Ion flow is governed by:
Membrane permeability.
Concentration gradients.
Electrical driving forces.
Membrane potential arises from:
Selective ion channels.
Active transport mechanisms.
Equilibrium potential ( Nernst ) defines the voltage at which ion movement is balanced.
Capacitance
A capacitor stores charge by separating it across an insulating layer.
Cell membrane acts as a capacitor with intracellular and extracellular fluid as conductors.
Stored charge :
The capacitive current is:
Faster voltage change
Time constant (
Determines how fast the voltage changes in response to current.
Represents the time to reach 63% of final voltage after a step current
Steps to Calculate Electrical Properties
Measure the time constant (
Measure steady-state voltage response to injected current.
Use
Resistance:
Calculate capacitance using:
Determine cell area:
Find specific membrane resistance:
Cell membrane behaves as an RC circuit, affecting signal transmission speed.
Larger capacitance slows down voltage changes.
Time constant (
Membrane resistance and capacitance influence action potential dynamics.
Voltage-Gated Channel Structure
S4 segment:
Contains positively charged amino acids.
Functions as the voltage sensor.
Ion channels exhibit high selectivity through steric and electrostatic mechanisms.
Ion movement replaces hydration shell interactions with channel protein interactions.
Voltage-gated channels rely on charged amino acids to detect voltage changes.
Inward rectifiers regulate resting membrane potential by blocking outward K⁺ flow at positive voltages.
Electrostatic forces, steric fit, and hydration energy govern ion flow through channels.
Membrane as an Electrical Circuit
Capacitor: lipid bilayer stores charge.
Resistor: ion channels regulate current.
Battery: Nernst potential represents ion concentration gradient.
Equivalent Circuits & Passive Electrical Properties
Membrane as an RC Circuit
Capacitance (
Resistance (
Ohm’s Law:
Input Resistance
Higher
Time Constant
Governs how quickly membrane voltage responds to current steps. Numerically, the time to reach ~63% of the final voltage after a step.
Length Constant
Determines how far a passive potential spreads along a cable-like structure (dendrite/axon) before decaying significantly.
Electrophysiology allows precise measurement of membrane currents and potentials.
Voltage-clamp and patch-clamp techniques revolutionized understanding of ion channels.
Membrane properties can be modeled as electrical circuits.
Time constant (
Single-channel and whole-cell recordings provide insights into ion channel function.
Current-Voltage ( I / V ) Plots
x-intercept = reversal potential for ion
slope = conductance
conductance ( g ) = flow of ions through some separation channel
linear slope portion = can assume Ohm's Law = constant conductance
positive slope portion = leak channels , normal voltage gated K+ and Na+ channels
negative slope portion = inward rectifier currents , inactivation time
Chord Conductance
Evaluate at various voltages to map how channels open with depolarization.
Separating Na/K Currents
Under voltage clamp, set
Examine each current’s activation, inactivation, reversal potential, etc.
Conductance-Voltage ( G / V ) Plots
Derived from I-V data using :
Normalized conductance shows sigmoidal activation curve.
we just need Gmax
then divide in half ( Gmax / 2 )
we then want the corresponding voltage point on the curve , we call this ( V1/2 )
V1/2 is the voltage at which 50% of the channels are open
if V1/2 is more negative , the channel activates at lower voltages ( probably sodium )
if V1/2 is more positive , the channel requires a stronger depolarization to activate ( probably potassium )
Single-Channel Events & Stochastic Gating
Microscopic Currents
Individual channels open in discrete, square-step events.
The macroscopic current is the sum of many channels.
Na
Na
K
Voltage Dependence
Higher depolarization usually increases open probability (especially for K
Open-Time / Closed-Time
By repeated single-channel recordings, you measure average open times, closed times, gating rate constants, etc.
Studying Na⁺ Channel Activation
Prepulses at negative voltages relieve inactivation.
Studying Na⁺ Channel Inactivation
Inactivation protocol:
Prepulse at various voltages to control inactivation.
Test pulse applies the same voltage to assess remaining available channels.
Inactivation range:
Na⁺ channels inactivate between -80 mV and -50 mV.
Observations:
More negative prepulse → More channels available to open.
Less negative prepulse → More channels remain inactivated.
Consequences of Na⁺ Channel Inactivation
Transient Na⁺ currents:
Even with sustained depolarization, Na⁺ channels inactivate.
Refractory period:
Prevents immediate reactivation of Na⁺ channels after an action potential.
Unidirectional action potential propagation:
Ensures nerve impulses travel in one direction.
Prolonged depolarization leads to paralysis:
Na⁺ channel inactivation prevents further excitation.
Voltage-gated Na⁺ channels exhibit rapid activation and inactivation.
I-V and G-V plots help analyze activation kinetics.
Inactivation prevents sustained Na⁺ influx, crucial for action potentials.
Refractory periods and unidirectional propagation depend on Na⁺ inactivation.
Influence of Ion Concentrations
Lower
Higher
Voltage Clamp Protocols (Activation/Inactivation)
(Relevant to Guides #6, #7)
Voltage-Step Protocols
Condition at a certain potential (e.g. -90 mV), then step to a test potential (e.g. -20 mV).
Measure current amplitude to see how many channels activate or remain inactivated.
Midpoint of Activation
The
Midpoint of Inactivation
The conditioning
Found by applying various prepulses, then stepping to a fixed test potential.
Na vs. K
Na
Skeletal Muscle Weakness & Inexcitability
Shifts in Resting
Disease can produce abnormal channel gating, changing
Input Resistance & Time Constant
If
Pathophysiological Relevance
Small disruptions in channel function or ion gradients can cause muscle weakness or periodic paralysis.
Electrical Propagation in Axons
Passive Spread (Cable Properties)
Voltage decays with distance if no regenerative channels are involved.
Larger diameter reduces
Unmyelinated Axons
Conduction velocities can be a few to ~20 m/s (like in squid giant axon).
Speed often scales with axon diameter.
Myelinated Axons
Myelin drastically lowers
Can reach 100+ m/s in large vertebrate axons.
Experimental Observations
Changing diameter,
More “wraps” (myelin) → less internodal capacitance → faster conduction.
Passive Axon Properties
Voltage spread along an axon without ion channels is insufficient for signaling.
Enhancement mechanisms:
Voltage-gated ion channels: amplify and regenerate the signal.
Myelin: insulates the axon and speeds up conduction.
Squid Giant Axon: An Evolutionary Solution
Diameter ~500 μm → increases conduction speed.
Unmyelinated, but large size compensates for slow conduction.
If all human neurons were this large, they would take up too much space.
Comparing Myelinated vs. Unmyelinated Axons
| Property | Unmyelinated Axons | Myelinated Axons |
|---|---|---|
| Conduction type | Continuous | Saltatory |
| Speed | Slow ( ~0.5–2 m/s ) | Fast ( ~50–100 m/s ) |
| Na⁺ channels | Spread along axon | Clustered at nodes |
| Capacitance | High | Low |
| Resistance | Low | High |
Myelin decreases capacitance and increases resistance:
Decreasing capacitance → Less charge needed to depolarize the next node.
Increasing resistance → Reduces current loss between nodes.
Voltage-gated Na⁺ and K⁺ channels enable action potential propagation.
Myelin dramatically increases conduction velocity while saving space.
Saltatory conduction allows for efficient and rapid neural signaling.
The balance between conduction speed, space, and metabolic cost drives axonal adaptations.
Calcium Action Potentials
Ca
Open upon depolarization but often inactivate slower, leading to longer-duration APs (plateau-like phases).
Replacing Na
Produces slower, longer spikes if Ca
Cardiac-Like APs
Characterized by a plateau due to sustained Ca
Increasing
Increasing
Role of K
Eventually repolarize the membrane, but if Ca
Properties of Voltage-Activated Calcium Channels
Subunit Structure :
main
has 4 domains
the main pore-forming subunit that determines ion selectivity, conductance, and gating properties
Four-transmembrane-spanning protein, initially found in skeletal muscle calcium channels but also present in the brain (e.g., stargazin).
Cytoplasmic protein that binds to the α1 subunit’s intracellular I-II linker, helping with trafficking to the plasma membrane and modulation
Derived from a single gene and proteolytically cleaved into an extracellular α2 portion and a membrane-spanning δ portion, which stabilizes the channel at the plasma membrane.
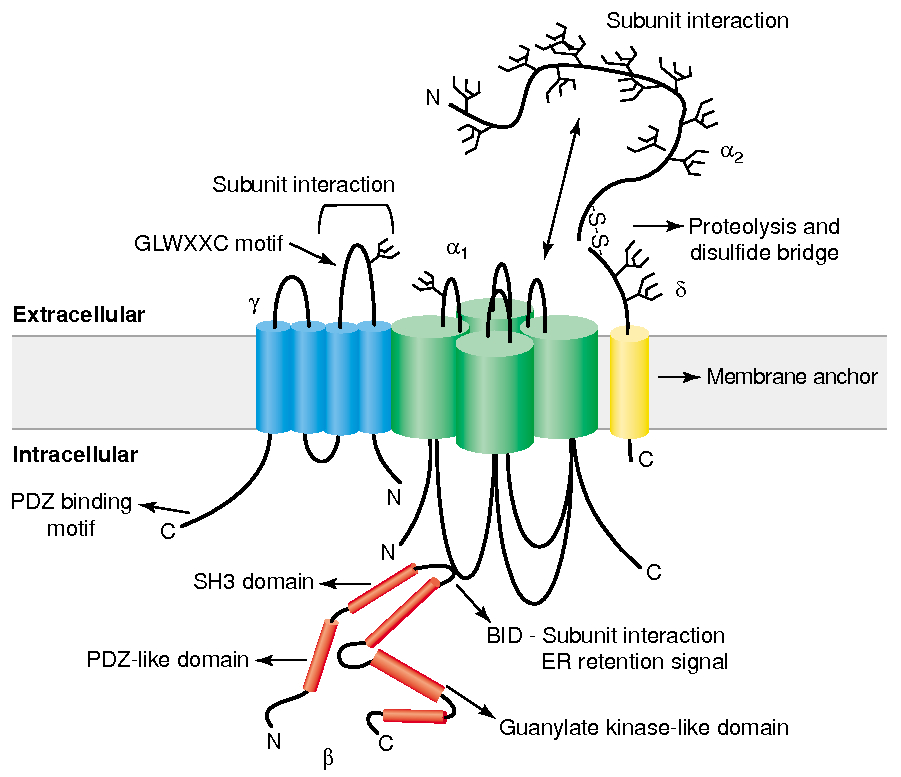
Gate / Voltage Sensor :
S4 segment :
Contains positively charged amino acids , arginine and lysine , every 3rd or 4th residue
unctions as the voltage sensor
Depolarization causes deformation in P-loops , creating a pore
Selectivity Filter for Calcium
Calcium selectivity is determined by the pore loop ( P-loop ) glutamates :
Glutamate residues ( E ) are conserved in each of the four domains ( I-IV ) of the α1 subunit
These residues form a high-affinity binding site for Ca2+ ,
ensuring preferential permeability over other ions
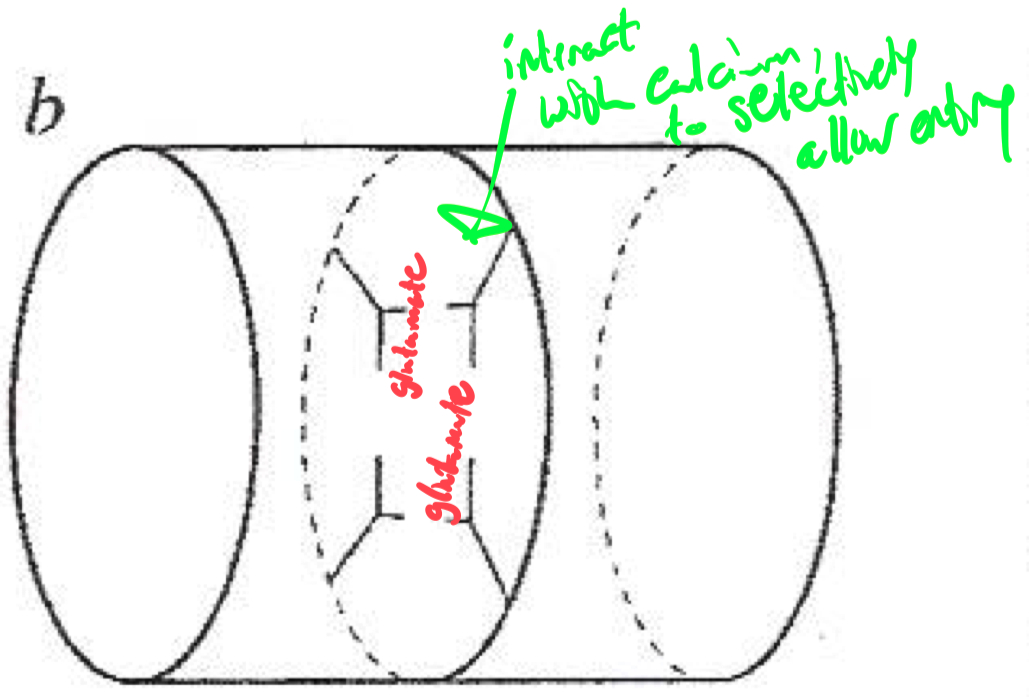
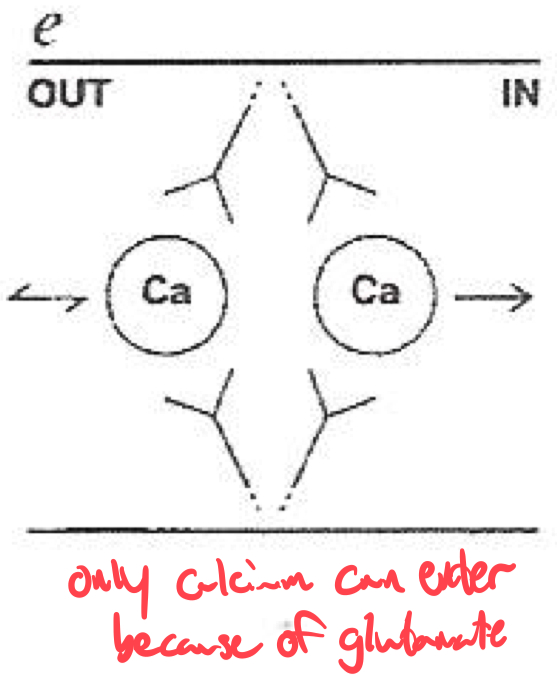
How would you classify or identify different subtypes of calcium channels ?
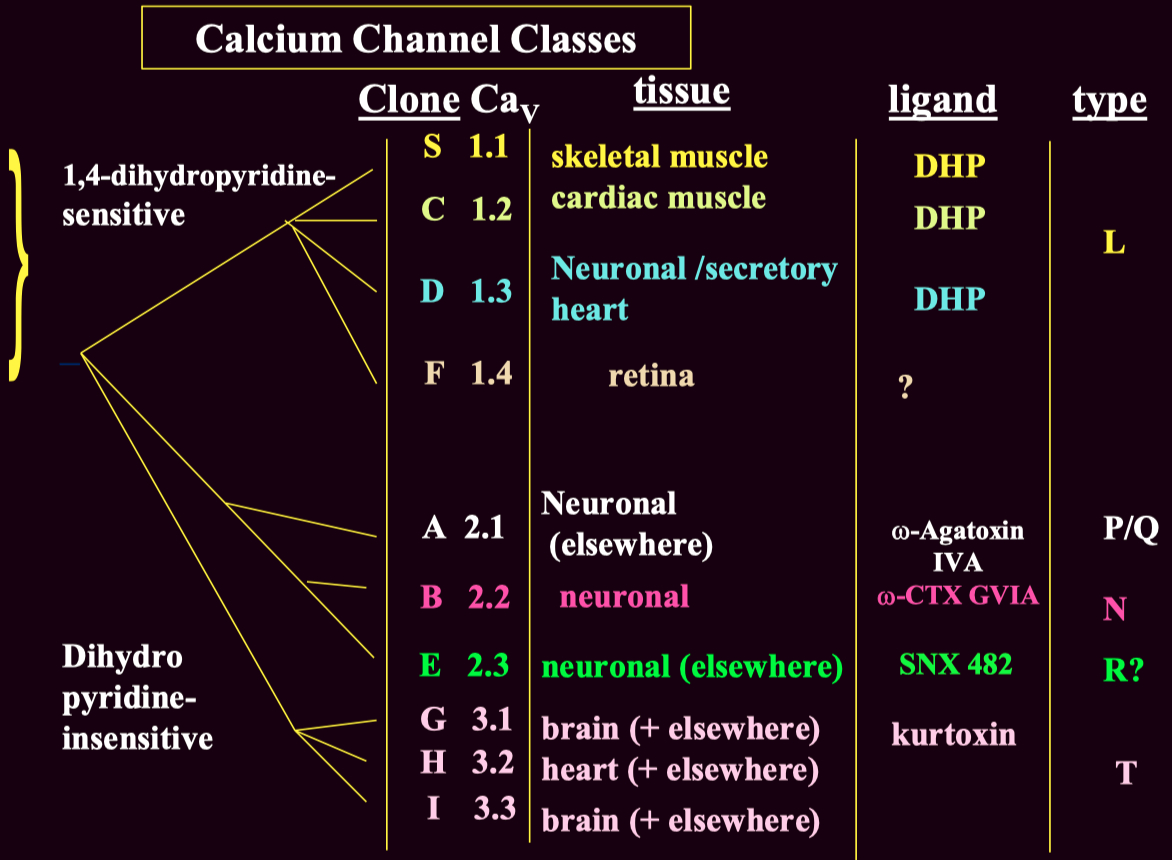
Voltage-gated calcium channels are classified into :
High-Voltage Activated ( HVA )
Low-Voltage Activated ( LVA ) ( T-type ) channels
These subtypes can be distinguished using :
Pharmacological blockers ( e.g., ω-Agatoxin IVA for P/Q-type channels )
Genetic expression ( different subtypes are present in different tissues )
Electrophysiology ( LVA T-type channels activate at lower voltages than HVA channels )
Channel Type Clone (CaV) Function Blockers L-type CaV1.1 - CaV1.4 Skeletal, cardiac muscle contraction, secretion DHP (nifedipine), verapamil P/Q-type CaV2.1 Neurotransmitter release (central) ω-Agatoxin IVA N-type CaV2.2 Neurotransmitter release (peripheral & central) ω-Conotoxin GVIA R-type CaV2.3 Uncertain SNX 482 T-type CaV3.1 - Cav3.3 Cell excitability Mibefradil, ethosuximide (controversial)
L-type blockers:
Dihydropyridines (DHPs): Nifedipine, Amlodipine.
Non-DHPs: Verapamil (cardiac selective).
P/Q-type blockers:
ω-Agatoxin IVA (funnel web spider toxin).
N-type blockers:
ω-Conotoxin GVIA (cone shell mollusk toxin).
Used for neuropathic pain management.
T-type blockers:
Mibefradil, ethosuximide (antiepileptics).
| Subunit | Type | Function |
|---|---|---|
| P/Q-type | CNS neurotransmitter release | |
| N-type | Neurotransmitter release (peripheral & central) | |
| L-type | Cardiac contraction | |
| L-type | Hormone secretion | |
| L-type | Skeletal muscle contraction | |
| T-type | Pacemaking, excitability |
Q: Explain why the resting
Q: Sketch/describe an RC charging curve and label
Q: On an
Q: How does changing
Q: Absolute vs. relative refractory periods in terms of Na
Q: Why might a muscle fiber at
Q: Raising
Q: A short current pulse doesn’t reach threshold, but a longer one does. Why? A: The membrane (RC circuit) needs enough time to charge up to threshold. A short pulse may end before hitting threshold.
Q: Multiple myelin wraps: effect on conduction velocity?
A: Myelin greatly reduces