7.5 Working with Carbohydrates
Oligosaccharide analysis is complicated by the fact that, unlike nucleic acids and proteins, oligosaccharides can be branched and are joined by a variety of linkages. The high charge density of many oligosaccharides and polysaccharides, and the relative lability of the sulfate esters in glycosaminoglycans, present further difficulties.
For simple, linear polymers such as amylose, the positions of the glycosidic bonds are determined by the classical method of exhaustive methylation: treating the intact polysaccharide with methyl iodide in a strongly basic medium to convert all free hydroxyls to acid-stable methyl ethers, then hydrolyzing the methylated polysaccharide in acid. The only free hydroxyls in the monosaccharide derivatives so produced are those that were involved in glycosidic bonds. To determine the sequence of monosaccharide residues, including any branches that are present, exoglycosidases of known specificity are used to remove residues one at a time from the nonreducing end(s). The known specificity of these exoglycosidases often allows deduction of the position and stereochemistry of the linkages.
For analysis of the oligosaccharide moieties of glycoproteins and glycolipids, the oligosaccharides are released by purified enzymes — glycosidases that specifically cleave O- or N-linked oligosaccharides, or lipases that remove lipid head groups. Alternatively, O-linked glycans can be released from glycoproteins by treatment with hydrazine.
The resulting mixtures of carbohydrates are resolved into their individual components by a variety of methods (Fig. 7-34), including the same techniques used in protein and amino acid separation: fractional precipitation by solvents, and ion-exchange and size-exclusion chromatography (see Fig. 3-17). Highly purified lectins, attached covalently to an insoluble support, can be used in affinity chromatography of carbohydrates.
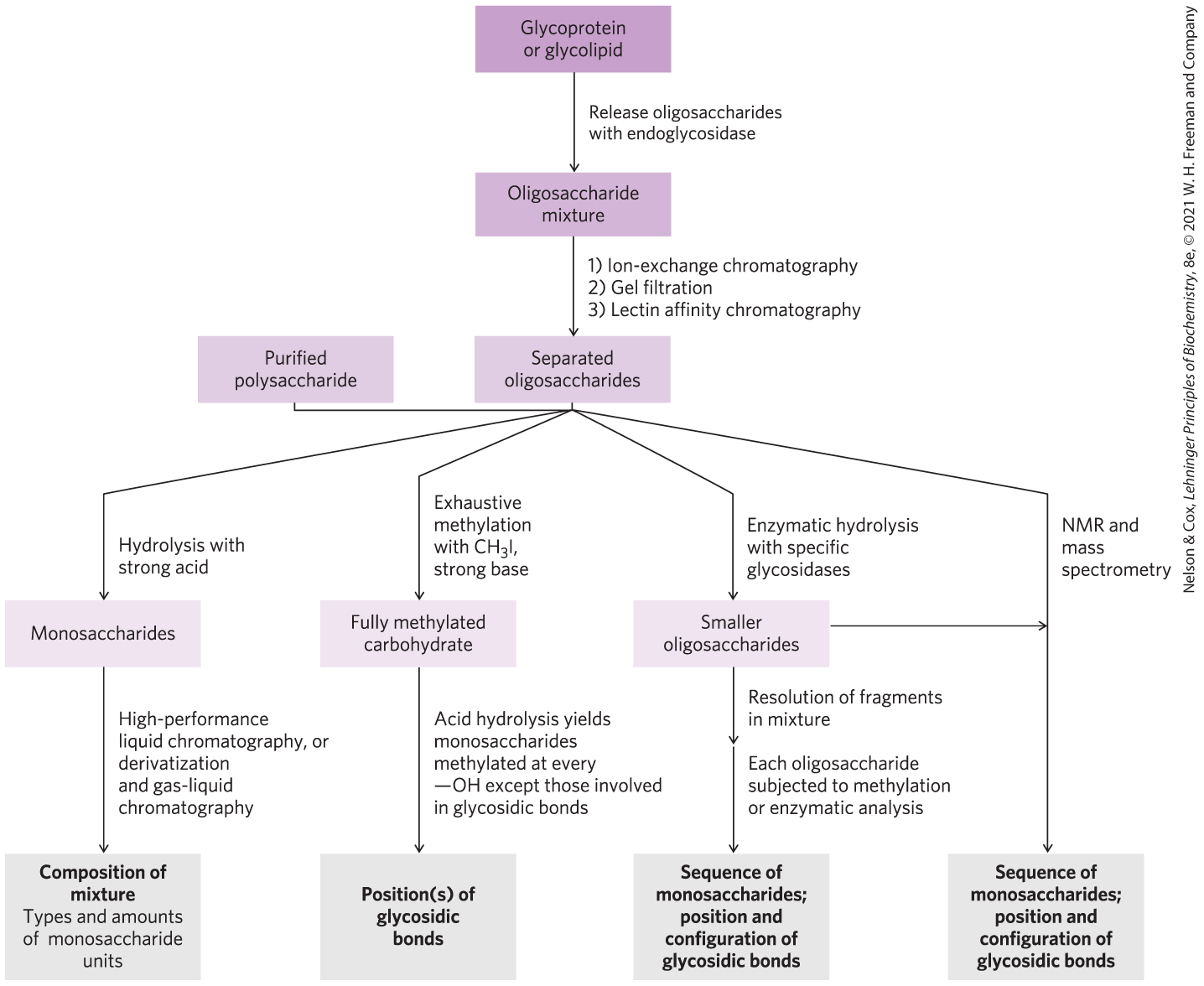
FIGURE 7-34 Methods of carbohydrate analysis. A carbohydrate purified in the first stage of the analysis often requires all four analytical routes for its complete characterization.
Hydrolysis of oligosaccharides and polysaccharides in strong acid yields a mixture of monosaccharides, which can be identified and quantified by chromatographic techniques to yield the overall composition of the polymer.
Oligosaccharide analysis relies heavily on mass spectrometry (see Figs 3-28 and 3-29) and high-resolution NMR spectroscopy (see Fig. 4-31). NMR analysis alone, especially for oligosaccharides of moderate size, can yield much information about sequence, linkage position, and anomeric carbon configuration. Automated procedures and commercial instruments are used for the routine determination of oligosaccharide structure, but the sequencing of branched oligosaccharides joined by more than one type of bond remains a far more formidable task than determining the linear sequences of proteins and nucleic acids.
Another important tool in working with carbohydrates is chemical synthesis, which has proved to be a powerful approach to understanding the biological functions of glycosaminoglycans and oligosaccharides. The chemistry involved in such syntheses is difficult, but carbohydrate chemists can now synthesize short segments of almost any glycosaminoglycan, with correct stereochemistry, chain length, and sulfation pattern, and oligosaccharides significantly more complex than those shown in Figure 7-27. Solid-phase oligosaccharide synthesis is based on the same principles (and has the same advantages) as peptide synthesis (see Fig. 3-30), but requires a set of tools unique to carbohydrate chemistry: blocking groups and activating groups that allow the synthesis of glycosidic linkages with the correct hydroxyl group. Synthetic approaches of this type currently represent an area of great interest, because it is difficult to purify defined oligosaccharides in adequate quantities from natural sources.
SUMMARY 7.5 Working with Carbohydrates
- Establishing the complete structure of oligosaccharides and polysaccharides is a more complex problem than protein and nucleic acid analysis. Traditional chemical and enzymatic approaches, as well as mass spectrometry and high-resolution NMR spectroscopy, applicable to small samples of carbohydrate, yield essential information about sequence, configuration at anomeric and other carbons, and positions of glycosidic bonds. Solid-phase synthetic methods yield defined oligosaccharides that are of great value in exploring lectin-oligosaccharide interactions and may prove clinically useful.
 Establishing the complete structure of oligosaccharides and polysaccharides is a more complex problem than protein and nucleic acid analysis. Traditional chemical and enzymatic approaches, as well as mass spectrometry and high-resolution NMR spectroscopy, applicable to small samples of carbohydrate, yield essential information about sequence, configuration at anomeric and other carbons, and positions of glycosidic bonds. Solid-phase synthetic methods yield defined oligosaccharides that are of great value in exploring lectin-oligosaccharide interactions and may prove clinically useful.
Establishing the complete structure of oligosaccharides and polysaccharides is a more complex problem than protein and nucleic acid analysis. Traditional chemical and enzymatic approaches, as well as mass spectrometry and high-resolution NMR spectroscopy, applicable to small samples of carbohydrate, yield essential information about sequence, configuration at anomeric and other carbons, and positions of glycosidic bonds. Solid-phase synthetic methods yield defined oligosaccharides that are of great value in exploring lectin-oligosaccharide interactions and may prove clinically useful.