12.5 Multivalent Adaptor Proteins and Membrane Rafts
Two generalizations have emerged from studies of signaling systems such as those we have discussed so far. First, protein kinases that phosphorylate Tyr, Ser, and Thr residues — and phosphatases that dephosphorylate them — are central to signaling, directly affecting the activities of a large number of protein substrates. Second, protein-protein interactions brought about by the reversible phosphorylation of Tyr, Ser, and Thr residues in signaling proteins create docking sites for other proteins that bring about indirect effects on proteins downstream in the signaling pathway. In fact, many signaling proteins are multivalent: they can interact with several different proteins simultaneously to form multiprotein signaling complexes. In this section we present a few examples to illustrate the general principles of phosphorylation-dependent protein interactions in signaling pathways.
Many of the proteins involved in signaling have intrinsically disordered regions (IDRs) that are flexible enough to allow specific interaction with more than one protein — and perhaps with many other proteins. Protein kinases, for example, have a well-conserved structure containing the substrate-binding and catalytic sites, but through evolution they have acquired additional sequences, typically 20–30 residues long, that are partially disordered and can fold to fit into various multienzyme regulatory networks. The activation loop found in most protein kinases is an IDR and is the universal regulator of kinase activity; its position changes when one or several residues become phosphorylated, switching on the kinase activity. Protein kinases of the PKA, PKB, and PKC families have a disordered carboxyl-terminal tail containing critical residues whose phosphorylation state switches between active and inactive kinase structures. In the MAPK cascade, an amino-terminal IDR plays a similar role as a docking site in the multienzyme complex. Given the ~1,000 protein kinase genes in humans, the many noncatalytic scaffold proteins, and the multiple interactions possible for IDRs in many of these proteins, the number of possible permutations and combinations available for switching and regulating metabolic processes by protein phosphorylation is impressively large.
Protein Modules Bind Phosphorylated Tyr, Ser, or Thr Residues in Partner Proteins
The protein Grb2 in the insulin signaling pathway (Figs. 12-22 and 12-27) binds through its SH2 domain to other proteins that have exposed –Tyr residues. The human genome encodes at least 87 SH2-containing proteins, many known to participate in signaling. The –Tyr residue is bound in a deep pocket in an SH2 domain, with each of its phosphate oxygens participating in hydrogen bonding or electrostatic interactions; the positive charges on two Arg residues figure prominently in the binding. Subtle differences in the structure of SH2 domains account for the specificities of the interactions of SH2-containing proteins with various –Tyr-containing proteins. The SH2 domain typically interacts with a –Tyr (which is assigned the index position 0) and the next three residues toward the carboxyl terminus (designated ). Some proteins with SH2 domains (Src, Fyn, Hck, Nck) favor negatively charged residues in the and positions; others (PLCγ 1, SHP2) have a long hydrophobic groove that binds to aliphatic residues in positions to . These differences define subclasses of SH2 domains that have different partner specificities.
Phosphotyrosine-binding domains, or PTB domains, are another binding partner for –Tyr proteins (Fig. 12-28), but their critical sequences and three-dimensional structure distinguish them from SH2 domains. The human genome encodes at least 24 proteins that contain PTB domains, including IRS1, which we have already encountered in its role as an adaptor protein in insulin-signal transduction (Fig. 12-23). The –Tyr-binding sites for SH2 and PTB domains on partner proteins are created by Tyr kinases and eliminated by protein tyrosine phosphatases (PTPs).
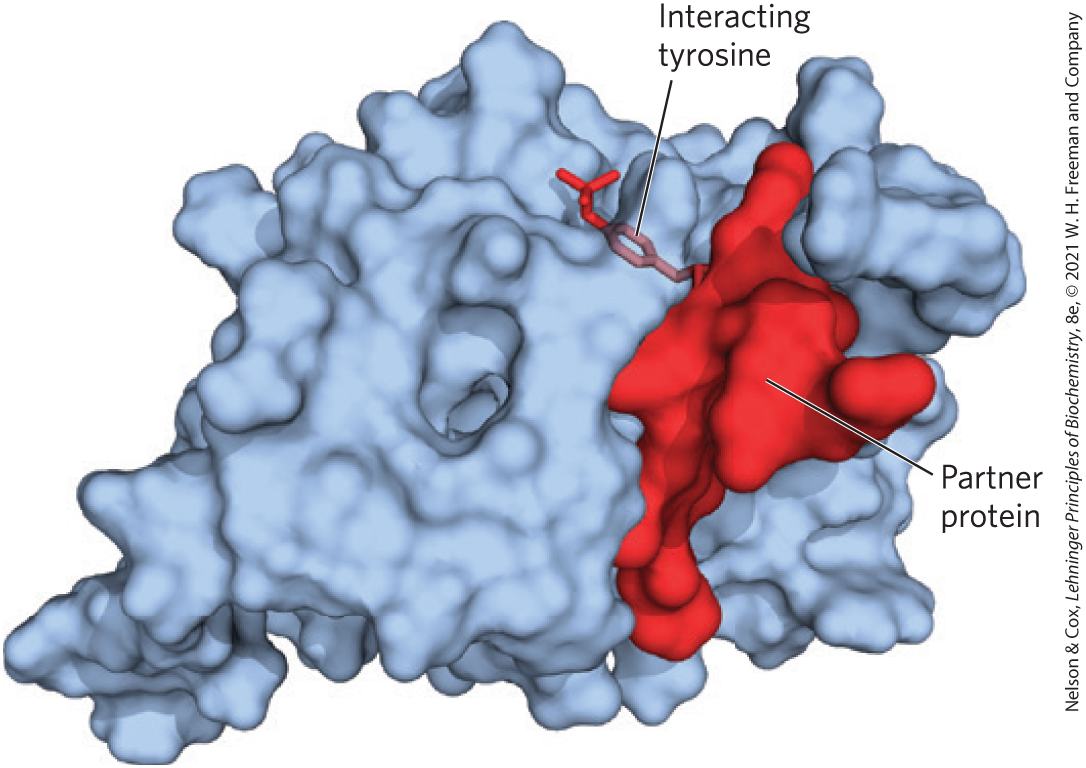
FIGURE 12-28 Interaction of a PTB domain with –Tyr residue in a partner protein. The PTB domain is represented as a blue surface contour. The partner protein is held to the kinase by multiple noncovalent interactions, which confer specificity on the interaction and position the –Tyr residue in a binding pocket at the enzyme’s active site. [Data from PDB ID 1SHC, M. M. Zhou et al., Nature 378:584, 1995.]
As we have seen, other signaling protein kinases, including PKA, PKC, PKG, and members of the MAPK cascade, phosphorylate Ser or Thr residues in their target proteins. In some cases, these proteins acquire the ability to interact with partner proteins through the phosphorylated residue, triggering a downstream process. An alphabet soup of domains that bind –Ser or –Thr residues has been identified, and more are sure to be found. Each domain favors a certain sequence around the phosphorylated residue, so proteins with that domain bind to and interact with a specific subset of phosphorylated proteins.
In some cases, the region on a protein that binds –Tyr of a substrate protein is masked by the region’s interaction with a –Tyr in the same protein. For example, the soluble protein Tyr kinase Src, when phosphorylated on a specific Tyr residue, is rendered inactive; an SH2 domain needed to bind the substrate protein instead binds the internal –Tyr. Removal of this –Tyr residue by a phosphoprotein phosphatase turns on the Tyr kinase activity of Src (Fig. 12-29a). Similarly, glycogen synthase kinase 3 (GSK3) is inactive when phosphorylated on a Ser residue in its autoinhibitory domain (Fig. 12-29b). Dephosphorylation of that domain frees the enzyme to bind (and then phosphorylate) its target proteins.
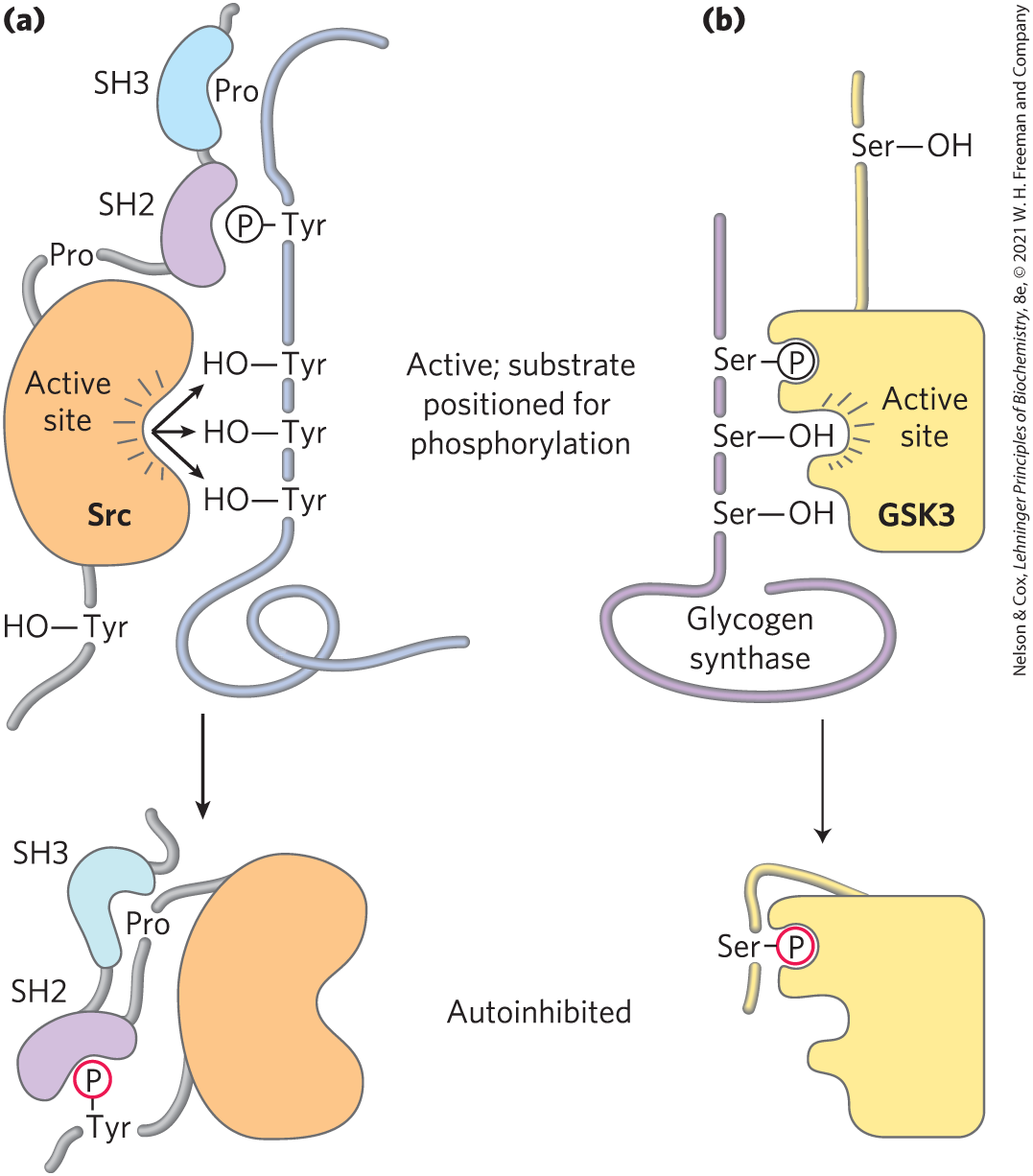
FIGURE 12-29 Mechanism of autoinhibition of Src and GSK3. (a) In the active form of the Tyr kinase Src, an SH2 domain binds a –Tyr in the protein substrate, and an SH3 domain binds a proline-rich region of the substrate, thus lining up the active site of the kinase with several target Tyr residues in the substrate (top). When Src is phosphorylated on a specific Tyr residue (bottom), the SH2 domain binds the internal –Tyr instead of the –Tyr of the substrate, and the SH3 domain binds an internal proline-rich region, preventing productive enzyme-substrate binding; the enzyme is thus autoinhibited. (b) In the active form of glycogen synthase kinase 3 (GSK3), an internal –Ser-binding domain is available to bind –Ser in its substrate (glycogen synthase) and to position the kinase to phosphorylate neighboring Ser residues (top). Phosphorylation of an internal Ser residue allows this internal kinase segment to occupy the –Ser-binding site, blocking substrate binding (bottom).
In addition to the three commonly phosphorylated residues in proteins (Tyr, Ser, Thr), there is a fourth phosphorylated structure that nucleates the formation of supramolecular complexes of signaling proteins: the phosphorylated head group of the membrane phosphatidylinositols. Many signaling proteins contain domains such as SH3 and PH (pleckstrin homology) that bind tightly to protruding on the cytoplasmic side of the plasma membrane. Wherever the enzyme PI3K creates this head group (as it does in response to the insulin signal), proteins that bind will cluster at the membrane surface.
Most of the proteins involved in signaling at the plasma membrane have one or more phosphoprotein- or phospholipid-binding domain; many have three or more, and thus are multivalent in their interactions with other signaling proteins. Figure 12-30 shows just a few of the multivalent proteins known to participate in signaling. Many of the complexes include components with membrane-binding domains. Given the location of so many signaling processes at the inner surface of the plasma membrane, the molecules that must collide to produce the signaling response are effectively confined to two-dimensional space — the membrane surface; collisions are far more likely here than in the three-dimensional space of the cytosol.
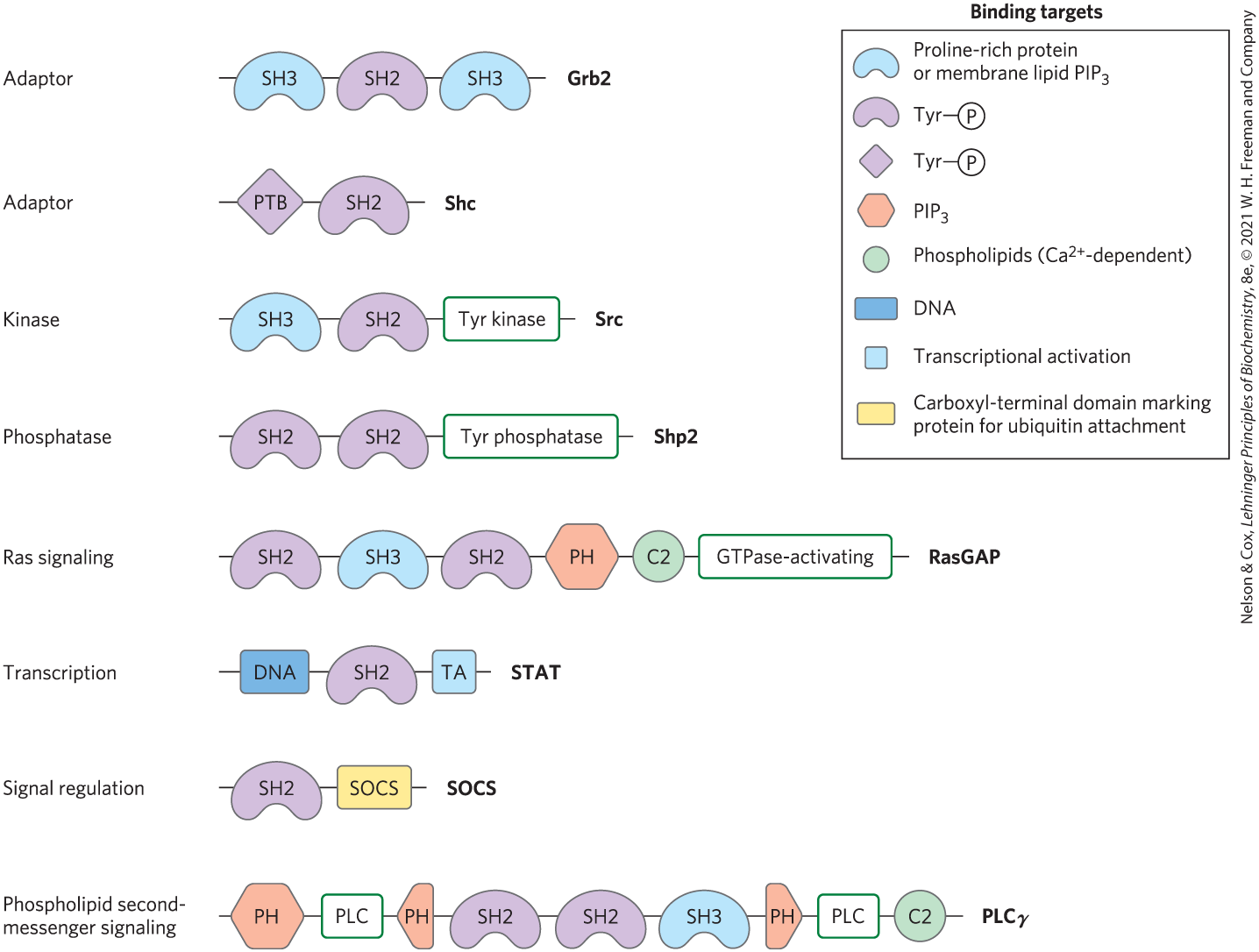
FIGURE 12-30 Some binding modules of signaling proteins. These signaling proteins interact with phosphorylated proteins or phospholipids in many permutations and combinations to form integrated signaling complexes. Each protein is represented by a line (with the amino terminus to the left); symbols indicate the location of conserved binding domains (with specificities as listed in the key; abbreviations are explained in the text); green boxes indicate catalytic activities. The name of each protein is given at its carboxyl-terminal end. [Information from T. Pawson et al., Trends Cell Biol. 11:504, 2001, Fig. 5.]
In summary, a remarkable picture of signaling pathways has emerged from studies of many signaling proteins and their multiple binding domains. An initial signal results in phosphorylation of the receptor or a target protein, triggering the assembly of large multiprotein complexes, held together on scaffolds with multivalent binding capacities. Some of these complexes contain several protein kinases that activate each other in turn, producing a cascade of phosphorylation and a great amplification of the initial signal. The interactions between cascade kinases are not left to the vagaries of random collisions in three-dimensional space. In the MAPK cascade, for example, a scaffold protein, KSR, binds all three kinases (MAPK, MAPKK, and MAPKKK), ensuring their proximity and correct orientation and even conferring allosteric properties on the interactions among the kinases, which makes their serial phosphorylation sensitive to very small stimuli (Fig. 12-31).
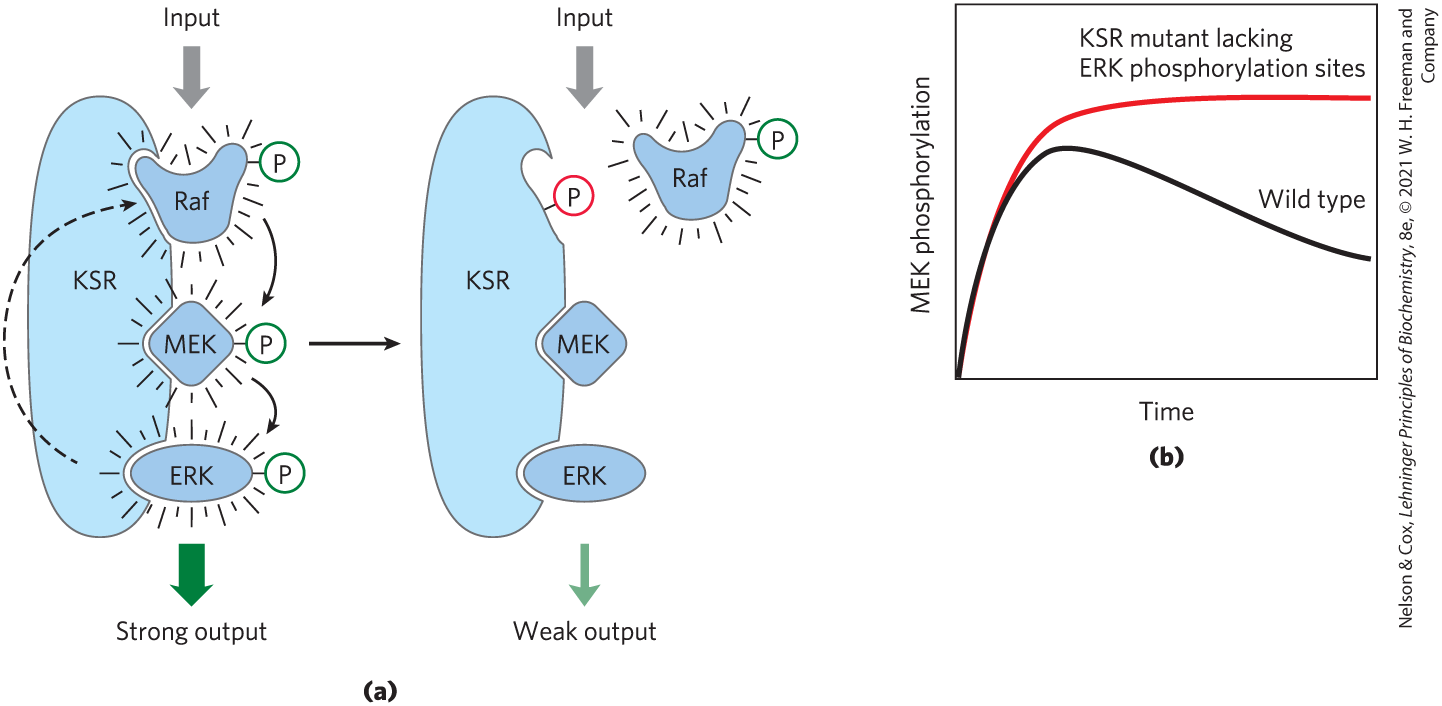
FIGURE 12-31 A scaffold protein from yeast that organizes and regulates a protein kinase cascade. (a) The scaffold protein KSR has binding sites for all three of the kinases in the Raf-MEK-ERK cascade. With the binding of all three in appropriate orientations, interactions among the proteins are rapid and efficient. When ERK has been activated (left), it phosphorylates the binding site for Raf (right), forcing a conformational change that displaces Raf and thereby prevents the phosphorylation of MEK. The result of this feedback regulation is that MEK phosphorylation is temporary. (b) In yeast cells with mutant KSR lacking the phosphorylation sites (red curve), no feedback occurs, producing a different time course of signaling. [Information from M. C. Good et al., Science 332:680, 2011, Fig. 2E.]
Phosphotyrosine phosphatases remove the phosphate from –Tyr residues, reversing the effect of phosphorylation. At least 37 genes in the human genome encode protein tyrosine phosphatases (PTPs). About half of these are receptorlike integral proteins with a single transmembrane domain; they are presumably controlled by extracellular factors not yet identified. Other PTPs are soluble and contain SH2 domains that determine their molecular partners and intracellular location. In addition, animal cells have protein –Ser and –Thr phosphatases such as PP1 that reverse the effects of Ser- and Thr-specific protein kinases.
We can see, then, that signaling occurs in protein circuits, which are effectively hardwired from signal receptor to response effector and can be switched off instantly by the hydrolysis of a single upstream phosphate ester bond. In these circuits, protein kinases are the writers, domains such as SH2 are the readers, and PTPs and other phosphatases are the erasers. The multivalency of signaling proteins allows the assembly of many different combinations of Lego-like signaling modules, each combination suited to particular signals, cell types, and metabolic circumstances, yielding diverse signaling circuits of extraordinary complexity.
Membrane Rafts and Caveolae Segregate Signaling Proteins
Membrane rafts (Chapter 11) are regions of the membrane bilayer enriched in sphingolipids, sterols, and certain proteins, including many proteins attached to the bilayer by GPI (glycosylated derivatives of phosphatidylinositol) anchors. The β-adrenergic receptor is segregated in rafts that also contain G proteins, adenylyl cyclase, PKA, and the protein phosphatase PP2, which together provide a highly integrated signaling unit. By segregating in a small region of the plasma membrane all of the elements required for responding to and ending the signal, the cell is able to produce a highly localized and brief “puff” of second messenger.
Some RTKs (EGFR and PDGFR) are also localized in rafts, and this sequestration very likely has functional significance. In isolated fibroblasts, EGFR is usually concentrated in specialized rafts called caveolae (see Fig. 11-23). When the cells are treated with EGF, the receptor leaves the raft, separating it from the other components of the EGF signaling pathway. This migration depends on the receptor’s protein kinase activity; mutant receptors lacking this activity remain in the raft during treatment with EGF. Such experiments suggest that spatial segregation of signaling proteins in rafts is yet another dimension of the already complex processes initiated by extracellular signals.
SUMMARY 12.5 Multivalent Adaptor Proteins and Membrane Rafts
- Many signaling proteins have domains that bind phosphorylated Tyr, Ser, or Thr residues in other proteins; the binding specificity for each domain is determined by sequences that adjoin the phosphorylated residue in the substrate. SH2 and PTB domains bind to proteins containing –Tyr residues; other domains bind –Ser and –Thr residues in various contexts. SH3 and PH domains bind the membrane phospholipid .
- Many signaling proteins are multivalent, with several different binding modules. By combining the substrate specificities of various protein kinases with the specificities of domains that bind phosphorylated Tyr, Ser, or Thr residues, and with phosphatases that can rapidly inactivate a signaling pathway, cells create a large number of multiprotein signaling complexes.
- Membrane rafts and caveolae sequester groups of signaling proteins in small regions of the plasma membrane, effectively raising their local concentrations and making signaling more efficient.
 Second,
Second, –Tyr residues. The human genome encodes at least 87 SH2-containing proteins, many known to participate in signaling. The
–Tyr residues. The human genome encodes at least 87 SH2-containing proteins, many known to participate in signaling. The  An initial signal results in phosphorylation of the receptor or a target protein, triggering the assembly of large multiprotein complexes, held together on scaffolds with multivalent binding capacities. Some of these complexes contain several protein kinases that activate each other in turn, producing a cascade of phosphorylation and a great amplification of the initial signal. The interactions between cascade kinases are not left to the vagaries of random collisions in three-dimensional space. In the MAPK cascade, for example, a scaffold protein, KSR, binds all three kinases (MAPK, MAPKK, and MAPKKK), ensuring their proximity and correct orientation and even conferring allosteric properties on the interactions among the kinases, which makes their serial phosphorylation sensitive to very small stimuli (
An initial signal results in phosphorylation of the receptor or a target protein, triggering the assembly of large multiprotein complexes, held together on scaffolds with multivalent binding capacities. Some of these complexes contain several protein kinases that activate each other in turn, producing a cascade of phosphorylation and a great amplification of the initial signal. The interactions between cascade kinases are not left to the vagaries of random collisions in three-dimensional space. In the MAPK cascade, for example, a scaffold protein, KSR, binds all three kinases (MAPK, MAPKK, and MAPKKK), ensuring their proximity and correct orientation and even conferring allosteric properties on the interactions among the kinases, which makes their serial phosphorylation sensitive to very small stimuli ( Many signaling proteins have domains that bind phosphorylated Tyr, Ser, or Thr residues in other proteins; the binding specificity for each domain is determined by sequences that adjoin the phosphorylated residue in the substrate. SH2 and PTB domains bind to proteins containing
Many signaling proteins have domains that bind phosphorylated Tyr, Ser, or Thr residues in other proteins; the binding specificity for each domain is determined by sequences that adjoin the phosphorylated residue in the substrate. SH2 and PTB domains bind to proteins containing