Chapter 4 THE THREE-DIMENSIONAL STRUCTURE OF PROTEINS
Proteins are big molecules. The covalent backbone of a typical protein contains hundreds of individual bonds. Because free rotation is possible around many of these bonds, the protein can, in principle, assume a virtually uncountable number of spatial arrangements, or conformations. In reality, however, each protein has a specific chemical or structural function, which suggests that each has a unique three-dimensional structure (Fig. 4-1). How stable is this structure, what factors guide its formation, and what holds it together? By the late 1920s, several proteins had been crystallized, including hemoglobin and the enzyme urease Given that, generally, the ordered array of molecules in a crystal can form only if the molecular units are identical, crystallization was evidence that even very large proteins are discrete chemical entities with unique structures. However, we now know that protein structure is always malleable, and in sometimes surprising ways. Changes in structure can be as important to a protein’s function as the structure itself.
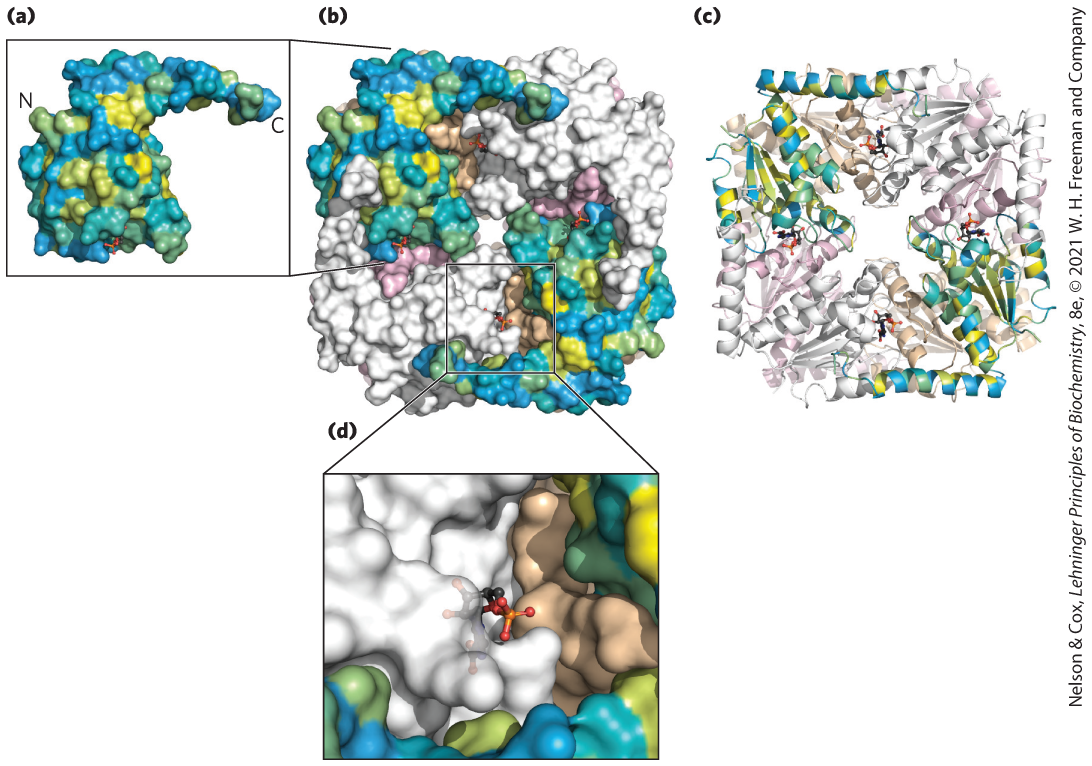
FIGURE 4-1 Relationship between protein structure and function. (a) The PurE enzyme from Escherichia coli catalyzes a reaction that forms carbon–carbon bonds in de novo purine biosynthesis. PurE is a small (17 kDa) single-domain protein. In this view, the protein surface of PurE has been modeled and colored by hydrophobicity: yellow for hydrophobic surfaces, blue for hydrophilic surfaces, and shades of green for those in between. It is apparent that the protein folds so that many of its polar groups are accessible to solvent. (b, c) The enzymatically active form of PurE is an octamer; eight PurE protomers combine to create a square-shaped quaternary structure with eight active sites. The structure in (b) is a surface representation; (c) is a ribbon diagram that traces the peptide backbone. Two protomers are colored by surface hydrophobicity. Others are shown in single colors (two each in gray, tan, and pink). (d) Each active site is formed using segments of three different protomers. A molecule of the reaction product carboxyaminoimidazole ribonucleotide bound at the active site is shown as a stick structure. [Data from PDB ID 2NSL, A. A. Hoskins et al., Biochemistry 46:2842, 2007.]
In this chapter, we examine the structure of proteins. We emphasize five principles:
Protein structures are stabilized by noncovalent interactions and forces. Formation of a thermodynamically favorable structure depends on the influences of the hydrophobic effect, hydrogen bonds, ionic interactions, and van der Waals forces. Natural protein structures are constrained by peptide bonds, whose configurations can be described by the dihedral angles ϕ and ψ.
Protein segments can adopt regular secondary structures such as the α helix and the β conformation. These structures are defined by particular values of ϕ and ψ and their formation is impacted by the amino acid composition of the segment. All of the ϕ and ψ values for a given protein structure can be visualized using a Ramachandran plot.
Tertiary structure describes the well-defined, three-dimensional fold adopted by a protein. Protein structures are often built by combinatorial use of common protein folds or motifs. Quaternary structure describes the interactions between components of a multisubunit assembly.
Tertiary structure is determined by amino acid sequence. Even though protein folding is complex, some denatured proteins can spontaneously refold into their active conformation based only on the chemical properties of their constituent amino acids. Cellular proteostasis involves numerous pathways that regulate the folding, unfolding, and degradation of proteins. Many human diseases arise from protein misfolding and defects in proteostasis.
The three-dimensional structures of proteins can be defined. Structural biologists use a variety of instruments and computational methods to solve biomolecular structures. The choice of method may depend on factors such as the size of the protein being studied, its properties, or the desired resolution of the final structure.
 Protein structures are stabilized by noncovalent interactions and forces. Formation of a thermodynamically favorable structure depends on the influences of the hydrophobic effect, hydrogen bonds, ionic interactions, and van der Waals forces. Natural protein structures are constrained by peptide bonds, whose configurations can be described by the dihedral angles ϕ and ψ.
Protein structures are stabilized by noncovalent interactions and forces. Formation of a thermodynamically favorable structure depends on the influences of the hydrophobic effect, hydrogen bonds, ionic interactions, and van der Waals forces. Natural protein structures are constrained by peptide bonds, whose configurations can be described by the dihedral angles ϕ and ψ. Protein segments can adopt regular secondary structures such as the α helix and the β conformation. These structures are defined by particular values of ϕ and ψ and their formation is impacted by the amino acid composition of the segment. All of the ϕ and ψ values for a given protein structure can be visualized using a Ramachandran plot.
Protein segments can adopt regular secondary structures such as the α helix and the β conformation. These structures are defined by particular values of ϕ and ψ and their formation is impacted by the amino acid composition of the segment. All of the ϕ and ψ values for a given protein structure can be visualized using a Ramachandran plot. Tertiary structure describes the well-defined, three-dimensional fold adopted by a protein. Protein structures are often built by combinatorial use of common protein folds or motifs. Quaternary structure describes the interactions between components of a multisubunit assembly.
Tertiary structure describes the well-defined, three-dimensional fold adopted by a protein. Protein structures are often built by combinatorial use of common protein folds or motifs. Quaternary structure describes the interactions between components of a multisubunit assembly. Tertiary structure is determined by amino acid sequence. Even though protein folding is complex, some denatured proteins can spontaneously refold into their active conformation based only on the chemical properties of their constituent amino acids. Cellular proteostasis involves numerous pathways that regulate the folding, unfolding, and degradation of proteins. Many human diseases arise from protein misfolding and defects in proteostasis.
Tertiary structure is determined by amino acid sequence. Even though protein folding is complex, some denatured proteins can spontaneously refold into their active conformation based only on the chemical properties of their constituent amino acids. Cellular proteostasis involves numerous pathways that regulate the folding, unfolding, and degradation of proteins. Many human diseases arise from protein misfolding and defects in proteostasis. The three-dimensional structures of proteins can be defined. Structural biologists use a variety of instruments and computational methods to solve biomolecular structures. The choice of method may depend on factors such as the size of the protein being studied, its properties, or the desired resolution of the final structure.
The three-dimensional structures of proteins can be defined. Structural biologists use a variety of instruments and computational methods to solve biomolecular structures. The choice of method may depend on factors such as the size of the protein being studied, its properties, or the desired resolution of the final structure.