High Level Overview
holding cell more negative forces sodium channels to complete cycle faster
more of them moving from inactivated to closed state
Passive ( no voltage gated channels , seen in dendrites ) :
voltage decays with distance if no regenerative channels are involved
Dynamic ( voltage gated channels present , seen in unmyelinated axons ) :
voltage-gated Na⁺ and K⁺ channels enable action potential propagation
Myelin drastically lowers
Reverse Calculate The Intercellular Concentration ( denominator ) from Some Random Voltage
lets say they tell you
what must
Example Cell , Finding Conductance Ratio and gTotal
pick random values for :
Resting Membrane Potential =
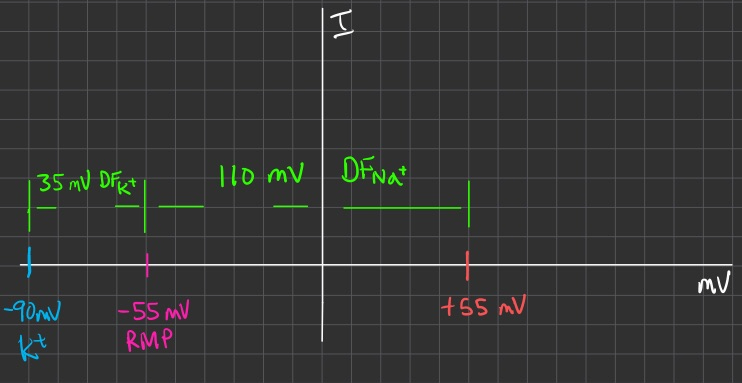
At steady state , you set the sum of all currents equal to zero
we are solving for ratio
and we expect it to be near
because we are at resting membrane potential ( current is zero )
so sodium channels are not very conductive ( small g )
potassium channels are 5 times more conductive than sodium at resting membrane potential
for this example :
we could do the algebra again , but a shortcut is just to remember to flip them from the way you would naturally write it like above :
we can also pick some random current value for
we expect current at RMP to be zero
so
let
let
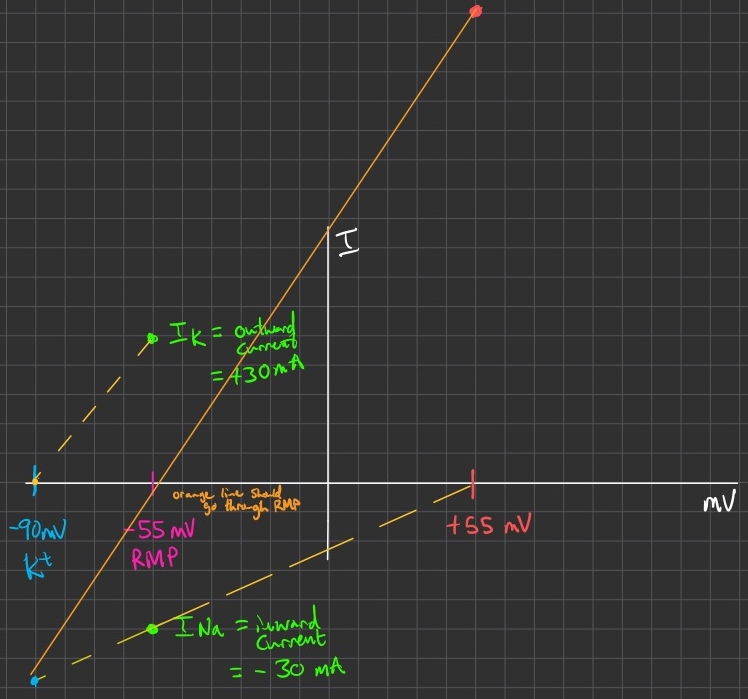
we then project out from the
then we can project outward for potassium , and downward for sodium ,
allowing you to draw the orange line in the middle
the orange line in the middle = total current
the slope of it = total conductance
Capacitance ( C )
adding myelin to an axon increases the diameter
so this decreases the capacitance
decreasing the capacitance , decreases the time constant
decreasing the time constant = charging and discharging times are faster for the cell
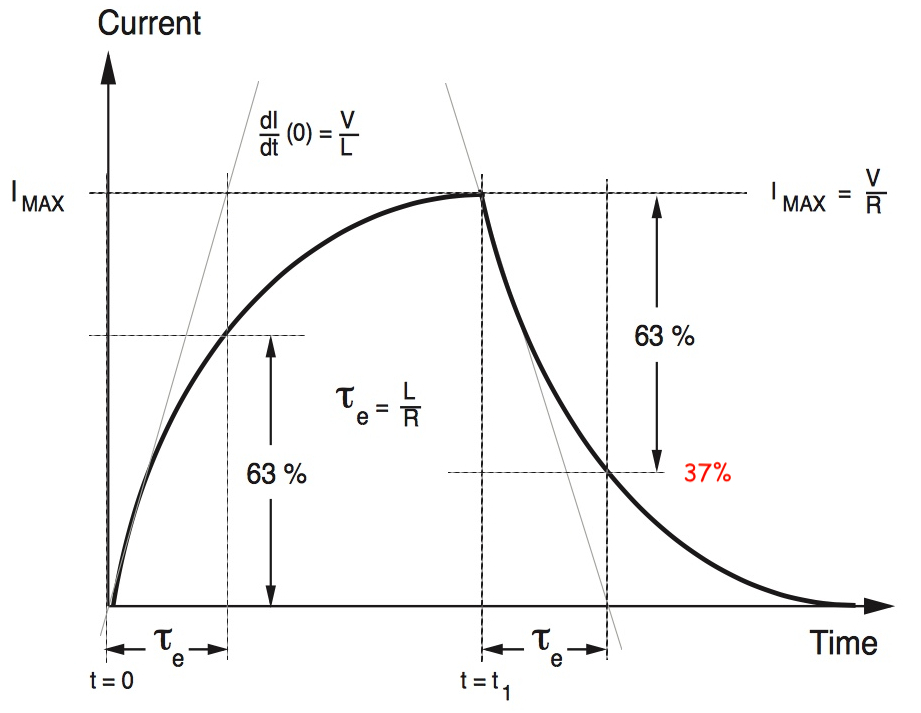
Time Constant ( τ )
Determines how fast the voltage changes in response to current.
Represents the time to reach 63% of the charging voltage after a step current
scaled by
Also represents the time to reach 63% of discharging voltage after a step current
you can also think of this as 37% of the initial value
scaled by
this image is for current
Length Constant ( λ )
tells us how far voltage spreads in an axon cylinder shape
so larger length constance = larger resistance
faster conduction
scale by
Chord Conductance ( g )
it is the chord conductance = the slope of the line connecting
Ion flow is governed by:
Membrane permeability.
Concentration gradients.
Electrical driving forces.
Membrane potential arises from:
Selective ion channels.
Active transport mechanisms.
Equilibrium potential ( Nernst ) defines the voltage at which ion movement is balanced.
Steps to Calculate Electrical Properties
Measure the time constant (
Measure steady-state voltage response to injected current
Use
Resistance is then the inverse
Calculate capacitance using:
Determine cell area:
Find specific membrane resistance (
Cell membrane behaves as an RC circuit , affecting signal transmission speed
Larger capacitance slows down voltage changes
Time constant (
Also note from the
smaller cells have higher resistance ,
what does this mean for charge/discharge times , the
higher resistance is higher
what does this mean for length constant ?
its proportional to
( square root is just a special scaling , doesn't impact proportionality statement )
so
therefore a larger
you could use :
and still
Squid Giant Axon: An Evolutionary Solution
Diameter ~500 μm → increases conduction speed.
Unmyelinated, but large size compensates for slow conduction.
If all human neurons were this large , they would take up too much space.
these larger axons have a very low intracellular resistance (
this increases the length constant by a lot
( decreasing denominator ➡️ increases result )
Voltage-Gated Channel Structure
S4 segment:
Contains positively charged amino acids.
Functions as the voltage sensor.
Membrane as an Electrical Circuit
Capacitor: lipid bilayer stores charge.
Resistor: ion channels regulate current.
Battery: Nernst potential represents ion concentration gradient.
Current-Voltage ( I / V ) Plots
x-intercept = reversal potential for ion
slope = conductance
conductance ( g ) = flow of ions through some separation channel
linear slope portion = can assume Ohm's Law = constant conductance
positive slope portion = leak channels , normal voltage gated K+ and Na+ channels
negative slope portion = inward rectifier currents , inactivation time
Conductance-Voltage ( G / V ) Plots
Derived from I-V data using :
Normalized conductance shows sigmoidal activation curve.
we just need Gmax
then divide in half ( Gmax / 2 )
we then want the corresponding voltage point on the curve , we call this ( V1/2 )
V1/2 is the voltage at which 50% of the channels are open
if V1/2 is more negative , the channel activates at lower voltages ( probably sodium )
if V1/2 is more positive , the channel requires a stronger depolarization to activate ( probably potassium )
Single-Channel Events & Stochastic Gating
Microscopic Currents
Individual channels open in discrete, square-step events.
The macroscopic current is the sum of many channels.
Na
Na
K
Voltage Dependence
Higher depolarization usually increases open probability ( especially for K
Open-Time / Closed-Time 9
By repeated single-channel recordings, you measure average open times, closed times, gating rate constants, etc.
Studying Na⁺ Channel Activation
Prepulses at negative voltages relieve inactivation.
Single-Channel Events & Stochastic Gating
Microscopic Currents
Individual channels open in discrete, square-step events.
The macroscopic current is the sum of many channels.
Na
Na
K
Voltage Dependence
Higher depolarization usually increases open probability (especially for K
Open-Time / Closed-Time
By repeated single-channel recordings, you measure average open times, closed times, gating rate constants, etc.
Studying Na⁺ Channel Activation
Prepulses at negative voltages relieve inactivation
this resets all the sodium channels to closed state , where they are ready to open as soon as possible
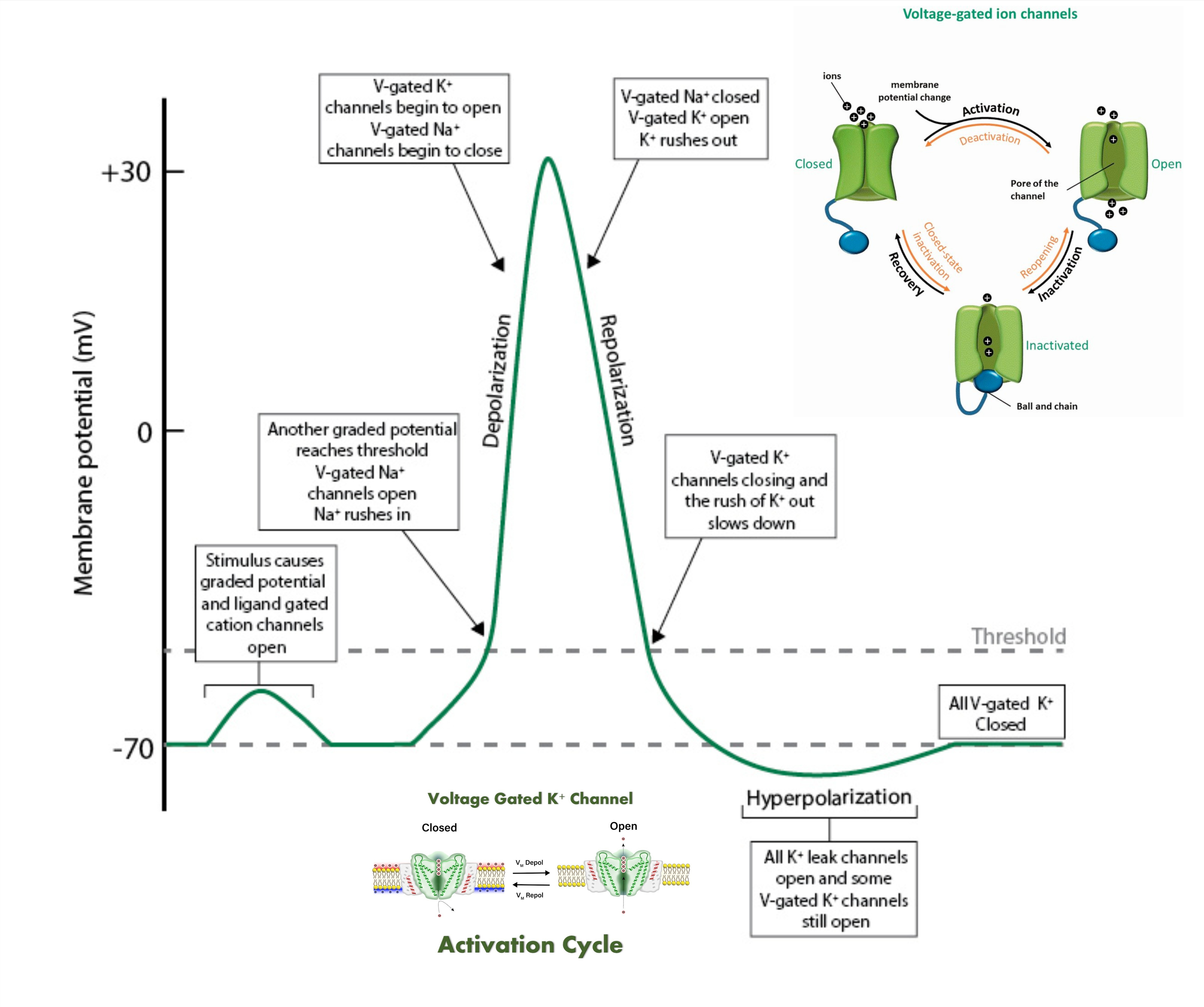
Studying Na⁺ Channel Inactivation
Inactivation protocol:
Prepulse at various voltages to control inactivation.
Test pulse applies the same voltage to assess remaining available channels.
Inactivation range:
Na⁺ channels inactivate between -80 mV and -50 mV.
Observations:
More negative prepulse → More channels available to open.
Less negative prepulse → More channels remain inactivated.
Consequences of Na⁺ Channel Inactivation
Transient Na⁺ currents:
Even with sustained depolarization, Na⁺ channels inactivate.
Refractory period:
Prevents immediate reactivation of Na⁺ channels after an action potential.
Unidirectional action potential propagation:
Ensures nerve impulses travel in one direction.
Prolonged depolarization leads to paralysis:
Na⁺ channel inactivation prevents further excitation.
Properties of Voltage-Activated Calcium Channels
Subunit Structure :
main
has 4 domains
the main pore-forming subunit that determines ion selectivity, conductance, and gating properties
Four-transmembrane-spanning protein, initially found in skeletal muscle calcium channels but also present in the brain (e.g., stargazin).
Cytoplasmic protein that binds to the α1 subunit’s intracellular I-II linker, helping with trafficking to the plasma membrane and modulation
Derived from a single gene and proteolytically cleaved into an extracellular α2 portion and a membrane-spanning δ portion, which stabilizes the channel at the plasma membrane.
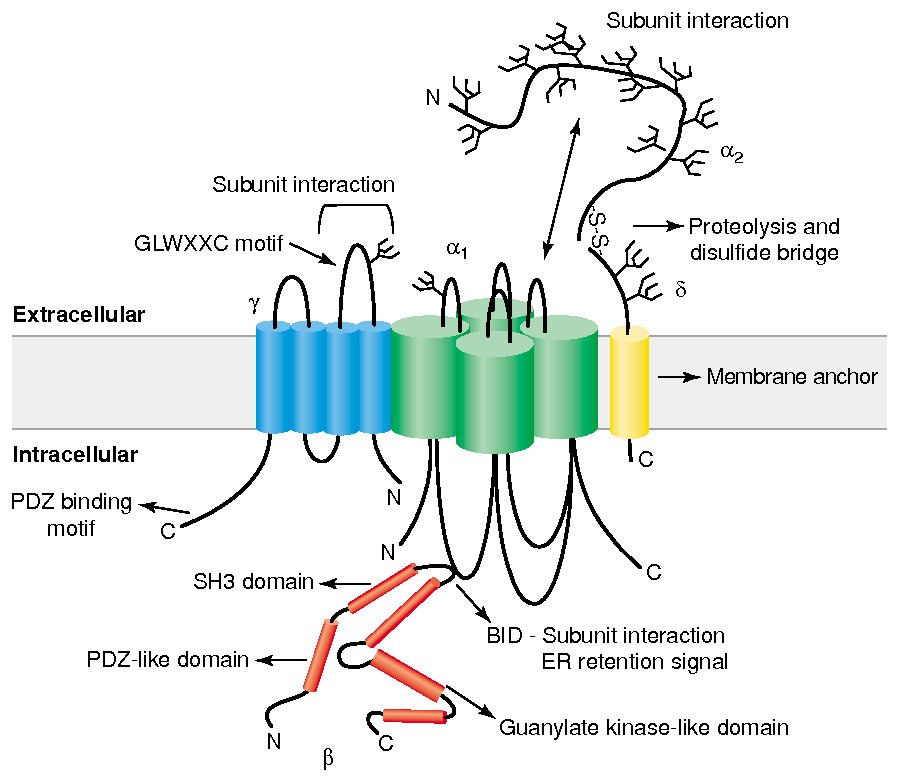
Gate / Voltage Sensor :
S4 segment :
Contains positively charged amino acids , arginine and lysine , every 3rd or 4th residue
unctions as the voltage sensor
Depolarization causes deformation in P-loops , creating a pore
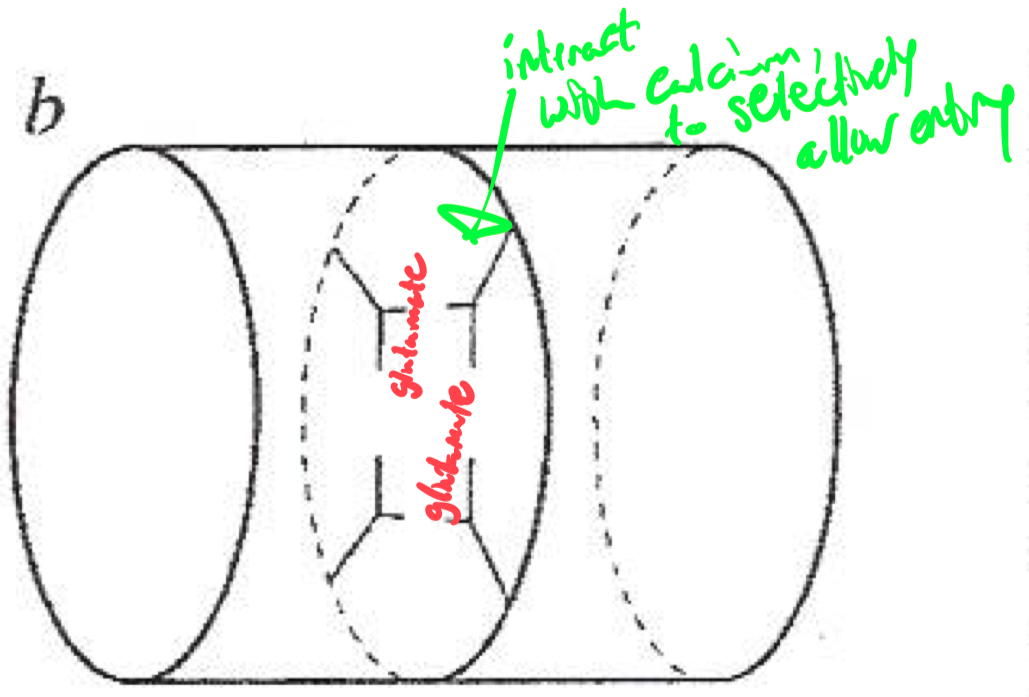
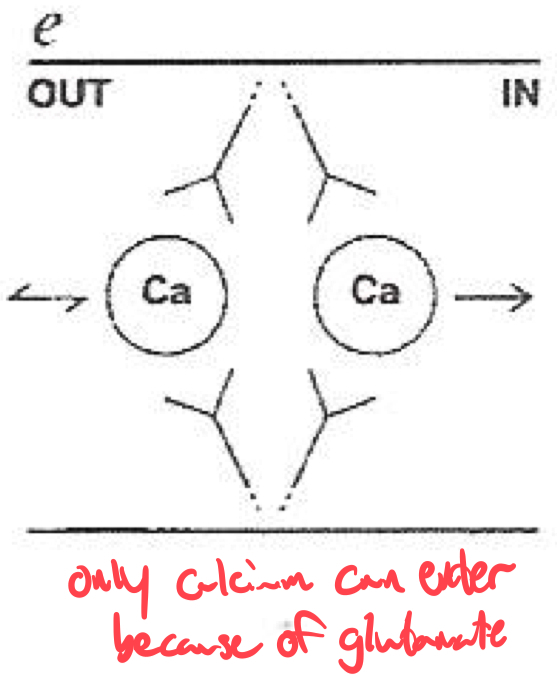
Selectivity Filter for Calcium
Calcium selectivity is determined by the pore loop ( P-loop ) glutamates :
Glutamate residues ( E ) are conserved in each of the four domains ( I-IV ) of the α1 subunit
These residues form a high-affinity binding site for Ca2+ ,
ensuring preferential permeability over other ions
How would you classify or identify different subtypes of calcium channels ?
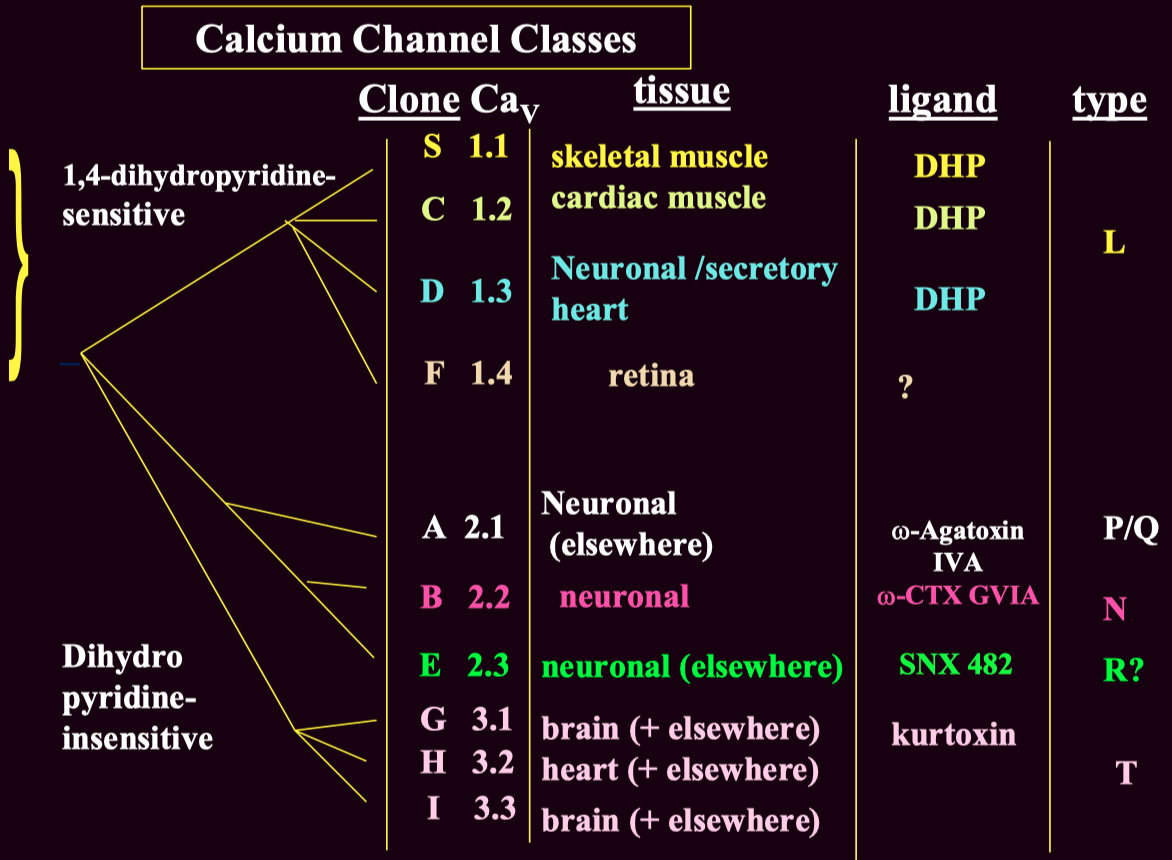
Voltage-gated calcium channels are classified into :
High-Voltage Activated ( HVA )
Low-Voltage Activated ( LVA ) ( T-type ) channels
These subtypes can be distinguished using :
Pharmacological blockers ( e.g., ω-Agatoxin IVA for P/Q-type channels )
Genetic expression ( different subtypes are present in different tissues )
Electrophysiology ( LVA T-type channels activate at lower voltages than HVA channels )
Channel Type Clone (CaV) Function Blockers L-type CaV1.1 - CaV1.4 Skeletal, cardiac muscle contraction, secretion DHP (nifedipine), verapamil P/Q-type CaV2.1 Neurotransmitter release (central) ω-Agatoxin IVA N-type CaV2.2 Neurotransmitter release (peripheral & central) ω-Conotoxin GVIA R-type CaV2.3 Uncertain SNX 482 T-type CaV3.1 - Cav3.3 Cell excitability Mibefradil, ethosuximide (controversial)
L-type blockers:
Dihydropyridines (DHPs): Nifedipine, Amlodipine.
Non-DHPs: Verapamil (cardiac selective).
P/Q-type blockers:
ω-Agatoxin IVA (funnel web spider toxin).
N-type blockers:
ω-Conotoxin GVIA (cone shell mollusk toxin).
Used for neuropathic pain management.
T-type blockers:
Mibefradil, ethosuximide (antiepileptics).
| Subunit | Type | Function |
|---|---|---|
| P/Q-type | CNS neurotransmitter release | |
| N-type | Neurotransmitter release (peripheral & central) | |
| L-type | Cardiac contraction | |
| L-type | Hormone secretion | |
| L-type | Skeletal muscle contraction | |
| T-type | Pacemaking, excitability |
Q: Explain why the resting
Q: Sketch/describe an RC charging curve and label
Q: On an
Q: How does changing
Q: Absolute vs. relative refractory periods in terms of Na
Q: Why might a muscle fiber at
Q: Raising
Q: A short current pulse doesn’t reach threshold, but a longer one does. Why? A: The membrane (RC circuit) needs enough time to charge up to threshold. A short pulse may end before hitting threshold.
Q: Multiple myelin wraps: effect on conduction velocity?
A: Myelin greatly reduces