 9 or 10 mm; little or no glucose appears in the urine. In diabetes, individuals assimilate the test dose of glucose poorly; their blood glucose level rises dramatically and returns to the fasting level very slowly. Because the blood glucose levels exceed the kidney threshold (about 10 mm), glucose also appears in the urine.
Carboxylic Acids (Ketone Bodies) Accumulate in the Blood of Those with Untreated Diabetes
With glucose unavailable to cells, fatty acids become the principal fuel, which leads to another characteristic metabolic change in diabetes: excessive but incomplete oxidation of fatty acids in the liver. The acetyl-CoA produced by β oxidation cannot be completely oxidized by the citric acid cycle, because the high ratio produced by β oxidation inhibits the cycle (recall that three steps of the cycle convert to ). Accumulation of acetyl-CoA leads to overproduction of the ketone bodies β-hydroxybutyrate and acetoacetate, which cannot be used by extrahepatic tissues as fast as they are made in the liver (see Figs. 17-15, 17-16). In addition to β-hydroxybutyrate and acetoacetate, the blood of individuals with diabetes contains small amounts of acetone, which results from the spontaneous decarboxylation of acetoacetate:
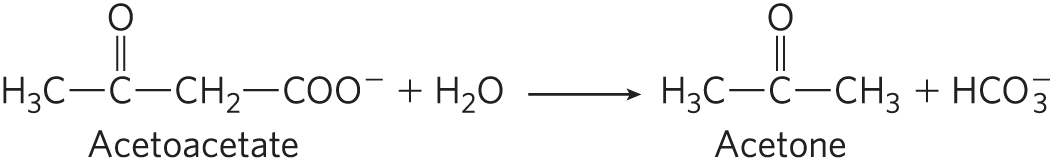
The overproduction of ketone bodies, called ketosis, results in greatly increased concentrations of ketone bodies in the blood (ketonemia) and urine (ketonuria).The ketone bodies are carboxylic acids, which ionize, releasing protons. In uncontrolled diabetes, this acid production can overwhelm the capacity of the blood’s bicarbonate buffering system and produce a lowering of blood pH called acidosis or, in combination with ketosis, ketoacidosis, a potentially life-threatening condition.
In Type 2 Diabetes the Tissues Become Insensitive to Insulin
In the industrialized world, where the food supply is more than adequate, there is a growing epidemic of obesity and the type 2 diabetes associated with it. The hallmark of type 2 diabetes is the development of insulin resistance, a state in which more insulin is needed to bring about the biological effects produced by a lower concentration of insulin in the normal, healthy state. In the early stages of the disease, pancreatic β cells secrete enough insulin to overcome the lower insulin sensitivity of muscle and liver. But the β cells eventually fail, and the lack of insulin becomes apparent in the body’s inability to regulate blood glucose. The intermediate stage, preceding type 2 diabetes mellitus, is sometimes called metabolic syndrome. This is typified by obesity, especially in the abdomen; hypertension (high blood pressure); abnormal blood lipids (high TAG and LDL, low HDL); slightly high fasting blood glucose; and a reduced ability to clear glucose in the glucose-tolerance test. Individuals with metabolic syndrome often also show changes in blood proteins associated with abnormal clotting (high fibrinogen concentration) or inflammation (high concentration of the C-reactive peptide, not to be confused with the C peptide generated during the proteolytic maturation of insulin). About 30% of the adult population in the United States has these indicators of metabolic syndrome.
What predisposes individuals with metabolic syndrome to develop type 2 diabetes? According to the “lipid toxicity” hypothesis (Fig. 23-42), the action of PPARδ on adipocytes normally keeps the cells ready to synthesize and store triacylglycerols — the adipocytes are insulin-sensitive and produce leptin, which leads to their continued intracellular deposition of TAGs. However, excess caloric intake in obese individuals causes adipocytes to become filled with TAGs, leaving adipose tissue unable to meet any further demand for TAG storage. Lipid-filled adipose tissue releases protein factors, including MCP-1 (monocyte chemotaxis protein-1), that attract macrophages, which infiltrate the tissue and may eventually represent as much as 50% of the adipose tissue by mass. Macrophages trigger the inflammatory response mediated by TNFα release, which impairs TAG deposition in adipocytes and favors release of free fatty acids into the blood. These excess fatty acids enter liver and muscle cells, where they are converted to TAGs that accumulate as lipid droplets. This ectopic (Greek ektopos, “out of place”) deposition of TAGs inhibits GLUT4 transporter movement to the plasma membrane, which leads to insulin insensitivity in liver and muscle, the hallmark of type 2 diabetes.
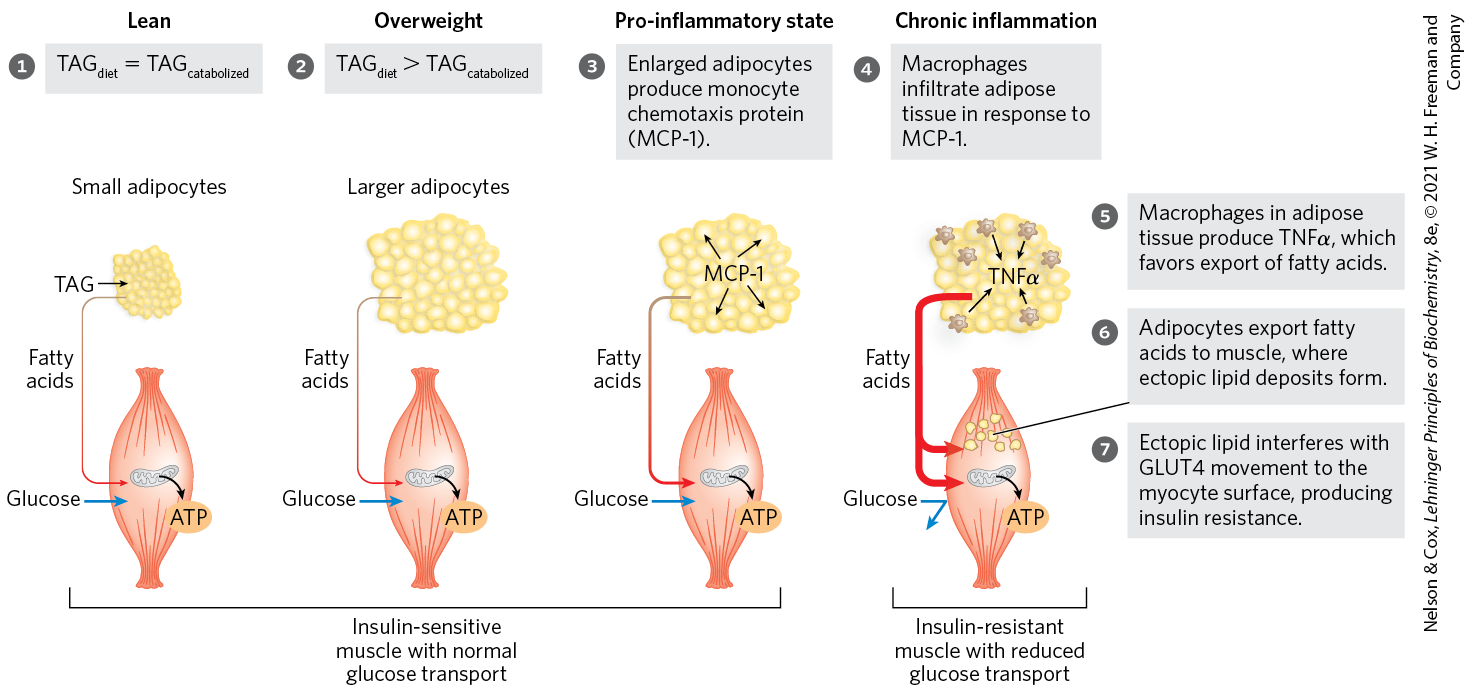
FIGURE 23-42 Overloading adipocytes with triacylglycerols triggers inflammation in fat tissue and ectopic lipid deposition and insulin resistance in muscle. In an individual of healthy body mass, dietary TAG uptake equals TAG oxidation for energy. In overweight individuals, excess caloric intake results in enlarged adipocytes, engorged with TAG and unable to store more. Enlarged adipocytes secrete MCP-1 (monocyte chemotaxis protein-1), attracting macrophages. Macrophages infiltrate the adipose tissue and produce TNFα (tumor necrosis factor α), which triggers lipid breakdown and release of fatty acids into the blood. The fatty acids enter myocytes, where they accumulate in small lipid droplets. This ectopic lipid storage in muscle somehow causes insulin resistance, perhaps by triggering lipid-activated protein kinases that inactivate some element in the insulin-signaling pathway. GLUT4 glucose transporters leave the myocyte surface, preventing glucose entry into muscle; the myocyte has now become insulin-resistant. It cannot use blood glucose for its fuel, so fatty acids are mobilized from adipose tissue and become the primary fuel. The increased influx of fatty acids into muscle leads to further deposition of ectopic lipids. In some individuals, insulin resistance develops into type 2 diabetes. [Information from A. Guilherme et al., Mol. Cell Biol. 9:367, 2008, Fig. 1.]
According to this hypothesis, excess stored fatty acids and TAGs are toxic to liver and muscle. Some individuals are less well equipped genetically to handle this burden of ectopic lipids and are more susceptible to the cellular damage that leads to development of type 2 diabetes. Insulin resistance probably involves impairment of several of the mechanisms by which insulin acts on metabolism, which include changes in protein levels and changes in the activities of signaling enzymes and transcription factors. For example, both adiponectin synthesis in adipocytes and adiponectin levels in the blood decrease with obesity and increase with weight loss.
There are genetic factors that predispose individuals to type 2 diabetes. Although 80% of people with type 2 diabetes are obese, most obese individuals do not develop type 2 diabetes. Given the complexity of the regulatory mechanisms we have discussed in this chapter, it is not surprising that the genetics of diabetes is complex, involving interactions among variant genes and environmental factors, including diet and lifestyle. A growing number of genetic loci have been reliably linked to type 2 diabetes; variation in any of these “diabetogenes” alone would cause a relatively small increase in the likelihood of developing type 2 diabetes.
Type 2 Diabetes Is Managed with Diet, Exercise, Medication, and Surgery
Studies show that at least four factors improve the health of individuals with type 2 diabetes: dietary restriction, regular exercise, drugs that increase insulin sensitivity or insulin production, and surgery that reroutes food passage through the gastrointestinal tract. Dietary restriction (and accompanying weight loss) reduces the overall burden of handling fatty acids. The lipid composition of the diet influences, through PPARs and other transcription factors, the expression of genes that encode proteins involved in burning fat.
Exercise contributes to weight loss directly by consuming calories. Exercise also increases release of irisin from muscle into the blood. Irisin increases the expression of UCP1 genes in white adipose tissue and also stimulates the development of beige adipocytes, so that even after the exercise ends, energy continues to be used in thermogenesis. Exercise activates AMPK, as does adiponectin; AMPK shifts metabolism toward fat oxidation and inhibits fat synthesis.
Several classes of drugs are used in the management of type 2 diabetes (Table 23-7). Their targets include AMPK, the channels in β cells, PPARs, and GLP receptors.
Intervention/treatment |
Direct target |
Effect of treatment |
|---|---|---|
Weight loss |
Adipose tissue; reduction in TAG content |
Reduces lipid burden; increases capacity for lipid storage in adipose tissue; restores insulin sensitivity |
Exercise |
AMPK, activated by increasing [AMP]/[ATP] |
Aids weight loss (see Fig. 23-34) |
Bariatric surgery |
Unknown |
Leads to weight loss, better control of blood glucose |
Sulfonylureas:
|
Pancreatic β cells; channels blocked |
Stimulates insulin secretion by pancreas (see Fig. 23-24) |
Biguanides:
|
AMPK, activated |
Increases glucose uptake by muscle; decreases glucose production in liver |
Thiazolidinediones:
|
PPARγ |
Stimulates expression of genes potentiating the action of insulin in liver, muscle, adipose tissue; increases glucose uptake; decreases glucose synthesis in liver |
GLP-1 modulators:
|
Glucagon-like peptide-1, dipeptide protease IV |
Enhances insulin secretion by pancreas |
In cases of extreme obesity, dramatic weight loss can be achieved by bariatric surgery, which reroutes food movement through the stomach and small intestine. In many cases, this procedure also moderates or even reverses type 2 diabetes. In Roux-en-Y gastric bypass (RYGBP, named for César Roux, the Swiss surgeon who developed the procedure), the stomach is reduced to a small pouch attached to the esophagus, and the middle region of the small intestine (the jejunum) is attached directly to the pouch. Food bypasses most of the stomach and the duodenum, and goes primarily to the “Roux limb” of the intestine. Stomach acid and digestive enzymes travel through bypassed portions of the gut to join the food in the common channel. People who undergo RYGBP surgery not only experience dramatic weight loss but also are less hungry. Remarkably, this surgery also reverses type 2 diabetes in many cases. The explanations for these effects are likely to lie in altered communication among the gut, the brain, and other organs. This may result from changes in the kind and amount of peptide hormones (such as GLP-1 and ) secreted in the intestine that signal satiety and inhibit feeding behavior. The last word has not been written on this issue.
SUMMARY 23.5 Diabetes Mellitus
- Metabolic syndrome, which includes obesity, hypertension, elevated blood lipids, and insulin resistance, is often the prelude to type 2 diabetes. Uncontrolled diabetes is characterized by high glucose levels in the blood and urine and the production and excretion of ketone bodies.
- In diabetes, insulin is either not produced or not recognized by the tissues, and the uptake of blood glucose is defective. Lacking access to glucose, cells rely on fatty acid oxidation, which results in ketone body formation, producing ketoacidosis.
- The insulin resistance that characterizes type 2 diabetes may be a consequence of abnormal lipid storage in muscle and liver, in response to a lipid intake that cannot be accommodated by adipose tissue.
- Effective treatments for type 2 diabetes include exercise, appropriate diet, and drugs that increase insulin sensitivity or insulin production. Surgical alteration of the digestive tract leads to weight loss and often reverses type 2 diabetes.


 Individuals with either type of diabetes are unable to take up glucose efficiently from the blood. Recall that insulin triggers the movement of GLUT4 glucose transporters to the plasma membrane in muscle and adipose tissue (see
Individuals with either type of diabetes are unable to take up glucose efficiently from the blood. Recall that insulin triggers the movement of GLUT4 glucose transporters to the plasma membrane in muscle and adipose tissue (see  Metabolic syndrome, which includes obesity, hypertension, elevated blood lipids, and insulin resistance, is often the prelude to type 2 diabetes. Uncontrolled diabetes is characterized by high glucose levels in the blood and urine and the production and excretion of ketone bodies.
Metabolic syndrome, which includes obesity, hypertension, elevated blood lipids, and insulin resistance, is often the prelude to type 2 diabetes. Uncontrolled diabetes is characterized by high glucose levels in the blood and urine and the production and excretion of ketone bodies.