20.5 Photorespiration and the and CAM Pathways
As we have seen, photosynthetic cells produce (by the splitting of ) during the light-driven reactions and use during the light-independent processes, so the net gaseous change during photosynthesis is the uptake of and release of :
In the dark, plants also carry out mitochondrial respiration, the oxidation of substrates to and the conversion of to . And there is another process in plants that, like mitochondrial respiration, consumes and produces and, like photosynthesis, is driven by light. This process, photorespiration, is a costly side reaction of photosynthesis, a result of the lack of specificity of the enzyme rubisco. In this section we describe this side reaction and the strategies plants use to minimize its metabolic consequences.
Photorespiration Results from Rubisco’s Oxygenase Activity
Rubisco is not absolutely specific for as a substrate. Molecular oxygen competes with at the active site, and about once in every three or four turnovers, rubisco catalyzes the condensation of with ribulose 1,5-bisphosphate to form 3-phosphoglycerate and 2-phosphoglycolate (Fig. 20-38), a metabolically unneeded product. This is the oxygenase activity referred to in the full name of rubisco: ribulose 1,5-bisphosphate carboxylase/oxygenase. The reaction with results in no fixation of and is presumably a net liability to the cell; salvaging the carbons from 2-phosphoglycolate (by the pathway outlined below) consumes significant amounts of cellular energy and releases some previously fixed .
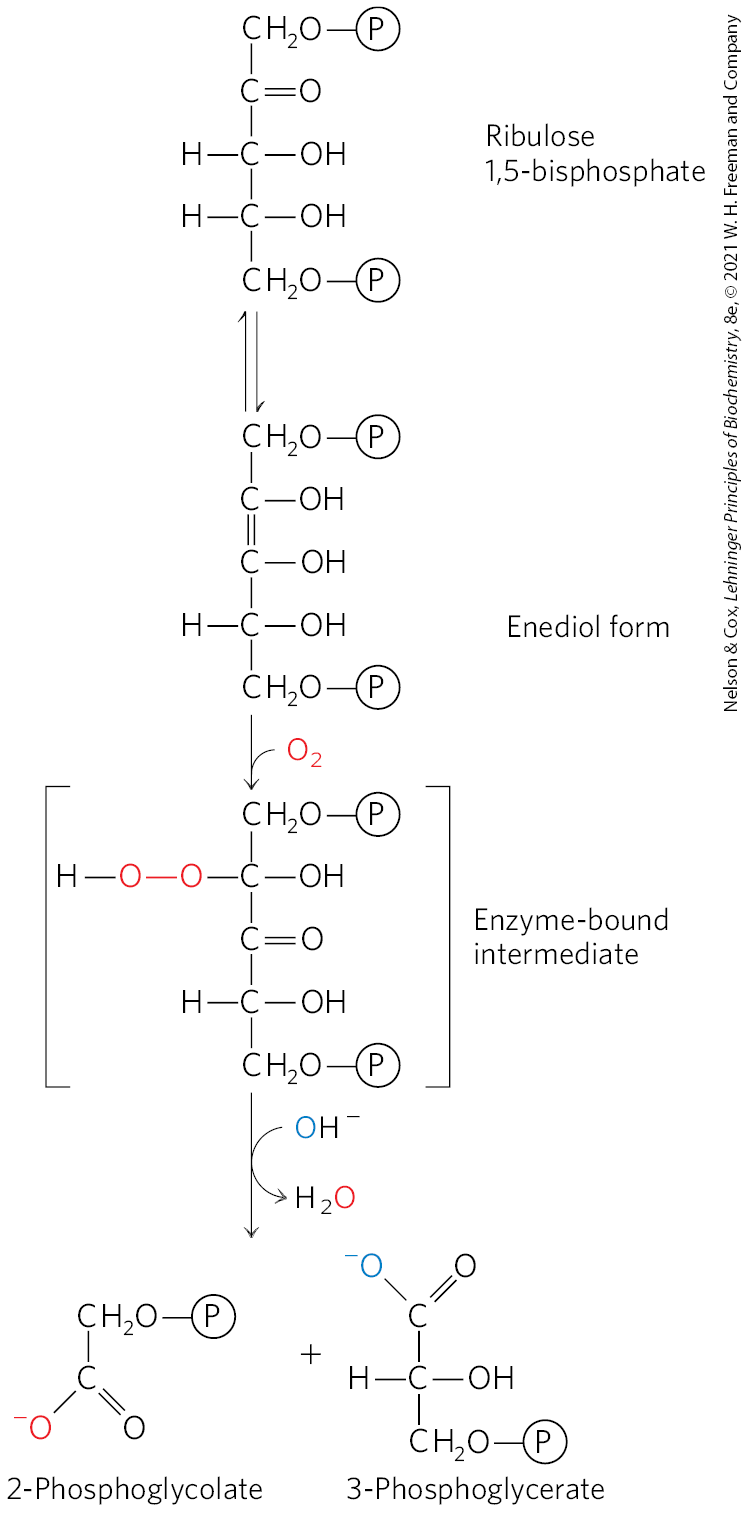
FIGURE 20-38 Oxygenase activity of rubisco. Rubisco can incorporate rather than into ribulose 1,5-bisphosphate. The unstable intermediate thus formed splits into 2-phosphoglycolate (recycled as described in Fig. 20-39) and 3-phosphoglycerate, which can reenter the Calvin cycle.
Phosphoglycolate Is Salvaged in a Costly Set of Reactions in Plants
The glycolate pathway converts two molecules of 2-phosphoglycolate to a molecule of serine (three carbons) and a molecule of (Fig. 20-39). In the chloroplast, a phosphatase converts 2-phosphoglycolate to glycolate, which is exported to the peroxisome. There, glycolate is oxidized by molecular oxygen, and the resulting aldehyde (glyoxylate) undergoes transamination to glycine. The hydrogen peroxide formed as a side product of glycolate oxidation is rendered harmless by peroxidases in the peroxisome. Glycine passes from the peroxisome to the mitochondrial matrix, where it undergoes oxidative decarboxylation by the glycine decarboxylase complex, an enzyme similar in structure and mechanism to two mitochondrial complexes we have already encountered: the pyruvate dehydrogenase complex and the α-ketoglutarate dehydrogenase complex (Chapter 16). The glycine decarboxylase complex oxidizes glycine to and , with the concomitant reduction of to NADH and transfer of the remaining carbon from glycine to the cofactor tetrahydrofolate. The one-carbon unit carried on tetrahydrofolate is then transferred to a second glycine by serine hydroxymethyltransferase, producing serine. The net reaction catalyzed by the glycine decarboxylase complex and serine hydroxymethyltransferase is
The serine is converted to hydroxypyruvate, then to glycerate, and finally to 3-phosphoglycerate, which is used to regenerate ribulose 1,5-bisphosphate, completing the long, expensive cycle (Fig. 20-39).
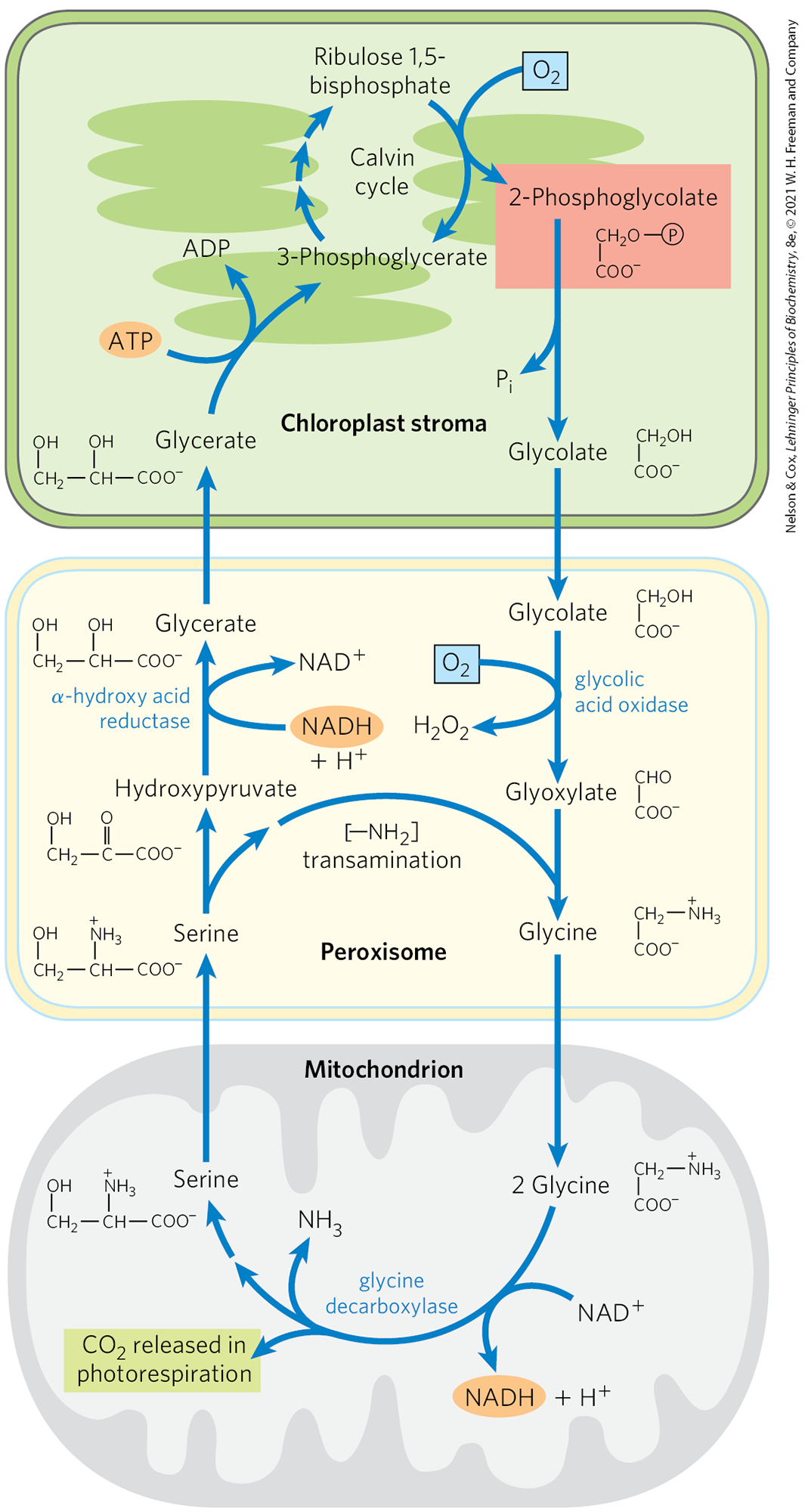
FIGURE 20-39 Glycolate pathway. This pathway, which salvages 2-phosphoglycolate (shaded light red) by converting it to serine and, eventually, to 3-phosphoglycerate, involves three cellular compartments. Glycolate formed by dephosphorylation of 2-phosphoglycolate in chloroplasts is oxidized to glyoxylate and transaminated to glycine in peroxisomes. In mitochondria, two glycine molecules condense to form serine and , released in photorespiration. This reaction is catalyzed by glycine decarboxylase, an enzyme present at very high levels in the mitochondria of plants. The serine is converted to hydroxypyruvate and then to glycerate in peroxisomes; glycerate reenters the chloroplasts to be phosphorylated, rejoining the Calvin cycle. Oxygen is consumed at two steps during photorespiration.
In bright sunlight, the carbon flux through the glycolate salvage pathway can be very high, producing about five times more than is typically produced by all the oxidations of the citric acid cycle. To generate this large flux, mitochondria contain prodigious amounts of the glycine decarboxylase complex: the four proteins of the complex make up half of all the protein in the mitochondrial matrix in the leaves of pea and spinach plants. In nonphotosynthetic parts of a plant, such as potato tubers, mitochondria have very low concentrations of the glycine decarboxylase complex.
The practical effects of this inefficiency are large and costly. The average yield of soybeans and wheat in the United States is reduced by an estimated 36% and 20%, respectively, by the necessity of recycling glycolate from photorespiration.
The combined activity of the rubisco oxygenase and the glycolate salvage pathway consumes and produces — hence the name photorespiration. Unlike mitochondrial respiration, photorespiration does not conserve energy and actually inhibits net biomass formation. This inefficiency has led to evolutionary adaptations in the -assimilation processes, particularly in plants that have evolved in warm climates. The apparent inefficiency of rubisco, and its effect in limiting biomass production, has inspired efforts to genetically engineer a “better” rubisco, but this goal is not, as yet, within reach (Box 20-1).
In Plants, Fixation and Rubisco Activity Are Spatially Separated
In many plants that grow in the tropics (and in temperate-zone crop plants native to the tropics, such as maize, sugarcane, and sorghum) a mechanism has evolved to circumvent the problem of wasteful photorespiration. The step in which is fixed into a three-carbon product, 3-phosphoglycerate, is preceded by several steps, one of which is temporary fixation of into oxaloacetate, a four-carbon compound. Plants that use this process are referred to as plants, and the assimilation process is known as the pathway, by comparison to the pathway in which is first fixed in the three-carbon compound 3-phosphoglycerate.
The plants, which typically grow at high light intensity and high temperatures, have several important characteristics: high photosynthetic rates, high growth rates, low photorespiration rates, low rates of water loss, and a specialized leaf structure. Photosynthesis in the leaves of plants involves two cell types: mesophyll and bundle-sheath cells (Fig. 20-40a).
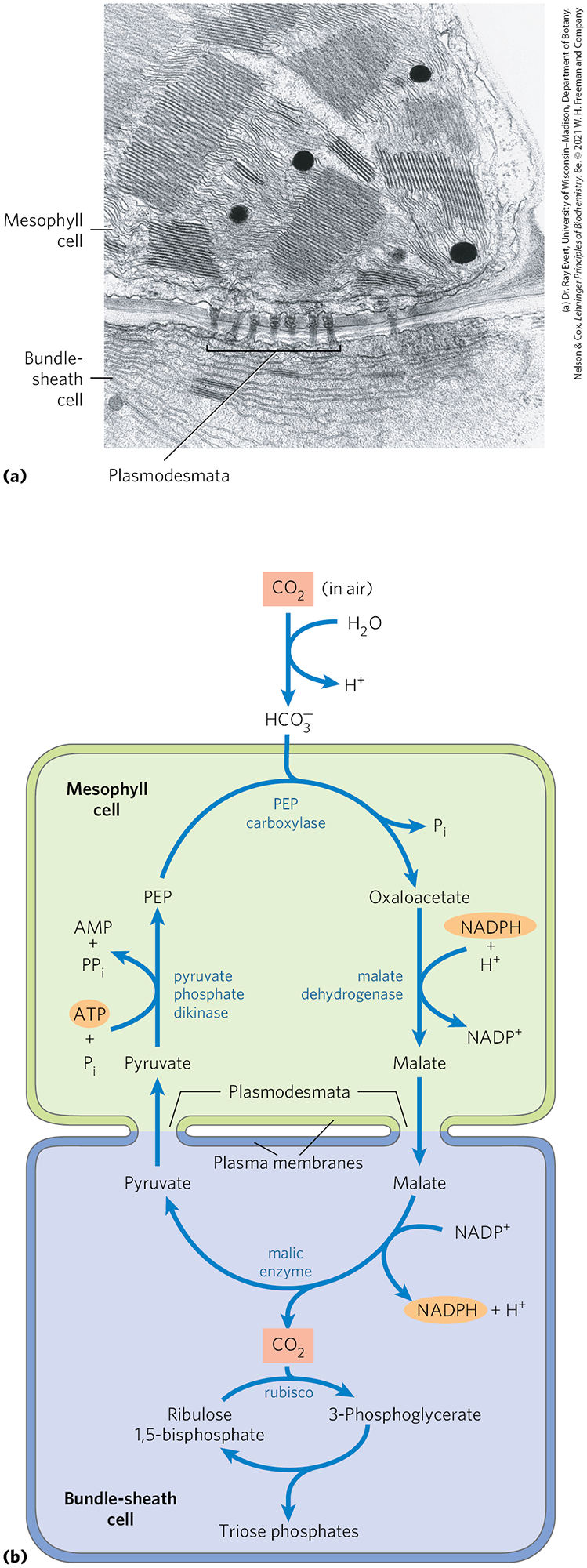
FIGURE 20-40 assimilation in plants. The pathway, involving mesophyll cells and bundle-sheath cells, predominates in plants of tropical origin. (a) Electron micrograph showing chloroplasts of adjacent mesophyll and bundle-sheath cells. The bundle-sheath cell contains starch granules. Plasmodesmata connecting the two cells are visible. (b) The pathway of assimilation, which occurs through a four-carbon intermediate.
Part a is a micrograph that shows a mesophyll on top that has several square to rectangular stacks of horizontally aligned membranes with many membranes in between and several large black circles. There is a pale space between the mesophyll cell and a bundle sheath cell below. A series of seven vertical dark bands connecting the two cells are labeled plasmodesmata. The bundle sheath cell has layers of membranes that are more spread out and that do not form stacks. Part b shows a light red box labeled C O 2 (in air) with an arrow pointing down to H C O 3 minus accompanied by a curved arrow showing the addition of H 2 O and loss of H plus. An arrow points down from H C O 3 minus to join a cyclic series of reactions that begins in the mesophyll cell. This arrow joins at the twelve o’clock position. The arrow from H C O 3 minus meets an arrow labeled P E P carboxylase that runs from P E P at the ten o’clock position to oxaloacetate at the two o’clock position with an arrow branching away at the one o’clock position to show the loss of P subscript i end subscript. An arrow labeled malate dehydrogenase points down from oxaloacetate to malate and is accompanied by a curved arrow showing the addition of an orange oval labeled N A D P H plus H plus and loss of N A D P plus. An arrow points down from malate through a connection between the mesophyll cell to the bundle sheath cell below to another molecule of malate. The membranes between the plasmodesmata are labeled as plasma membranes. A curved arrow labeled malic enzyme points from malate to pyruvate at the eight o’clock position, just beneath the left-hand opening to the mesophyll cell above. This arrow is accompanied by a curved arrow showing the addition of N A D P plus and loss of an orange oval labeled N A D P plus H plus. A the bottom of the curved arrow, an arrow branches to show the loss of a light red box labeled C O 2 that joins with a curved arrow below labeled rubisco that curves from ribulose 1,5-bisphosphate on the left to 3-phosphoglycerate on the right. Another arrow curves from 3-phosphoglycerate back to ribulose 1,5-bisphosphate with an arrow branching off at the six o’clock position to show the loss of triose phosphates. Pyruvate at the upper left of the bundle sheath cell moves through the channel above into the bundle sheath cell, from which an arrow labeled pyruvate phosphate dikinase points up to P E P and is accompanied by an arrow showing the addition of an orange oval labeled A T P plus P lowercase i and loss of A M P plus P P subscript i end subscript. All data are approximate.
The fixation of into the four-carbon oxaloacetate occurs in the cytosol of leaf mesophyll cells. The reaction is catalyzed by phosphoenolpyruvate (PEP) carboxylase, for which the substrate is , not . The oxaloacetate thus formed is either reduced to malate at the expense of NADPH (as shown in Fig. 20-40b) or converted to aspartate by transamination:
The malate or aspartate formed in the mesophyll cells then passes into neighboring bundle-sheath cells through plasmodesmata, protein-lined channels that connect two plant cells and provide a path for movement of metabolites and even small proteins between cells. In the bundle-sheath cells, malate is oxidized and decarboxylated to yield pyruvate and by the action of malic enzyme, reducing . In plants that use aspartate as the carrier, aspartate arriving in bundle-sheath cells is transaminated to form oxaloacetate and reduced to malate, then the is released by malic enzyme or PEP carboxykinase. Labeling experiments show that the free released in the bundle-sheath cells is the same molecule originally fixed into oxaloacetate in the mesophyll cells. This is now fixed again, this time by rubisco, in exactly the same reaction that occurs in plants: incorporation of into C-1 of 3-phosphoglycerate.
The pyruvate formed by decarboxylation of malate in bundle-sheath cells is transferred back to the mesophyll cells, where it is converted to PEP by an unusual enzymatic reaction catalyzed by pyruvate phosphate dikinase (Fig. 20-40b). This enzyme is called a dikinase because two different molecules are simultaneously phosphorylated by one molecule of ATP: pyruvate to PEP, and phosphate to pyrophosphate. The pyrophosphate is subsequently hydrolyzed to phosphate, so two high-energy phosphate groups of ATP are used in regenerating PEP. The PEP is now ready to receive another molecule of in the mesophyll cell.
The PEP carboxylase of mesophyll cells has a high affinity for (which is favored relative to in aqueous solution) and can fix more efficiently than can rubisco. Unlike rubisco, it does not use as an alternative substrate, so there is no competition between and . The PEP carboxylase reaction, then, serves to fix and concentrate in the form of malate. Release of from malate in the bundle-sheath cells yields a sufficiently high local concentration of for rubisco to function near its maximal rate, and for suppression of the enzyme’s oxygenase activity.
Once is fixed into 3-phosphoglycerate in the bundle-sheath cells, the other reactions of the Calvin cycle take place exactly as described earlier. Thus in plants, mesophyll cells carry out assimilation by the pathway and bundle-sheath cells synthesize starch and sucrose by the pathway.
Three enzymes of the pathway are regulated by light, becoming more active in daylight. Malate dehydrogenase is activated by the thioredoxin-dependent reduction mechanism shown in Figure 20-37; PEP carboxylase is activated by phosphorylation of a Ser residue; and pyruvate phosphate dikinase is activated by dephosphorylation.
The pathway of assimilation has a greater energy cost in plants than in plants. For each molecule of assimilated in the pathway, a molecule of PEP must be regenerated at the expense of two phosphoanhydride bonds in ATP. Thus plants need five ATP molecules to assimilate one molecule of , whereas plants need only three (nine per triose phosphate). As the temperature increases (and the affinity of rubisco for decreases, as noted above), a point is reached, at about 28 to 30 °C, at which the gain in efficiency from the elimination of photorespiration more than compensates for this energetic cost. plants (crabgrass, for example) outgrow most plants during the summer, as any experienced gardener can attest.
In CAM Plants, Capture and Rubisco Action Are Temporally Separated
Succulent plants such as cactus and pineapple, which are native to very hot, very dry environments, have another variation on photosynthetic fixation, which reduces loss of water vapor through the pores (stomata) by which and must enter leaf tissue. Instead of separating the initial trapping of and its fixation by rubisco across space (as do the plants), they separate these two events over time. At night, when the air is cooler and moister, the stomata open to allow entry of , which is then fixed into oxaloacetate by PEP carboxylase. The oxaloacetate is reduced to malate and stored in the vacuoles, to protect cytosolic and plastid enzymes from the low pH produced by malic acid dissociation. During the day the stomata close, preventing the water loss that would result from high daytime temperatures, and the trapped overnight in malate is released as by the NADP-linked malic enzyme. This is now assimilated by the action of rubisco and the Calvin cycle enzymes. Because this method of fixation was first discovered in stonecrops, perennial flowering plants of the family Crassulaceae, it is called crassulacean acid metabolism, and the plants are called CAM plants. Table 20-1 compares characteristics of , , and CAM plants.
Plants |
Plants |
CAM Plants |
|
|---|---|---|---|
Examples |
Spinach, pea, rice, wheat, beans, most trees |
Maize (corn), sugarcane, crabgrass |
Cactus, prickly pear, orchid, pineapple |
Most efficient environment |
15 to 25 °C |
Hot and dry; 30 to 47 °C |
Extremely dry; 35 °C |
Path of fixation |
photosynthesis only |
Sequential and cycles spatially separated: in mesophyll cells followed by in bundle-sheath cells |
and cycles, separated spatially and temporally |
Cell type involved |
Mesophyll cells |
in mesophyll cells, in bundle-sheath cells |
and in the same mesophyll cells |
Light conditions |
Light |
Light |
in light; in dark |
Initial acceptor |
Ribulose 1,5-bisphosphate |
Phosphoenolpyruvate |
Ribulose 1,5-bisphosphate in light; phosphoenolpyruvate in dark |
-fixing enzyme |
Rubisco |
PEP carboxylase, then rubisco |
Rubisco in light; PEP carboxylase at night |
First stable product of fixation |
3-Phosphoglycerate |
Oxaloacetate in cycle |
3-Phosphoglycerate in light; oxaloacetate in dark |
Energy needed for complete reduction of one molecule of |
3 ATP, 2 NADPH |
5 ATP, 2 NADPH |
6.5 ATP, 2 NADPH |
Photorespiration |
Present |
Absent or suppressed |
Absent or suppressed |
SUMMARY 20.5 Photorespiration and the and CAM Pathways
- Rubisco is not completely specific for as its substrate; it can also use , producing 2-phosphoglycolate, which must be disposed of in an oxygen-dependent pathway. The result is increased consumption of — photorespiration.
- The 2-phosphoglycolate is converted to glyoxylate, to glycine, and then to serine in a pathway that involves enzymes in the chloroplast stroma, peroxisomes, and mitochondria.
- In plants, the -assimilation pathway minimizes photorespiration: is first fixed in mesophyll cells into a four-carbon compound, which passes into bundle-sheath cells and releases in high concentrations. The released is fixed by rubisco, and the remaining reactions of the Calvin cycle occur as in plants.
- In CAM plants, is fixed into malate in the dark and stored in vacuoles until daylight, when the stomata are closed (minimizing water loss), and the stored malate serves as a source of for rubisco.
 Rubisco is not absolutely specific for as a substrate. Molecular oxygen competes with at the active site, and about once in every three or four turnovers, rubisco catalyzes the condensation of with ribulose 1,5-bisphosphate to form 3-phosphoglycerate and 2-phosphoglycolate (
Rubisco is not absolutely specific for as a substrate. Molecular oxygen competes with at the active site, and about once in every three or four turnovers, rubisco catalyzes the condensation of with ribulose 1,5-bisphosphate to form 3-phosphoglycerate and 2-phosphoglycolate ( Rubisco is not completely specific for as its substrate; it can also use , producing 2-phosphoglycolate, which must be disposed of in an oxygen-dependent pathway. The result is increased consumption of — photorespiration.
Rubisco is not completely specific for as its substrate; it can also use , producing 2-phosphoglycolate, which must be disposed of in an oxygen-dependent pathway. The result is increased consumption of — photorespiration.