5.2 Complementary Interactions between Proteins and Ligands: The Immune System and Immunoglobulins
We have seen how the conformations of oxygen-binding proteins affect and are affected by the binding of small ligands ( or CO) to the heme group. However, most protein-ligand interactions do not involve a prosthetic group. Instead, the binding site for a ligand is more often like the hemoglobin binding site for BPG — a cleft in the protein lined with amino acid residues, arranged to make the binding interaction highly specific. Effective discrimination between ligands is the norm at binding sites, even when the ligands have only minor structural differences.
Almost all organisms have an immune system of some type that allows them to respond to challenges presented by environmental pathogens. The emergence of vertebrates about 500 million years ago was accompanied by the evolution of an adaptive immune system based on the generation of large numbers of distinct cell clones, each expressing a protein variant that could recognize and bind to a particular type of chemical structure. The system distinguishes molecular “self” from “nonself” and then destroys what is identified as nonself. In this way, the immune system eliminates viruses, bacteria, and other pathogens and molecules that may pose a threat to the organism. On a physiological level, the immune response is an intricate and coordinated set of interactions among many classes of proteins, molecules, and cell types. At the level of individual proteins, the immune response demonstrates how an acutely sensitive and specific biochemical system is built upon the reversible binding of ligands to proteins.
The Immune Response Includes a Specialized Array of Cells and Proteins
Immunity is brought about by a variety of leukocytes (white blood cells), including macrophages and lymphocytes, all of which develop from undifferentiated stem cells in the bone marrow. Leukocytes can leave the bloodstream and patrol the tissues, each cell producing one or more proteins capable of recognizing and binding to molecules that might signal an infection.
The immune response consists of two complementary systems, the humoral and cellular immune systems. The humoral immune system (Latin humor, “fluid”) is directed at bacterial infections and extracellular viruses (those found in the body fluids), but it can also respond to individual foreign proteins. The cellular immune system destroys host cells infected by viruses and also destroys some parasites and foreign tissues.
At the heart of the humoral immune response are soluble proteins called antibodies or immunoglobulins, often abbreviated Ig. Immunoglobulins bind bacteria, viruses, or large molecules identified as foreign and target them for destruction. Making up 20% of blood protein, the immunoglobulins are produced by B lymphocytes, or B cells, so named because they complete their development in the bone marrow.
The agents at the heart of the cellular immune response are a class of T lymphocytes, or T cells (so called because the latter stages of their development occur in the thymus), known as cytotoxic T cells ( cells, also called killer T cells). Recognition of infected cells or parasites involves proteins called T-cell receptors on the surface of cells. Receptors are proteins, usually found on the outer surface of cells and extending through the plasma membrane, that recognize and bind extracellular ligands, thus triggering changes inside the cell.
In addition to cytotoxic T cells, there are helper T cells ( cells), whose function it is to produce soluble signaling proteins called cytokines, which include the interleukins. cells interact with macrophages. The cells participate only indirectly in the destruction of infected cells and pathogens, stimulating the selective proliferation of those and B cells that can bind to a particular antigen. This process, called clonal selection, increases the number of immune system cells that can respond to a particular pathogen. A host organism needs time, often days, to mount an immune response against a new antigen, but memory cells permit a rapid response to pathogens previously encountered. A vaccine to protect against a particular viral infection often consists of weakened or killed virus or isolated proteins from a viral or bacterial protein coat. When injected into a person, the vaccine generally does not cause an infection and illness, but it effectively “teaches” the immune system what the viral particles look like, stimulating the production of memory cells. On subsequent infection, these cells can bind to the virus and trigger a rapid immune response.
The importance of cells is dramatically illustrated by the epidemic produced by HIV (human immunodeficiency virus), the virus that causes AIDS (acquired immune deficiency syndrome). cells are the primary targets of HIV infection; elimination of these cells progressively incapacitates the entire immune system.
Each recognition protein of the immune system, either a T-cell receptor or an antibody produced by a B cell, specifically binds some particular chemical structure, distinguishing it from virtually all others. Humans are capable of producing more than different antibodies with distinct binding specificities. Given this extraordinary diversity, any chemical structure on the surface of a virus or an invading cell will most likely be recognized and bound by one or more antibodies. Antibody diversity is derived from random reassembly of a set of immunoglobulin gene segments through genetic recombination mechanisms that are discussed in Chapter 25 (see Fig. 25-42).
A specialized lexicon is used to describe the unique interactions between antibodies or T-cell receptors and the molecules they bind. Any molecule or pathogen capable of eliciting an immune response is called an antigen. An antigen may be a virus, a bacterial cell wall, or an individual protein or other macromolecule. A complex antigen may be bound by several different antibodies. An individual antibody or T-cell receptor binds only a particular molecular structure within the antigen, called its antigenic determinant or epitope.
It would be unproductive for the immune system to respond to small molecules that are common intermediates and products of cellular metabolism. Molecules of are generally not antigenic. However, when small molecules are covalently attached to large proteins in the laboratory, they can be used to elicit an immune response. These small molecules are called haptens. The antibodies produced in response to protein-linked haptens will then bind to the same small molecules in their free form. Such antibodies are sometimes used in the development of analytical tests described later in this chapter or as a specific ligand in affinity chromatography (see Fig. 3-17c). We now turn to a more detailed description of antibodies and their binding properties.
Antibodies Have Two Identical Antigen-Binding Sites
Immunoglobulin G (IgG) is the major class of antibody molecule and one of the most abundant proteins in the blood serum. IgG has four polypeptide chains: two large ones, called heavy chains, and two smaller ones, called light chains, linked by noncovalent and disulfide bonds into a complex of 150,000. The heavy chains of an IgG molecule interact at one end, then branch to interact separately with the light chains, forming a Y-shaped molecule (Fig. 5-20). At the “hinges” separating the base of an IgG molecule from its branches, the immunoglobulin can be cleaved with proteases. Cleavage with the protease papain liberates the basal fragment, called Fc because it usually crystallizes readily, and the two branches, called Fab, the antigen-binding fragments. Each branch has a single antigen-binding site.
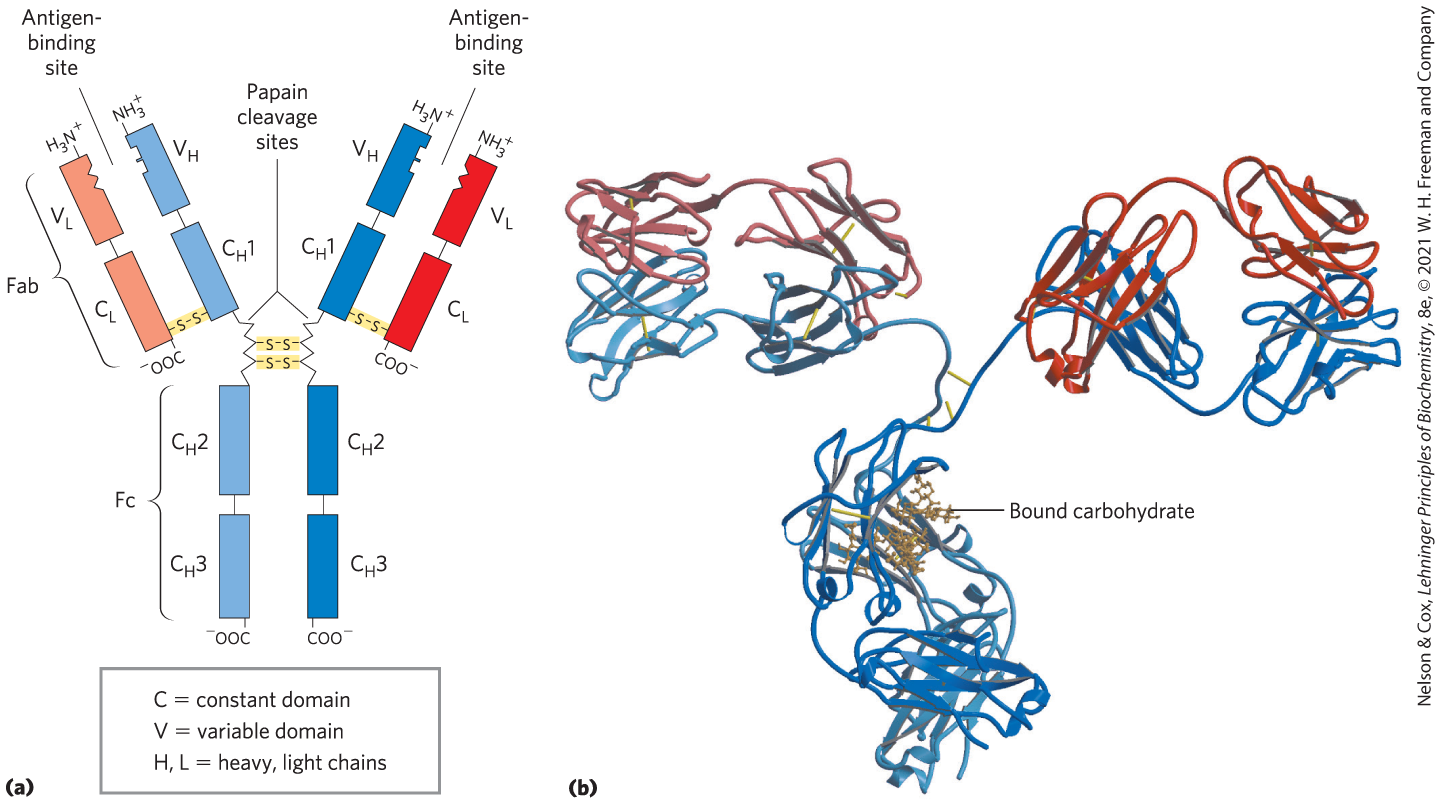
FIGURE 5-20 Immunoglobulin G. (a) Pairs of heavy and light chains combine to form a Y-shaped molecule. Two antigen-binding sites are formed by the combination of variable domains from one light () chain and one heavy () chain. Cleavage with papain separates the Fab and Fc portions of the protein in the hinge region. The Fc portion also contains bound carbohydrate (shown in (b)). (b) A ribbon model of an IgG molecule. Although the molecule has two identical heavy chains (two shades of blue) and two identical light chains (two shades of red), it crystallized in the asymmetric conformation shown here. Conformational flexibility is important to the function of immunoglobulins. [(b) Data from PDB ID 1IGT, L. J. Harris et al., Biochemistry 36:1581, 1997.]
The fundamental structure of immunoglobulins was first established by Gerald Edelman and Rodney Porter in the 1960s. Each chain is made up of identifiable domains. Some are constant in sequence and structure from one IgG to the next; others are variable. The constant domains have a characteristic structure known as the immunoglobulin fold, a well-conserved structural motif in the all-β class of proteins (Chapter 4). There are three of these constant domains in each heavy chain and one in each light chain. The heavy and light chains also have one variable domain each, in which most of the variability in amino acid sequence is found. The variable domains associate to create the antigen-binding site (Fig. 5-20), allowing formation of an antigen-antibody complex (Fig. 5-21).
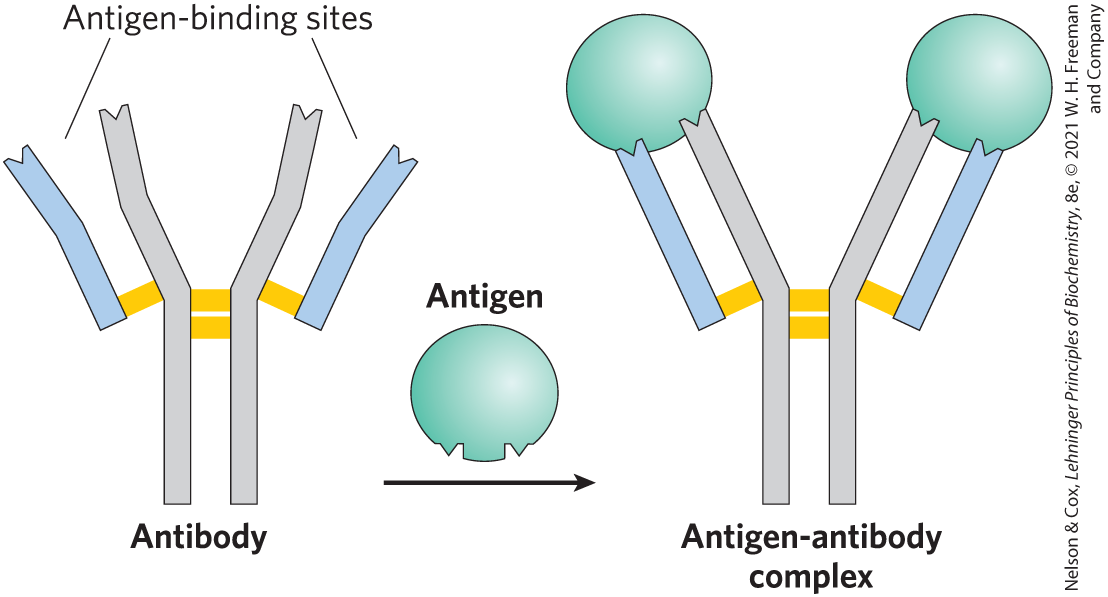
FIGURE 5-21 Binding of IgG to an antigen. To generate an optimal fit for the antigen, the binding sites of IgG often undergo slight conformational changes. Such induced fit is common to many protein-ligand interactions.
In many vertebrates, IgG is but one of five classes of immunoglobulins. Each class has a characteristic type of heavy chain, denoted α, δ, ε, γ, and μ for IgA, IgD, IgE, IgG, and IgM, respectively. Two types of light chain, κ and λ, occur in all classes of immunoglobulins. The overall structures of IgD and IgE are similar to that of IgG. IgM occurs either in a monomeric, membrane-bound form or in a secreted form that is a cross-linked pentamer of this basic structure (Fig. 5-22). IgA, found principally in secretions such as saliva, tears, and milk, can be a monomer, a dimer, or a trimer. IgM is the first antibody to be made by B lymphocytes and the major antibody in the early stages of a primary immune response. Some B cells soon begin to produce IgD (with the same antigen-binding site as the IgM produced by the same cell), but the particular function of IgD is less clear.
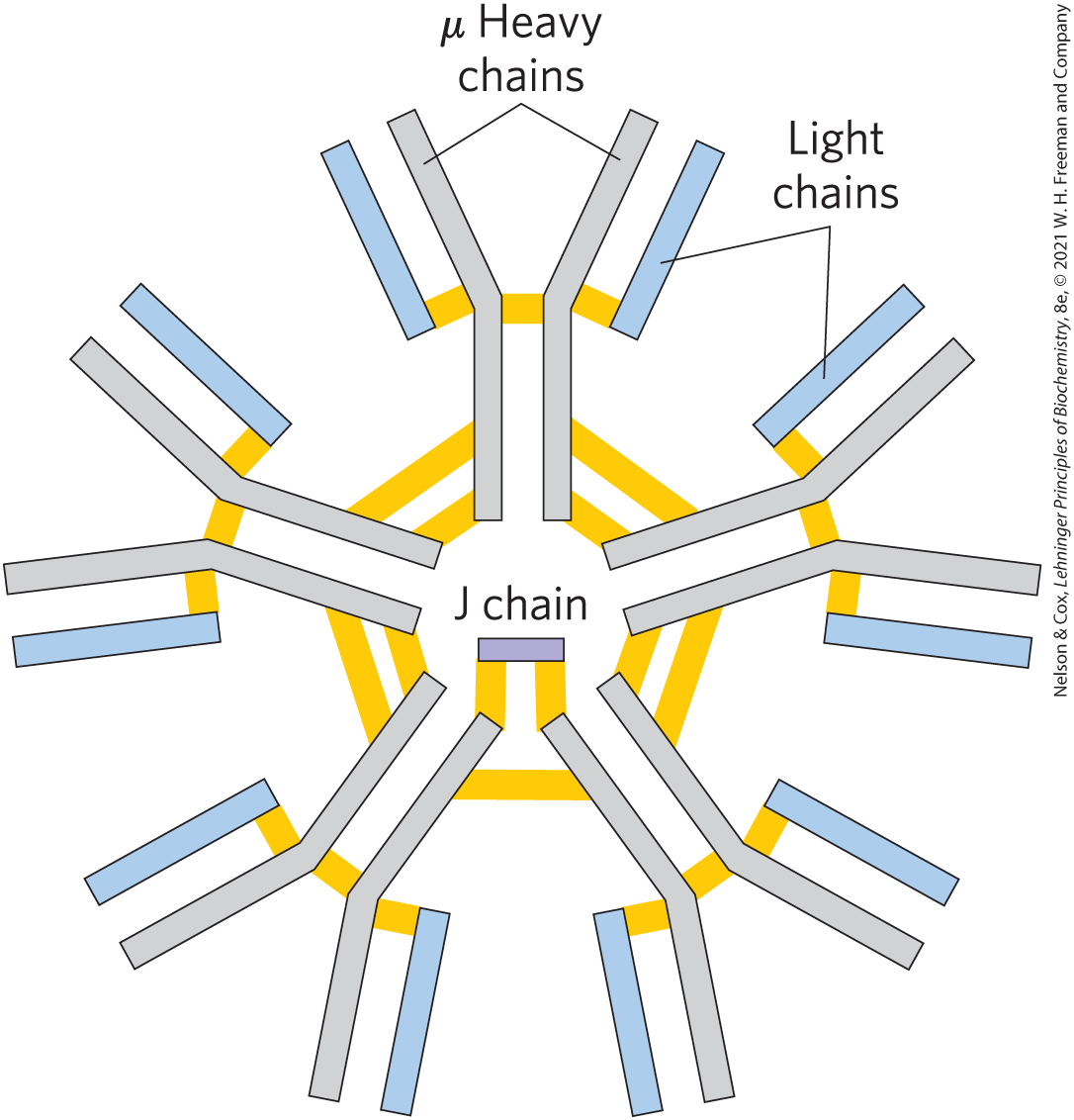
FIGURE 5-22 IgM pentamer of immunoglobulin units. The pentamer is cross-linked with disulfide bonds (yellow). The J chain is a polypeptide of 20,000 found in both IgA and IgM.
The IgG described above is the major antibody in secondary immune responses, which are initiated by a class of B cells called memory B cells. As part of the organism’s ongoing immunity to antigens already encountered and dealt with, IgG is the most abundant immunoglobulin in the blood. When IgG binds to an invading bacterium or virus, it activates certain leukocytes such as macrophages to engulf and destroy the invader, and it also activates some other parts of the immune response. Receptors on the macrophage surface recognize and bind the Fc region of IgG. When these Fc receptors bind an antibody-pathogen complex, the macrophage engulfs the complex by phagocytosis (Fig. 5-23).
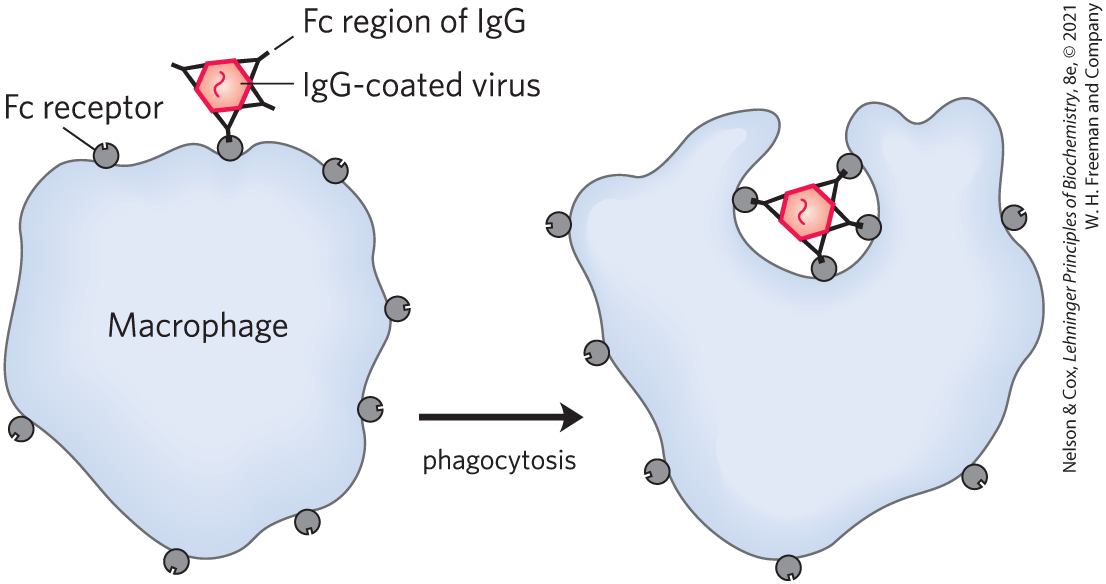
FIGURE 5-23 Phagocytosis of an antibody-bound virus by a macrophage. The Fc regions of antibodies bound to the virus now bind to Fc receptors on the surface of a macrophage, triggering the macrophage to engulf and destroy the virus.
IgE plays an important role in the allergic response, interacting with basophils (phagocytic leukocytes) in the blood and with histamine-secreting cells called mast cells, which are widely distributed in tissues. This immunoglobulin binds, through its Fc region, to special Fc receptors on the basophils or mast cells. In this form, IgE serves as a receptor for antigen. If antigen is bound, the cells are induced to secrete histamine and other biologically active amines that cause the dilation and increased permeability of blood vessels. These effects on the blood vessels are thought to facilitate the movement of immune system cells and proteins to sites of inflammation. They also produce the symptoms normally associated with allergies. Pollen and other allergens are recognized as foreign, triggering an immune response normally reserved for pathogens.
Antibodies Bind Tightly and Specifically to Antigen
The binding specificity of an antibody is determined by the amino acid residues in the variable domains of its heavy and light chains. Some of those residues, particularly those lining the antigen-binding site, are hypervariable — especially likely to differ. Specificity is conferred by chemical complementarity between the antigen and its specific binding site. For example, a binding site with a negatively charged group may bind an antigen with a positive charge in the complementary position. In many instances, complementarity is achieved interactively as the structures of antigen and binding site influence each other as they come closer together. Conformational changes in the antibody and/or the antigen then allow the complementary groups to interact fully. This is an example of induced fit. The complex of a peptide derived from HIV (a model antigen) and an Fab molecule, shown in Figure 5-24, illustrates some of these properties. The changes in structure observed on antigen binding are particularly striking in this example.
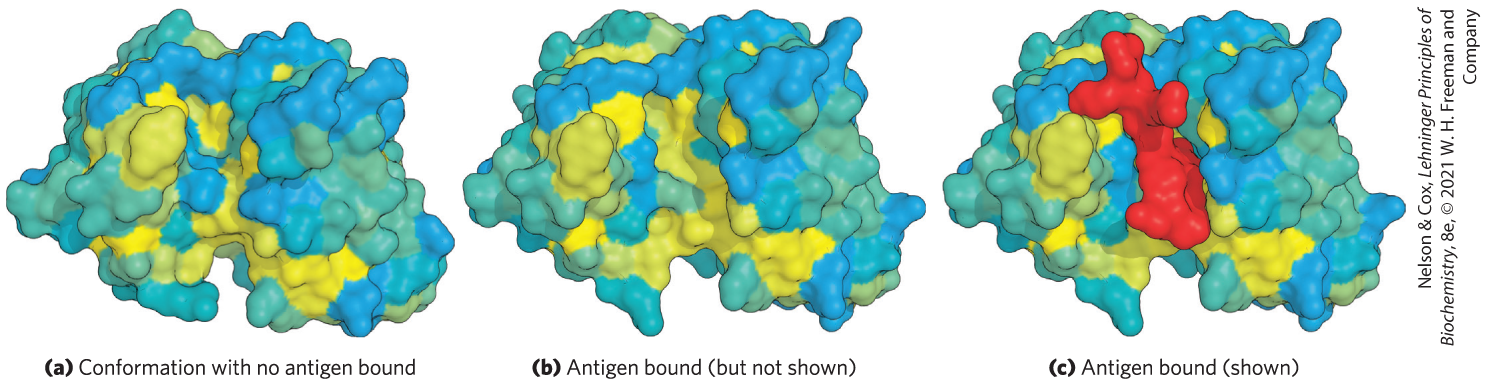
FIGURE 5-24 Induced fit in the binding of an antigen to IgG. The Fab fragment of an IgG molecule is shown here with the surface contour colored to represent hydrophobicity. Hydrophobic surfaces are yellow and hydrophilic surfaces are blue, with shades of blue to green to yellow in between. (a) View of the Fab fragment in the absence of antigen (a small peptide derived from HIV), looking down on the antigen binding site. (b) The same view, but with the Fab fragment in the “bound” conformation with the antigen omitted to provide an unobstructed view of the altered binding site. Note how the hydrophobic binding cavity has enlarged and several groups have shifted position. (c) The same view as (b) but with the antigen (red) in the binding site. [Data from (a) PDB ID 1GGC, R. L. Stanfield et al., Structure 1:83, 1993; (b, c) PDB ID 1GGI, J. M. Rini et al., Proc. Natl. Acad. Sci. USA 90:6325, 1993.]
A typical antibody-antigen interaction is quite strong, characterized by values as low as (recall that a lower corresponds to a stronger binding interaction; see Table 5-1). The reflects the energy derived from the hydrophobic effect and the various ionic, hydrogen-bonding, and van der Waals interactions that stabilize the binding. The binding energy required to produce a of is about 65 kJ/mol.
The Antibody-Antigen Interaction Is the Basis for a Variety of Important Analytical Procedures
The extraordinary binding affinity and specificity of antibodies make them valuable analytical reagents. Two types of antibody preparations are in use: polyclonal and monoclonal. Polyclonal antibodies are those produced by many different B lymphocytes responding to one antigen, such as a protein injected into an animal. Cells in the population of B lymphocytes produce antibodies that bind specific, different epitopes within the antigen. Thus, polyclonal preparations contain a mixture of antibodies that recognize different parts of the protein. Monoclonal antibodies, in contrast, are synthesized by a population of identical B cells (a clone) grown in cell culture. These antibodies are homogeneous, all recognizing the same epitope.
The specificity of antibodies has practical uses. In a versatile analytical technique, an antibody is attached to a reagent that makes it easy to detect (Fig. 5-25). When the antibody binds the target protein, the label reveals the presence of the protein in a solution or its location in a gel, or even in a living cell (Fig. 5-25a). In one application of this technique, an immunoblot or Western blot assay (Fig. 5-26b), proteins that have been separated by gel electrophoresis are transferred electrophoretically to a nitrocellulose membrane. After washing, the membrane is treated successively with primary antibody, secondary antibody linked to enzyme, and substrate. A colored precipitate forms only along the band containing the protein of interest. Immunoblotting allows the detection of a minor component in a sample and provides an approximation of its molecular weight.
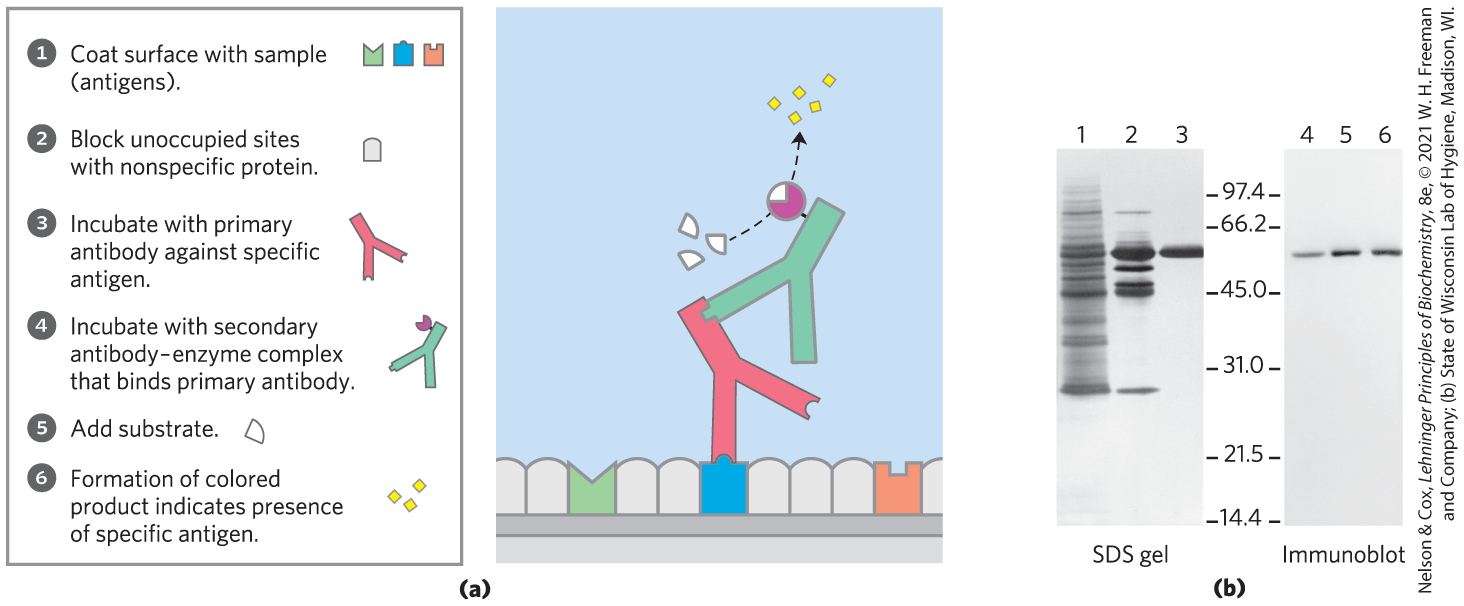
FIGURE 5-25 Antibodies as analytical reagents. (a) A schematic representation of the use of the specific reaction of an antibody with its antigen to identify and quantify a specific protein in a complex sample. (b) One common application is the immunoblot. Lanes 1 to 3 are from an SDS gel; samples from successive stages in the purification of a protein kinase were separated and stained with Coomassie blue. Lanes 4 to 6 show the same samples, but these were electrophoretically transferred to a nitrocellulose membrane after separation on an SDS gel. The membrane was then “probed” with antibody against the protein kinase. The numbers between the SDS gel and the immunoblot indicate in thousands.
We will encounter other aspects of antibodies in later chapters. They are extremely important in medicine and can tell us much about the structure of proteins and the action of genes.
SUMMARY 5.2 Complementary Interactions between Proteins and Ligands: The Immune System and Immunoglobulins
- The immune response is mediated by interactions among an array of specialized leukocytes and their associated proteins. T lymphocytes produce T-cell receptors. B lymphocytes produce immunoglobulins.
- Humans have five classes of immunoglobulins, each with different biological functions. The most abundant class is IgG, a Y-shaped protein with two heavy chains and two light chains. The domains near the upper ends of the Y are hypervariable within the broad population of IgGs and form two antigen-binding sites.
- A given immunoglobulin generally binds to only a part, called the epitope, of a large antigen. Binding often involves a conformational change in the IgG, an induced fit to the antigen.
- The exquisite binding specificity of immunoglobulins is exploited in analytical techniques such as immunoblotting.
 Instead, the binding site for a ligand is more often like the hemoglobin binding site for BPG — a cleft in the protein lined with amino acid residues, arranged to make the binding interaction highly specific. Effective discrimination between ligands is the norm at binding sites, even when the ligands have only minor structural differences.
Instead, the binding site for a ligand is more often like the hemoglobin binding site for BPG — a cleft in the protein lined with amino acid residues, arranged to make the binding interaction highly specific. Effective discrimination between ligands is the norm at binding sites, even when the ligands have only minor structural differences. Each recognition protein of the immune system, either a T-cell receptor or an antibody produced by a B cell, specifically binds some particular chemical structure, distinguishing it from virtually all others. Humans are capable of producing more than different antibodies with distinct binding specificities. Given this extraordinary diversity, any chemical structure on the surface of a virus or an invading cell will most likely be recognized and bound by one or more antibodies. Antibody diversity is derived from random reassembly of a set of immunoglobulin gene segments through genetic recombination mechanisms that are discussed in
Each recognition protein of the immune system, either a T-cell receptor or an antibody produced by a B cell, specifically binds some particular chemical structure, distinguishing it from virtually all others. Humans are capable of producing more than different antibodies with distinct binding specificities. Given this extraordinary diversity, any chemical structure on the surface of a virus or an invading cell will most likely be recognized and bound by one or more antibodies. Antibody diversity is derived from random reassembly of a set of immunoglobulin gene segments through genetic recombination mechanisms that are discussed in  IgE plays an important role in the allergic response, interacting with basophils (phagocytic leukocytes) in the blood and with histamine-secreting cells called mast cells, which are widely distributed in tissues. This immunoglobulin binds, through its Fc region, to special Fc receptors on the basophils or mast cells. In this form, IgE serves as a receptor for antigen. If antigen is bound, the cells are induced to secrete histamine and other biologically active amines that cause the dilation and increased permeability of blood vessels. These effects on the blood vessels are thought to facilitate the movement of immune system cells and proteins to sites of inflammation. They also produce the symptoms normally associated with allergies. Pollen and other allergens are recognized as foreign, triggering an immune response normally reserved for pathogens.
IgE plays an important role in the allergic response, interacting with basophils (phagocytic leukocytes) in the blood and with histamine-secreting cells called mast cells, which are widely distributed in tissues. This immunoglobulin binds, through its Fc region, to special Fc receptors on the basophils or mast cells. In this form, IgE serves as a receptor for antigen. If antigen is bound, the cells are induced to secrete histamine and other biologically active amines that cause the dilation and increased permeability of blood vessels. These effects on the blood vessels are thought to facilitate the movement of immune system cells and proteins to sites of inflammation. They also produce the symptoms normally associated with allergies. Pollen and other allergens are recognized as foreign, triggering an immune response normally reserved for pathogens. 
 The immune response is mediated by interactions among an array of specialized leukocytes and their associated proteins. T lymphocytes produce T-cell receptors. B lymphocytes produce immunoglobulins.
The immune response is mediated by interactions among an array of specialized leukocytes and their associated proteins. T lymphocytes produce T-cell receptors. B lymphocytes produce immunoglobulins.