19.4 Mitochondria in Thermogenesis, Steroid Synthesis, and Apoptosis
Although ATP production is a central role for the mitochondrion, this organelle has other functions that, in specific tissues or under specific circumstances, are also crucial. In adipose tissue, mitochondria generate heat to protect vital organs from low ambient temperature; in the adrenal glands and the gonads, mitochondria are the sites of steroid hormone synthesis; and in most or all tissues, they are key participants in apoptosis (programmed cell death).
Uncoupled Mitochondria in Brown Adipose Tissue Produce Heat
We noted above that respiration slows when the cell is adequately supplied with ATP. There is a remarkable and instructive exception to this general rule. Most newborn mammals, including humans, have a type of adipose tissue called brown adipose tissue (BAT; p. 852), in which fuel oxidation serves not to produce ATP but to generate heat to keep the newborn warm. This specialized adipose tissue is brown because of the presence of large numbers of mitochondria and thus high concentrations of cytochromes, with heme groups that are strong absorbers of visible light.
There are at least two mechanisms of thermogenesis. The mitochondria of brown adipocytes are much like those of other mammalian cells, except in having a unique protein in their inner membrane. Uncoupling protein 1 (UCP1), a long-chain fatty symporter, provides a path for protons to return to the matrix without passing through the complex (Fig. 19-36). As a result of this short-circuiting of protons, the energy of oxidation is not conserved by ATP formation but is dissipated as heat, which contributes to maintaining body temperature. However, UCP1 is only part of the story, and heat generation occurs in mammals even when it is absent. The thermogenic action of UCP1 is supplemented by a futile cycle involving creatine and phosphocreatine (Fig. 19-36). ATP synthesized in the mitochondrial matrix is exported to the intermembrane space via the adenine nucleotide translocator, an ADP/ATP antiporter. There, the ATP is used to phosphorylate creatine to create phosphocreatine and ADP. The ADP is transported back into the matrix. Hydrolysis of phosphocreatine completes a futile cycle that liberates heat.
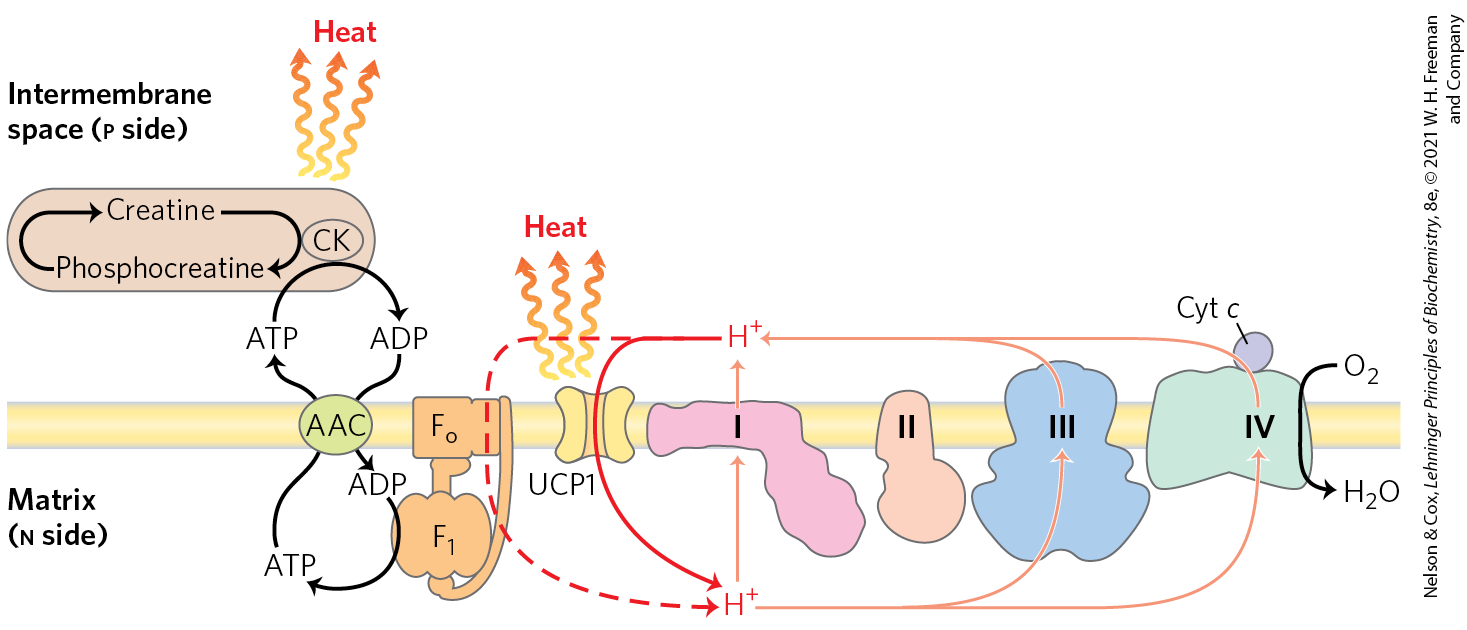
FIGURE 19-36 Two mechanisms of thermogenesis in mitochondria. UCP1, an uncoupling protein in the mitochondria of brown adipose tissue, causes the energy conserved by proton pumping to be dissipated as heat by providing an alternative route for protons to reenter the mitochondrial matrix. A futile cycle in which creatine is phosphorylated by creatine kinase (CK), using ATP and producing ADP transported by the ATP/ADP carrier (AAC), also generates heat.
Hibernating animals also depend on the activity of uncoupled BAT mitochondria to generate heat during their long dormancy (see Box 17-1). We will return to the role of UCP1 when we discuss the regulation of body mass in Chapter 23 (pp. 867–869).
Mitochondrial P-450 Monooxygenases Catalyze Steroid Hydroxylations
Mitochondria are the site of biosynthetic reactions that produce steroid hormones, including the sex hormones, glucocorticoids, mineralocorticoids, and vitamin D hormone. These compounds are synthesized from cholesterol or a related sterol in a series of hydroxylations catalyzed by enzymes of the cytochrome P-450 family (see Box 21-1), all of which have a critical heme group (its absorption at 450 nm gives this family its name). In the hydroxylation reactions, one atom of molecular oxygen is incorporated into the substrate and the second is reduced to , making cytochrome P-450 enzymes monooxygenases:
In this reaction, two species are oxidized: NADPH and .
There are dozens of P-450 enzymes, all situated in the inner mitochondrial membrane with their catalytic site exposed to the matrix. Steroidogenic cells are packed with mitochondria specialized for steroid synthesis; the mitochondria are generally larger than those in other tissues and have more extensive and highly convoluted inner membranes (Fig. 19-37).

FIGURE 19-37 Mitochondria of adrenal gland, specialized for steroid synthesis. As seen in this electron micrograph of a thin section of adrenal gland, mitochondria are profuse and have extensive cristae, providing a large surface for the P-450 enzymes of the inner membrane.
The path of electron flow in the mitochondrial P-450 system is complex, involving a flavoprotein and an iron-sulfur protein that carry electrons from NADPH to the P-450 heme (Fig. 19-38). All P-450 enzymes have a heme that interacts with and a substrate-binding site that confers specificity.
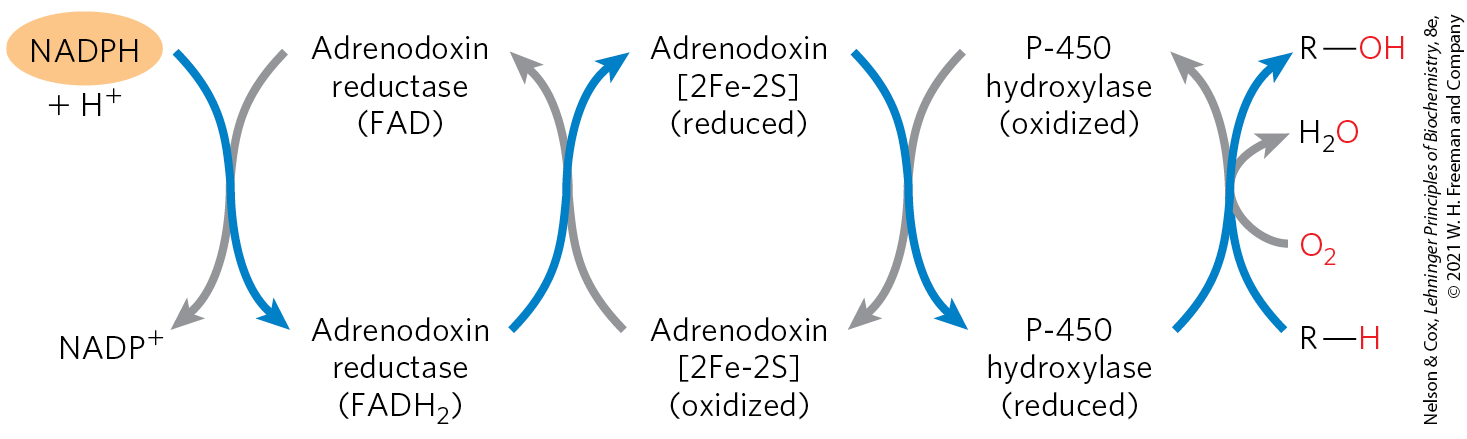
FIGURE 19-38 Path of electron flow in mitochondrial cytochrome P-450 reactions in adrenal gland. Two electrons are transferred from NADPH to the FAD-containing flavoprotein adrenodoxin reductase, which passes the electrons, one at a time, to adrenodoxin, a small, soluble 2Fe-2S protein. Adrenodoxin passes single electrons to the cytochrome P-450 hydroxylase, which interacts directly with and the substrate to form the products, and .
Another large family of P-450 enzymes is found in the endoplasmic reticulum of hepatocytes. These enzymes catalyze reactions similar to the mitochondrial P-450 reactions, but their substrates include a wide variety of hydrophobic compounds, many of which are xenobiotics — compounds not found in nature but synthesized industrially. The P-450 enzymes of the ER have very broad and overlapping substrate specificities. Hydroxylation of the hydrophobic compounds makes them more water-soluble, and they can then be cleared by the kidneys and excreted in urine. Among the substrates for these P-450 oxygenases are many commonly used prescription drugs. Metabolism by P-450 enzymes limits a drug’s lifetime in the bloodstream and thus its therapeutic effects. Humans differ in their genetic complement of P-450 enzymes in the ER, as well as in the extent to which certain P-450 enzymes have been induced, such as by a history of ethanol ingestion. In principle, therefore, an individual’s genetics and personal history should be considered in determining therapeutic drug doses. In practice, this precise tailoring of dosage is not yet economically feasible, but it may become so.
Mitochondria Are Central to the Initiation of Apoptosis
Apoptosis, also called programmed cell death, is a process in which individual cells die for the good of the organism, such as in the course of normal embryonic development, and the organism conserves the cells’ molecular components (amino acids, nucleotides, and so forth). Apoptosis may be triggered by an external signal, acting at a plasma membrane receptor, or by internal events such as DNA damage, viral infection, oxidative stress from the accumulation of ROS, or other stress such as a heat shock.
Mitochondria play a critical role in triggering apoptosis. When a stressor gives the signal for cell death, one early consequence is an increase in the permeability of the outer mitochondrial membrane, allowing cytochrome c to escape from the intermembrane space into the cytosol (Fig. 19-39). The increased permeability is due to the opening of the permeability transition pore complex (PTPC), a multisubunit protein in the outer membrane; its opening and closing are affected by several proteins that stimulate or suppress apoptosis. When released into the cytosol, cytochrome c interacts with monomers of the protein Apaf-1 (apoptosis protease activating factor-1), causing the formation of an apoptosome composed of seven Apaf-1 and seven cytochrome c molecules. The apoptosome provides the platform on which the proenzyme procaspase-9 is activated to caspase-9, a member of a family of highly specific proteases, called the caspases, involved in apoptosis. These cysteine proteases cleave proteins only on the carboxyl-terminal side of Asp residues, thus the name “caspases.” Caspase-9 initiates a cascade of proteolytic activations, with one caspase activating a second, and this in turn activating a third, and so forth (see Fig. 12-42). Note that this role of cytochrome c in apoptosis is a clear case of “moonlighting,” in that one protein plays two very different roles in the cell (see Box 16-1).
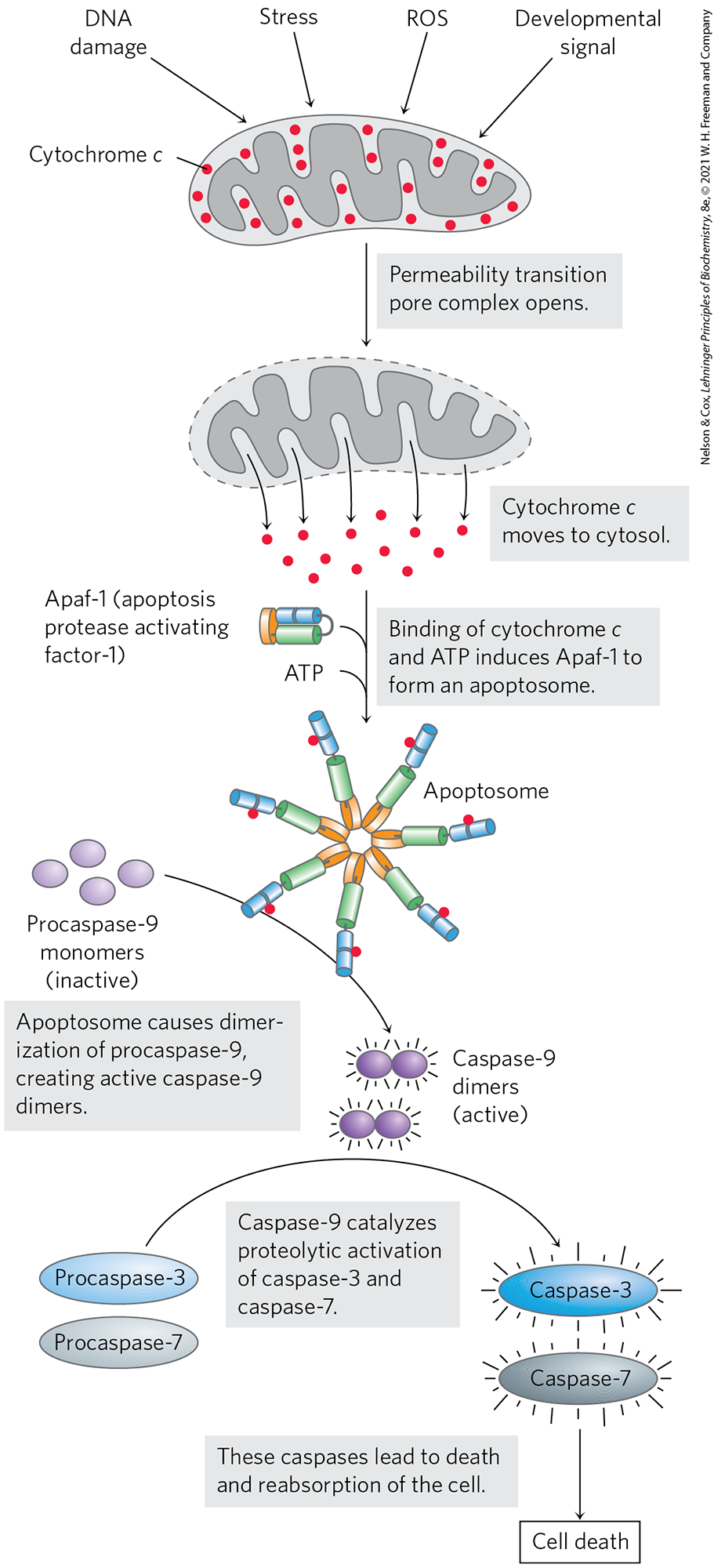
FIGURE 19-39 Role of cytochrome c in apoptosis. Cytochrome c is a small, soluble, mitochondrial protein, located in the intermembrane space, that carries electrons between Complex III and Complex IV during respiration. In a completely separate role, as outlined here, it acts as a trigger for apoptosis by stimulating the activation of a family of proteases called caspases. [Information from S. J. Riedl and G. S. Salvesen, Nat. Rev. Mol. Cell Biol. 8:409, 2007, Fig. 3.]
SUMMARY 19.4 Mitochondria in Thermogenesis, Steroid Synthesis, and Apoptosis
- In the brown adipose tissue of newborns, electron transfer is uncoupled from ATP synthesis, and the energy of fuel oxidation is dissipated as heat. Hibernating animals use this strategy to avoid freezing.
- Hydroxylation reaction steps in the synthesis of steroid hormones in steroidogenic tissues (adrenal gland, gonads, liver, and kidney) take place in specialized mitochondria. Key reactions are catalyzed by a family of P-450 monooxygenases.
- Mitochondria play a central role in apoptosis. Mitochondrial cytochrome c, released into the cytosol, participates in activation of caspase-9, one of the proteases involved in apoptosis.
 fuel oxidation serves not to produce ATP but to generate heat to keep the newborn warm. This specialized adipose tissue is brown because of the presence of large numbers of mitochondria and thus high concentrations of cytochromes, with heme groups that are strong absorbers of visible light.
fuel oxidation serves not to produce ATP but to generate heat to keep the newborn warm. This specialized adipose tissue is brown because of the presence of large numbers of mitochondria and thus high concentrations of cytochromes, with heme groups that are strong absorbers of visible light. As a result of this short-circuiting of protons, the energy of oxidation is not conserved by ATP formation but is dissipated as heat, which contributes to maintaining body temperature. However, UCP1 is only part of the story, and heat generation occurs in mammals even when it is absent. The thermogenic action of UCP1 is supplemented by a futile cycle involving creatine and phosphocreatine (
As a result of this short-circuiting of protons, the energy of oxidation is not conserved by ATP formation but is dissipated as heat, which contributes to maintaining body temperature. However, UCP1 is only part of the story, and heat generation occurs in mammals even when it is absent. The thermogenic action of UCP1 is supplemented by a futile cycle involving creatine and phosphocreatine ( Another large family of P-450 enzymes is found in the endoplasmic reticulum of hepatocytes. These enzymes catalyze reactions similar to the mitochondrial P-450 reactions, but their substrates include a wide variety of hydrophobic compounds, many of which are xenobiotics — compounds not found in nature but synthesized industrially. The P-450 enzymes of the ER have very broad and overlapping substrate specificities. Hydroxylation of the hydrophobic compounds makes them more water-soluble, and they can then be cleared by the kidneys and excreted in urine. Among the substrates for these P-450 oxygenases are many commonly used prescription drugs. Metabolism by P-450 enzymes limits a drug’s lifetime in the bloodstream and thus its therapeutic effects. Humans differ in their genetic complement of P-450 enzymes in the ER, as well as in the extent to which certain P-450 enzymes have been induced, such as by a history of ethanol ingestion. In principle, therefore, an individual’s genetics and personal history should be considered in determining therapeutic drug doses. In practice, this precise tailoring of dosage is not yet economically feasible, but it may become so.
Another large family of P-450 enzymes is found in the endoplasmic reticulum of hepatocytes. These enzymes catalyze reactions similar to the mitochondrial P-450 reactions, but their substrates include a wide variety of hydrophobic compounds, many of which are xenobiotics — compounds not found in nature but synthesized industrially. The P-450 enzymes of the ER have very broad and overlapping substrate specificities. Hydroxylation of the hydrophobic compounds makes them more water-soluble, and they can then be cleared by the kidneys and excreted in urine. Among the substrates for these P-450 oxygenases are many commonly used prescription drugs. Metabolism by P-450 enzymes limits a drug’s lifetime in the bloodstream and thus its therapeutic effects. Humans differ in their genetic complement of P-450 enzymes in the ER, as well as in the extent to which certain P-450 enzymes have been induced, such as by a history of ethanol ingestion. In principle, therefore, an individual’s genetics and personal history should be considered in determining therapeutic drug doses. In practice, this precise tailoring of dosage is not yet economically feasible, but it may become so. 
 In the brown adipose tissue of newborns, electron transfer is uncoupled from ATP synthesis, and the energy of fuel oxidation is dissipated as heat. Hibernating animals use this strategy to avoid freezing.
In the brown adipose tissue of newborns, electron transfer is uncoupled from ATP synthesis, and the energy of fuel oxidation is dissipated as heat. Hibernating animals use this strategy to avoid freezing.