Misc
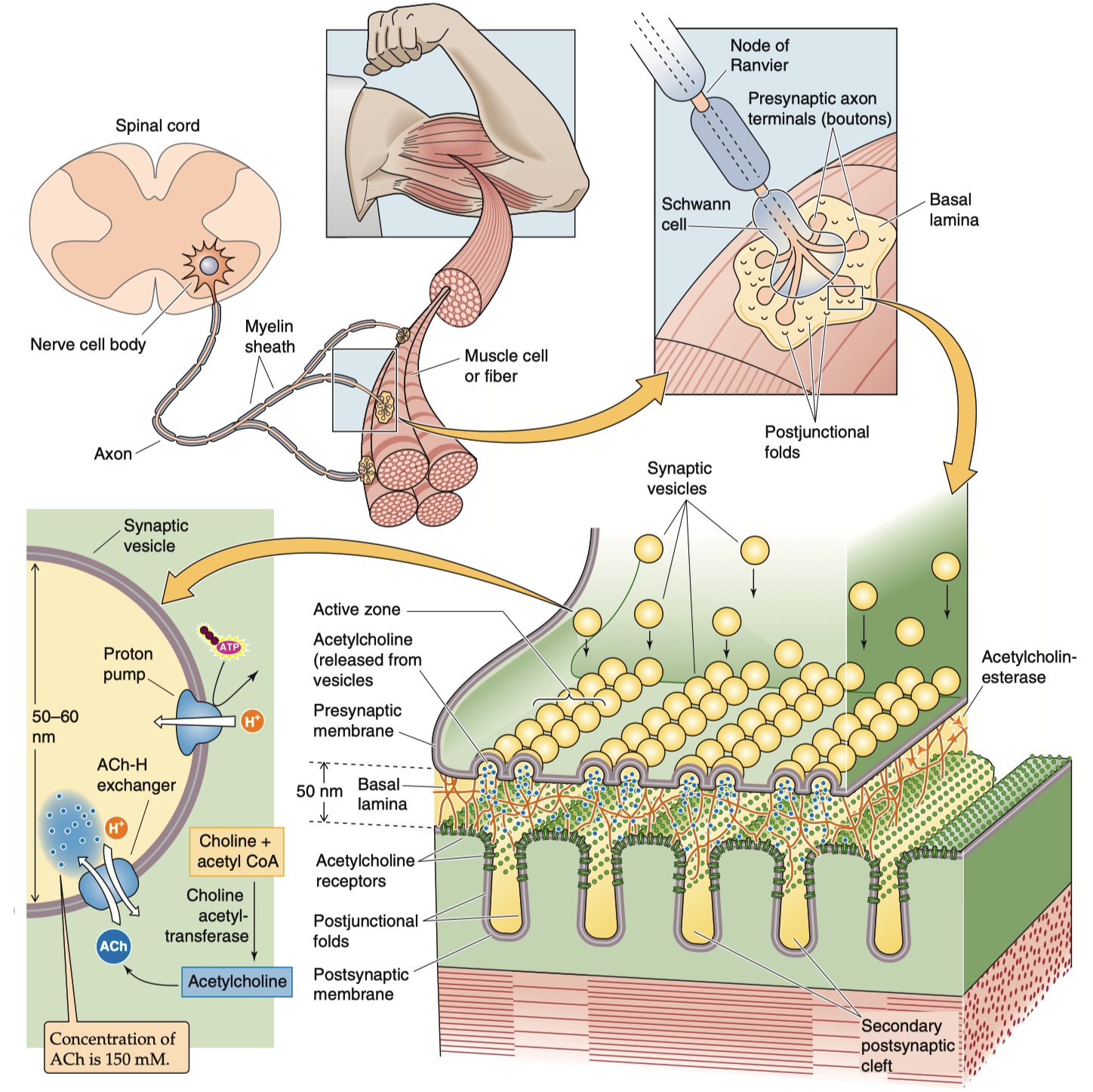
Neuronal Structure ( important for synaptic transmission ) :
Axon
Axon initial segment
Presynaptic terminal/bouton
synaptic vesicles
mitochondria
active zones
Synaptic cleft
Postsynaptic cell ( dendrite )
receptors signaling molecules scaffolding molecules
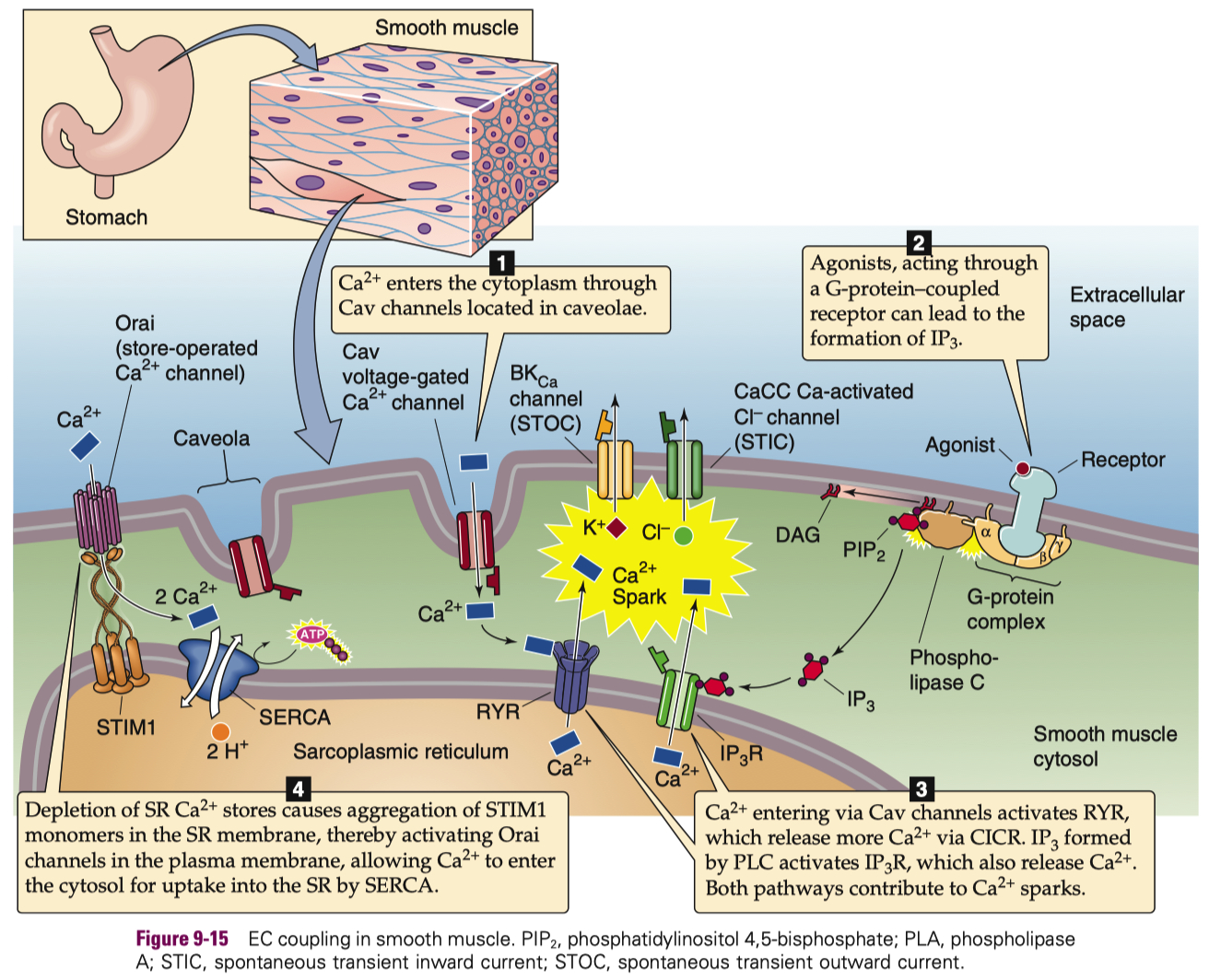
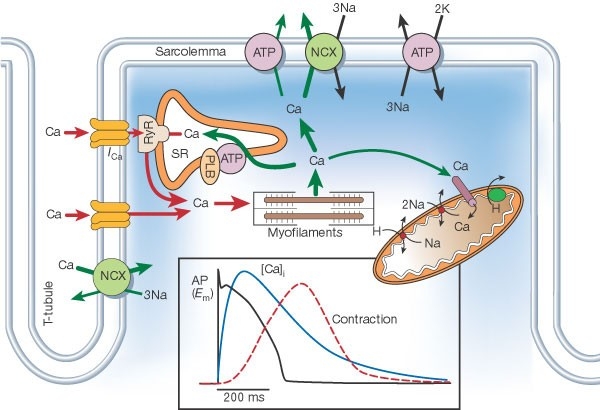
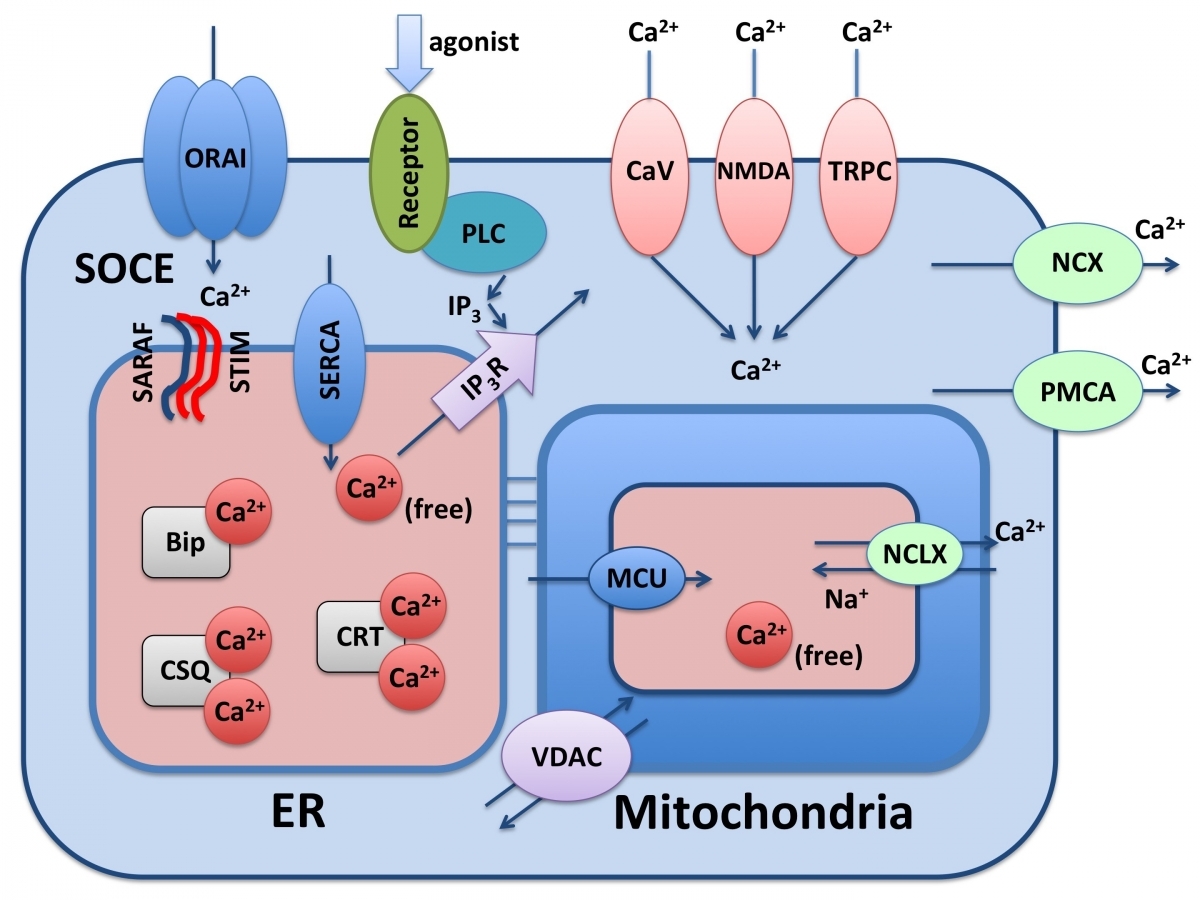
the heart does calcium-induced-calcium-release , skeletal muscle uses direct contact of ryanodine receptors instead
Susuki
Adding myelin increases resistance ( membrane )
increasing resistance increases conduction velocity
Adding myelin decreases capacitance
decreasing capacitance decreases time constant
cell is quicker to charge / discharge
Adding myelin increases Conduction Velocity :
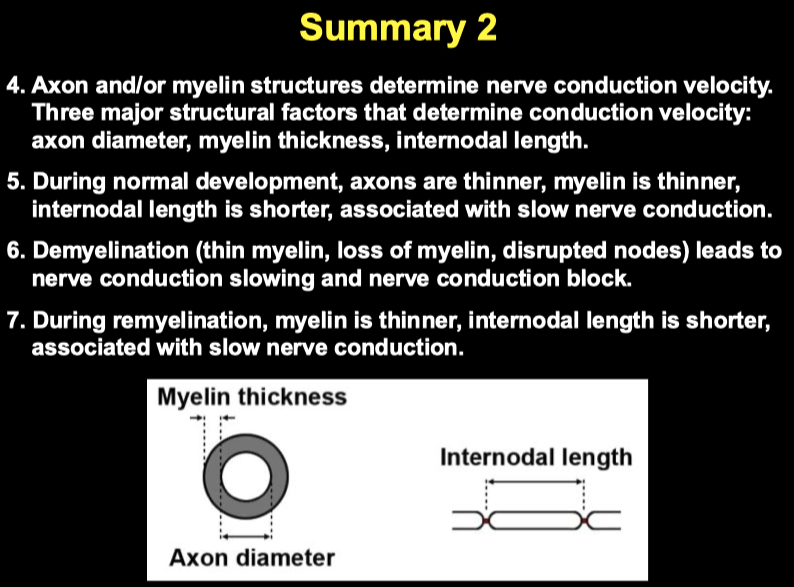
Axon Diameter :
Larger axons have lower axial resistance ( Ri ) , allowing current to spread more easily down the axon
Conduction velocity = √(axon diameter) for unmyelinated axons
and more linearly proportional to changes in diameter for myelinated axons
Myelin Thickness :
Thicker myelin → faster conduction, up to an optimal point.
Myelin increases membrane resistance (Rm) and decreases membrane capacitance (Cm):
High Rm: Prevents leak of ions.
Low Cm: Faster charging of the membrane (shorter time constant τ = Rm × Cm).
Together, this allows action potentials to jump faster (saltatory conduction) between nodes
"g-ratio" matters:
g-ratio = inner axon diameter / outer fiber diameter (including myelin).
Optimal g-ratio ≈ 0.6–0.7.
Too little myelin = poor insulation.
Too much myelin = slows it down by increasing internodal capacitance.
Internodal Length ( Distance between nodes of Ranvier )
Longer internodes → faster conduction ( to a limit )
Fewer nodes = fewer sites where the AP needs to regenerate, saving time.
But if internodes are too long, signal may fail to reach the next node (passive depolarization too small).
Optimal internodal length depends on axon size — typically ~100 times the axon diameter.
Axon diameter and myelin thickness increase during development
Internodal length increases during development
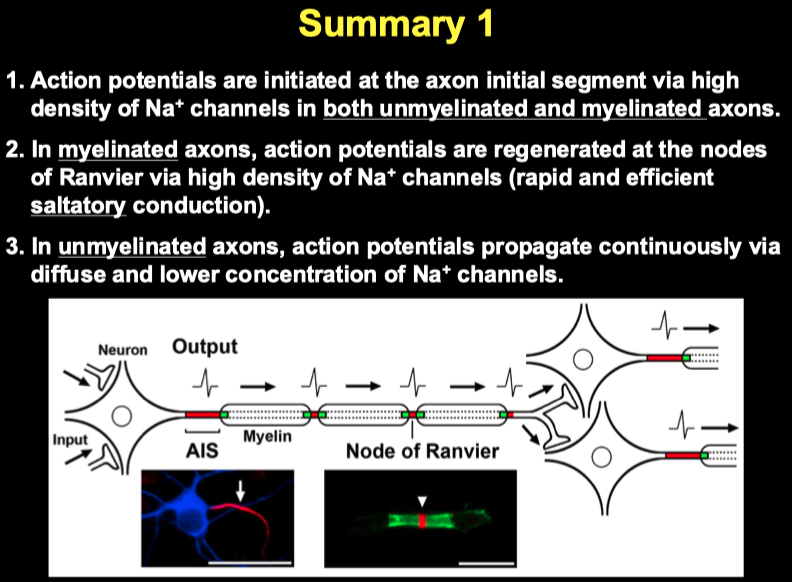
Where is the site of action potential initiation?
axon initial segment
Ion channels are uniformly distributed along the unmyelinated axons
High density of Na + channels at the axon initial segment
Diffuse and lower concentration of Na + channels along the distal unmyelinated axons
Ion channels are highly accumulated at the AIS and nodes along myelinated axons
High density of Na + channels at the axon initial segment and nodes of Ranvier
No or little Na + channels in internodal segments
Propagation :
In unmyelinated axons, action potential propagation is continuous along the length of the axons.
In myelinated axons, action potential propagation is saltatory: the action potential jumps from node to node.
Myelin decreases capacitance and increases resistance, both contributing to saltatory conduction.
Demyelination leads to conduction slowing / block
Decreases membrane resistance (Rm) ➔ more current leaks out of the axon.
Increases membrane capacitance (Cm) ➔ slower membrane charging, so the axon can't depolarize quickly.
Slows conduction velocity dramatically because passive spread of depolarization is weakened.
K + channel blocker restores conduction in experimentally demyelinated nerves
Lysolecithin = artificial demyelination
Acute Inflammatory Demyelinating Polyradiculoneuropathy ( AIDP )
autoimmune attack demyelination on PNS nerves
Guillain-Barre type
Multiple Sclerosis ( MS ) :
autoimmune attack on oligodendrocytes ( CNS )
loss of myelin
disrupted paranodal and nodal organization
treatment = K+ channel blocker
Rich
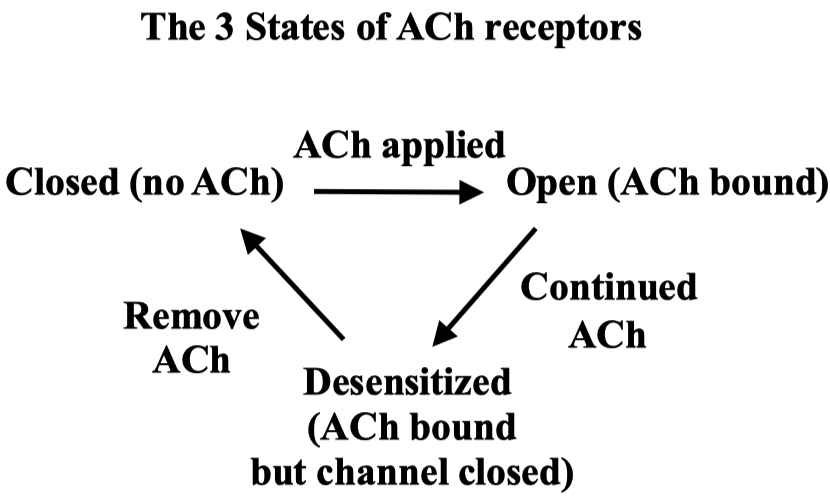
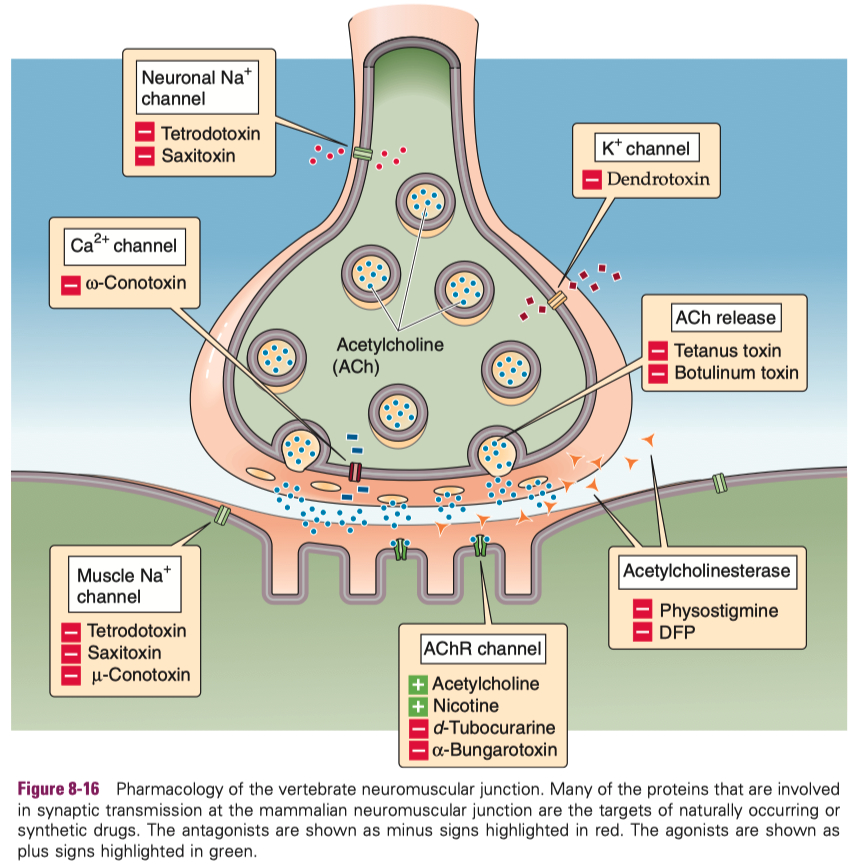
Myasthenia Gravis ( MG ) :
autoimmune attack on skeletal muscle neuromuscular junction ACh receptors
treatment = acytlecholine esterase inhibitors
but the balance is tricky , because too much inhibitor will cause too much available acetylcholine
then the receptors will naturally become desensitized on their own due to excess ACh
If you voltage clamp some muscle fiber at -90 mV vs clamping it at -20 mV ,
the one clamped at -90 will have a much larger current measured
there is a larger driving force for acetylcholine
Bennett
Cardiac Action Potential
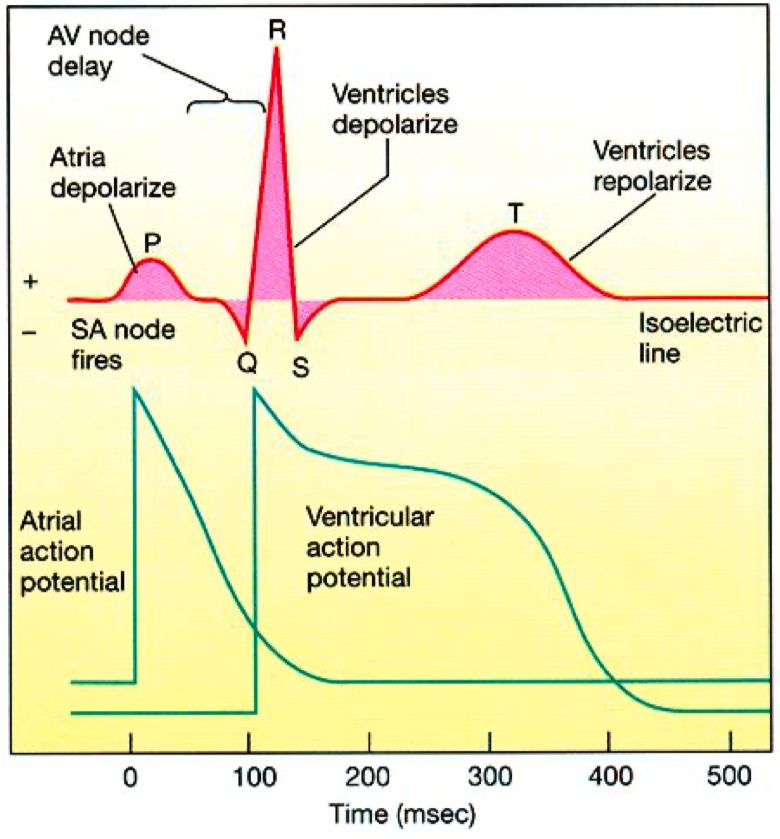
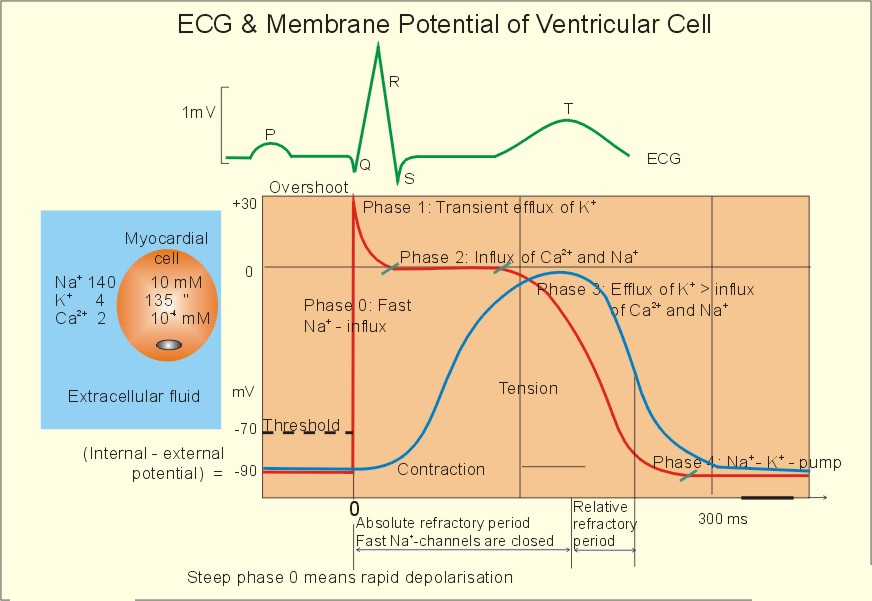
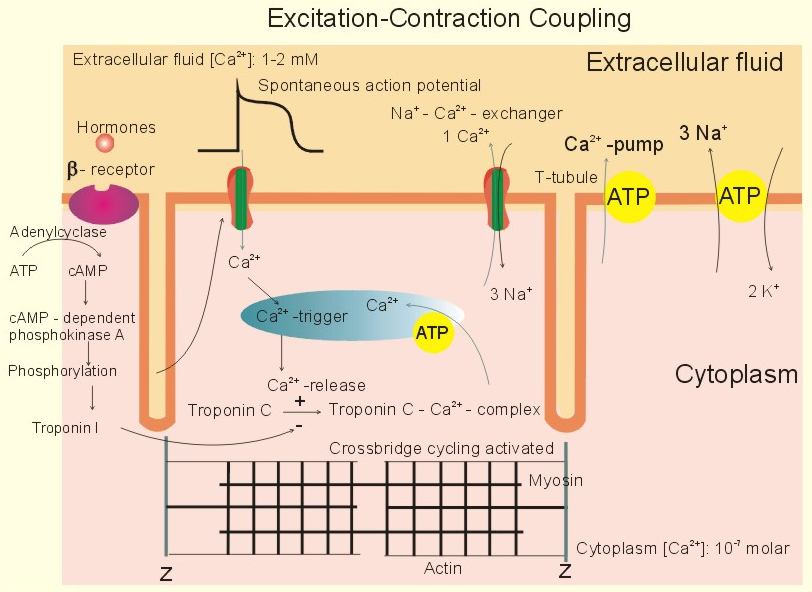
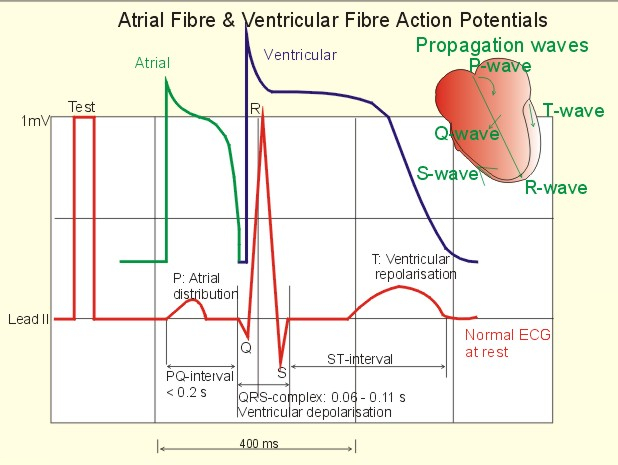
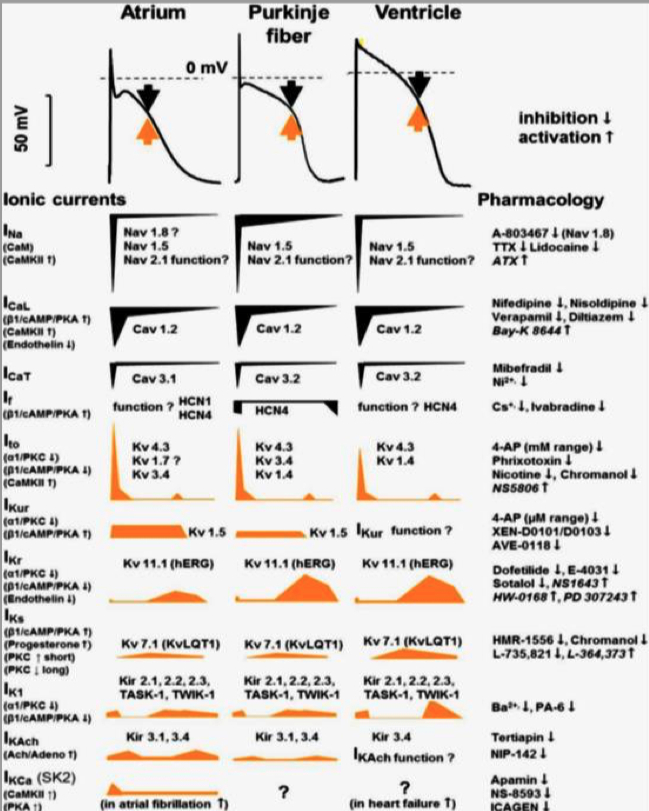
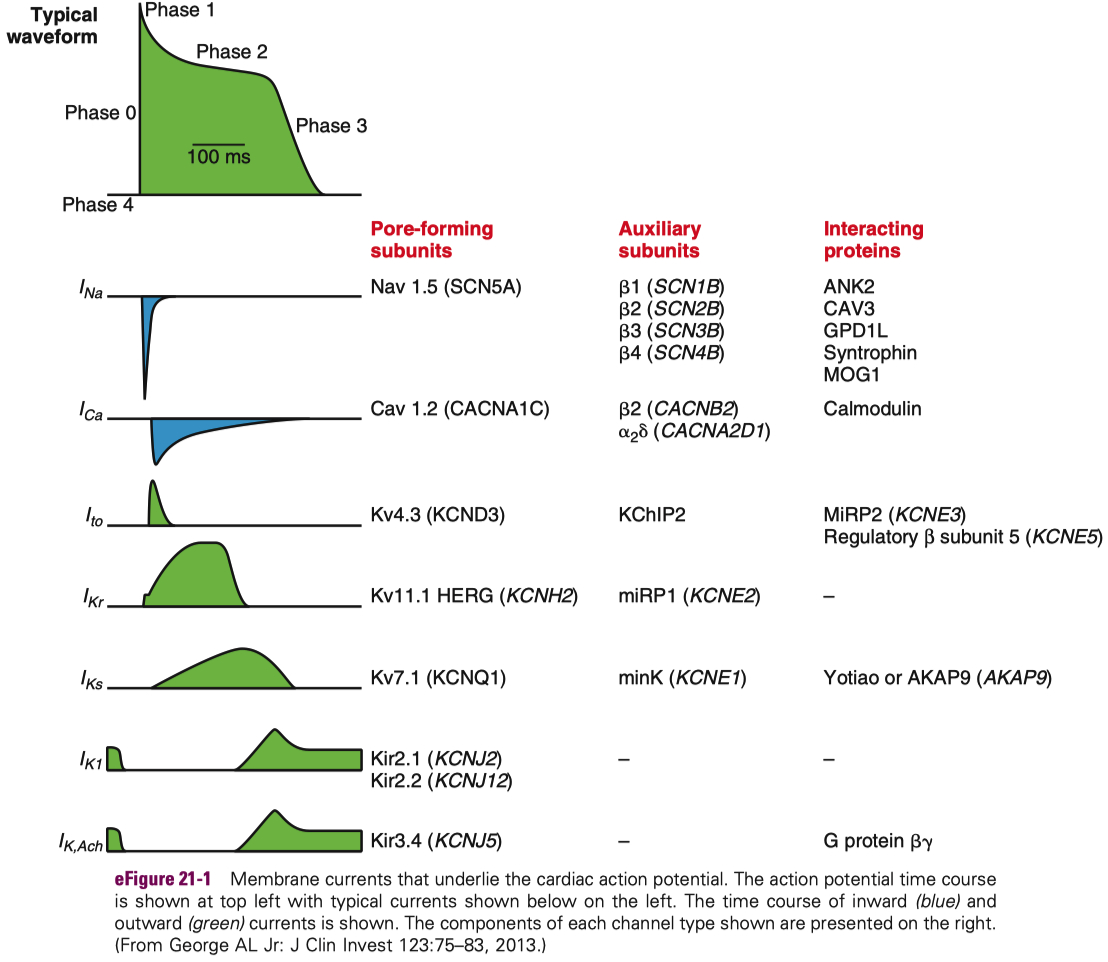
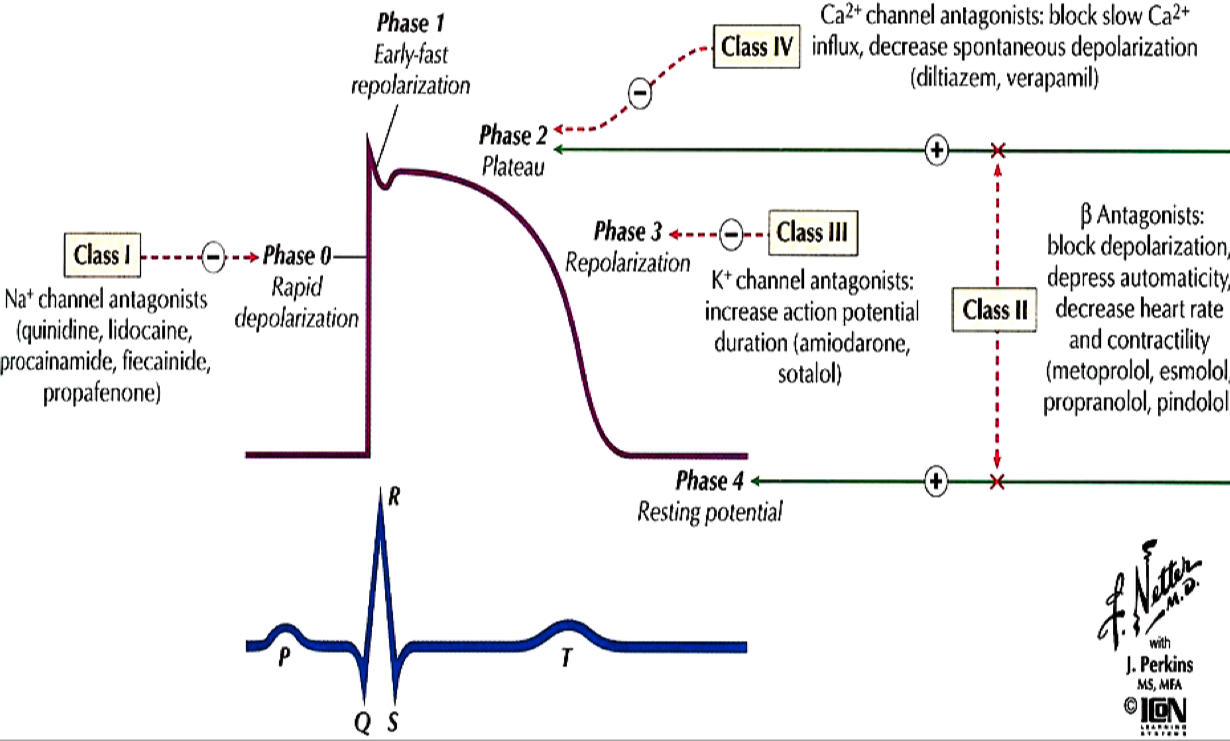
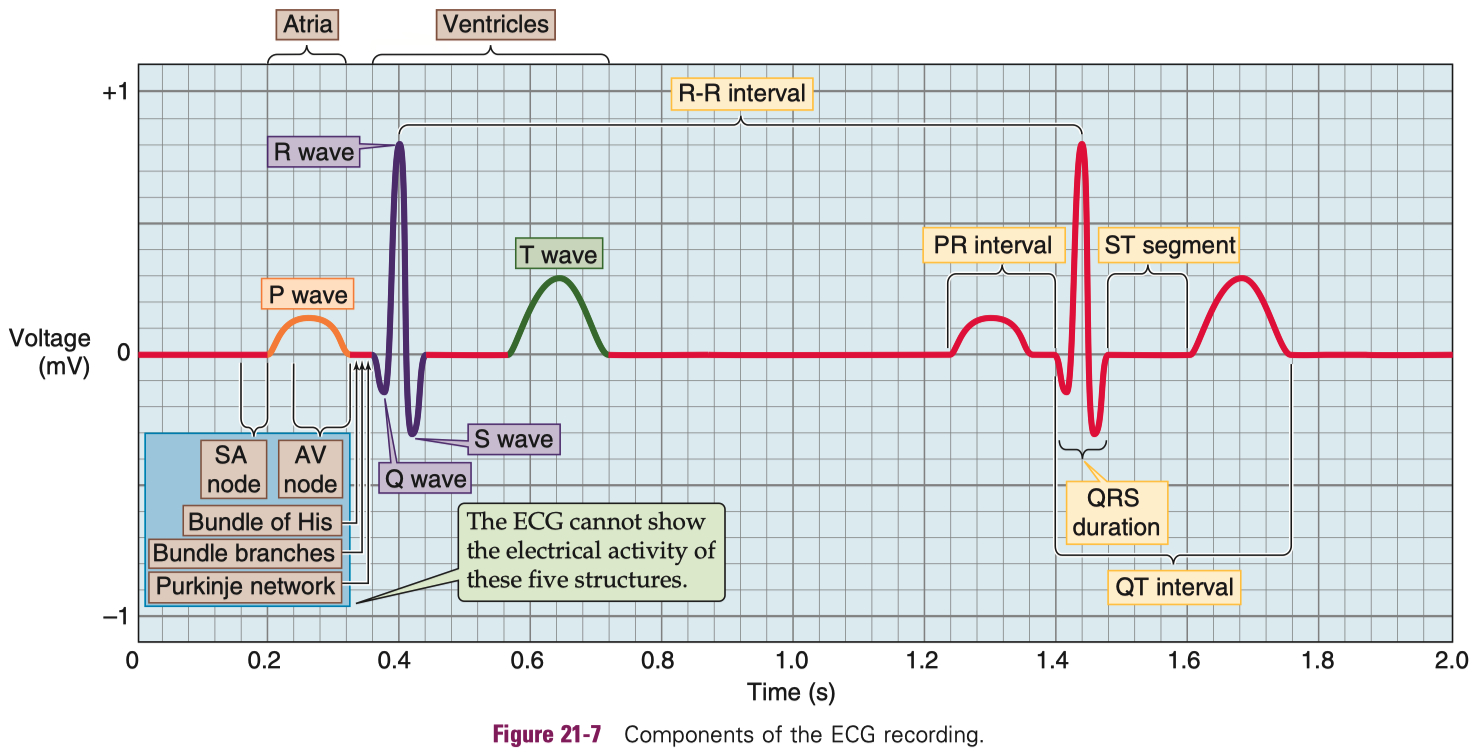
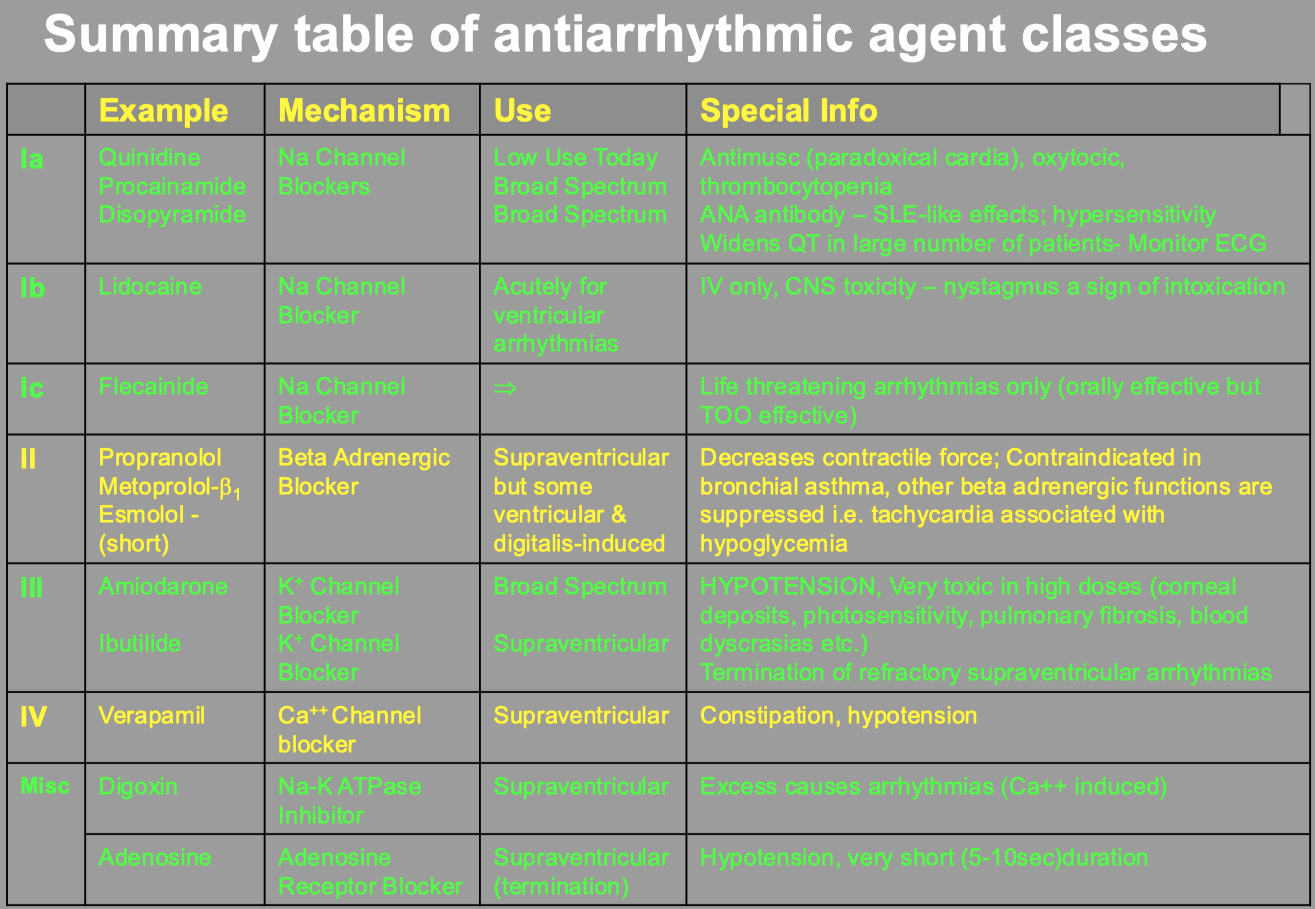
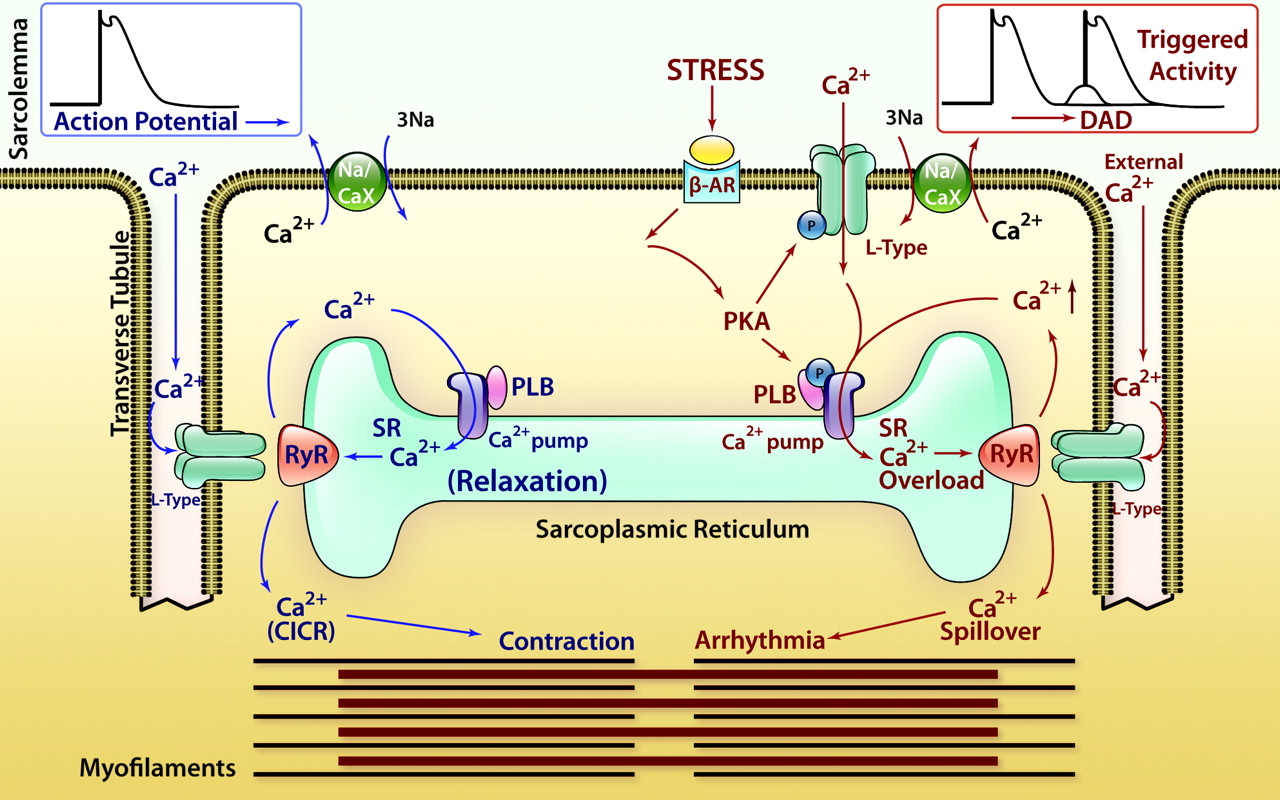
Figure 1. CICR, SOICR, and triggered arrhythmia. Left (in blue) depicts the mechanism of CICR, in which an action potential activates the voltage-dependent L-type Ca2+ channel, leading to a small Ca2+ influx. This Ca2+ entry opens the RyR2 channel in the SR, resulting in SR Ca2+ release and muscle contraction. Right (in red) denotes the mechanism of SOICR, in which spontaneous SR Ca2+ release or Ca2+ spillover occurs under conditions of SR Ca2+ overload caused, for example, by stress via the β-adrenergic receptor (b-AR)/PKA/phospholamban (PLB) signaling pathway. SOICR can activate the NCX, which, in turn, can lead to DADs and triggered activities.
Ladle
| Neurotransmitter | Receptor Type | Subtype Name | Other Names / Notes | Ions Conducted | Effect |
|---|---|---|---|---|---|
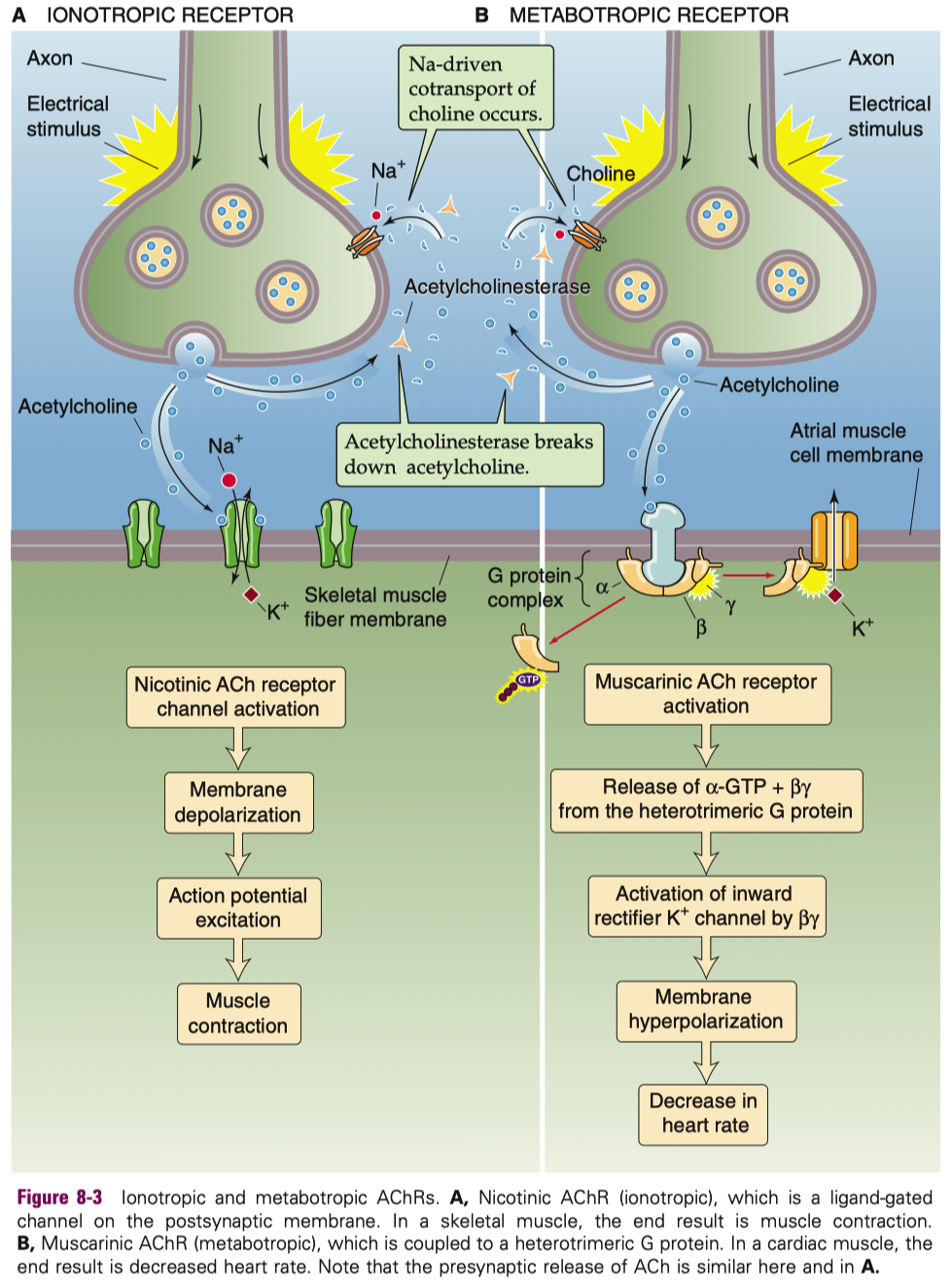
| Acetylcholine (ACh) | Ionotropic | Nicotinic | Ligand-gated ion channel | Na+ in , K+ out ( some also Ca2+ permeable ) | Excitatory |
| Metabotropic | Muscarinic | GPCR ( M1–M5 subtypes ) | — ( via second messengers ) | Usually excitatory ( M1 , M3 , M5 ) or inhibitory ( M2 , M4 ) depending on subtype | |
| Glutamate | Ionotropic | AMPA , Kainate | Ligand-gated ion channels | Na+ in, K+ out | Excitatory |
| Ionotropic | NMDA | Requires glycine co-agonist , voltage-gated Mg2+ block | Na+, Ca2+ in, K+ out | Strong excitatory , Ca2+-dependent signaling | |
| Metabotropic | mGluR ( types 1–8 ) | GPCRs | — ( via second messengers ) | Modulatory ( can be excitatory or inhibitory ) | |
| GABA | Ionotropic | GABAA | Ligand-gated Cl– channel | Cl– in ( hyperpolarizes ) | Inhibitory |
| Metabotropic | GABAB | GPCR activating K+ channels, inhibiting Ca2+ channels | ↑ K+ out , ↓ Ca2+ in ( via second messenger ) | Inhibitory | |
| Glycine | Ionotropic | Glycine receptor | Ligand-gated Cl– channel | Cl– in ( hyperpolarizes ) | Inhibitory |
| Metabotropic | — ( none ) | No known metabotropic receptor | — | — |
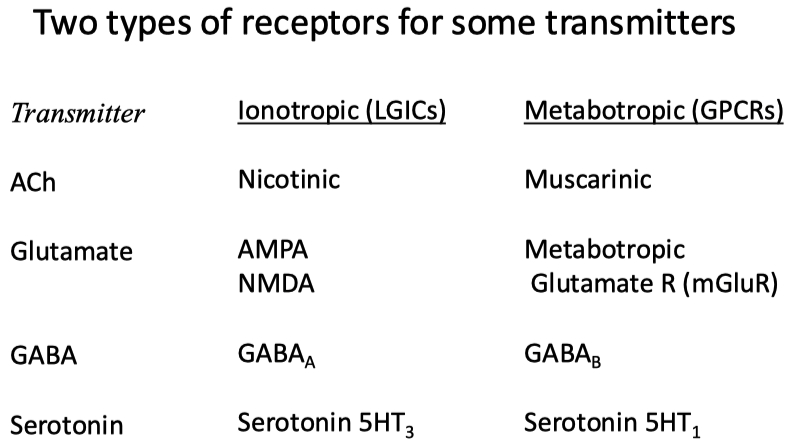
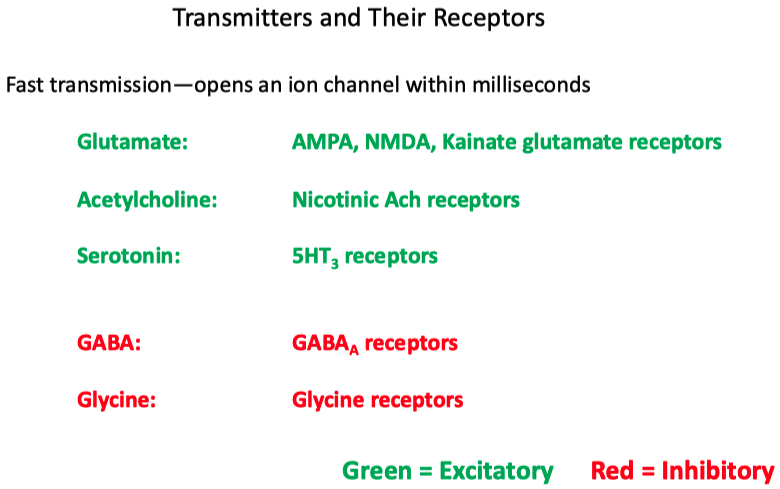
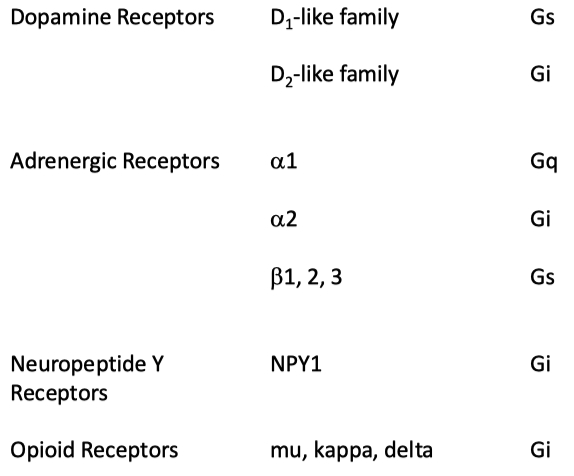
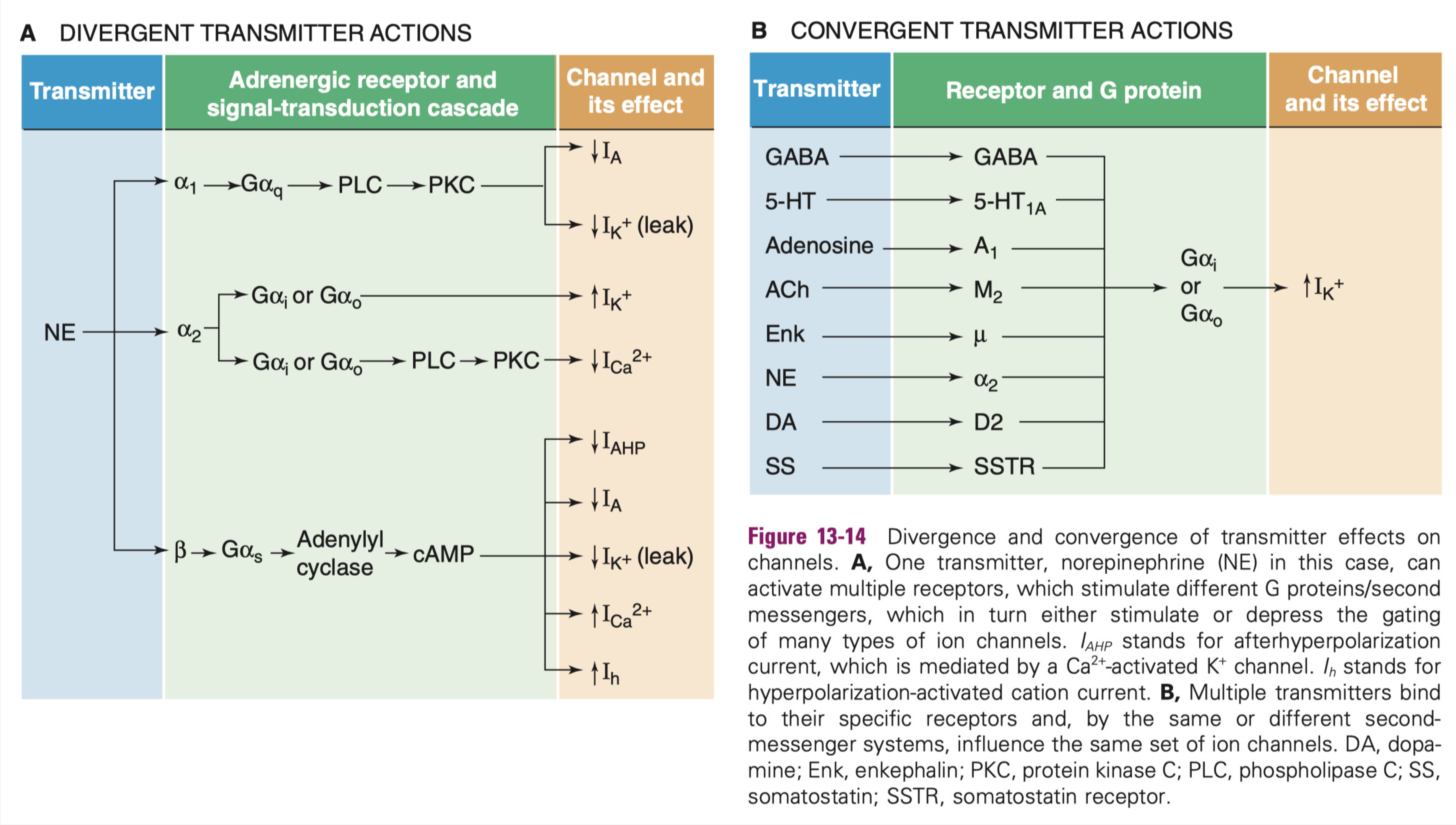
Dopamine for example can bind to both stimulatory and inhibitory GPCRs
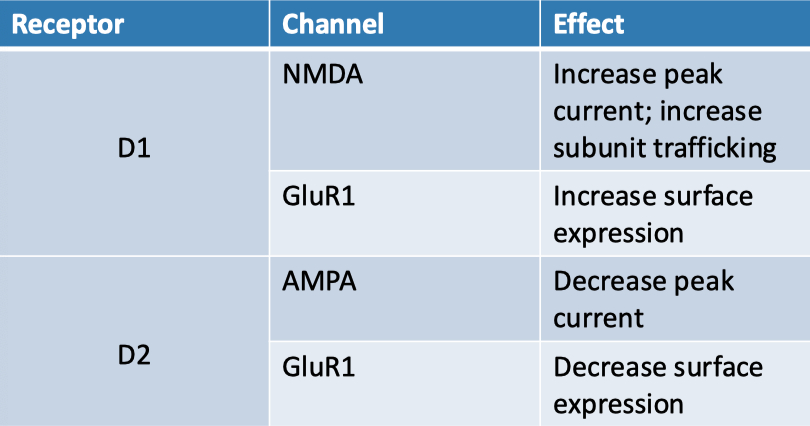
| Receptor | Kinetics | Calcium Permeable ? | Voltage Dependent ? |
|---|---|---|---|
| AMPA | Fast | No | No |
| NMDA | Slow | Yes | Yes ( via magnesium block ) |
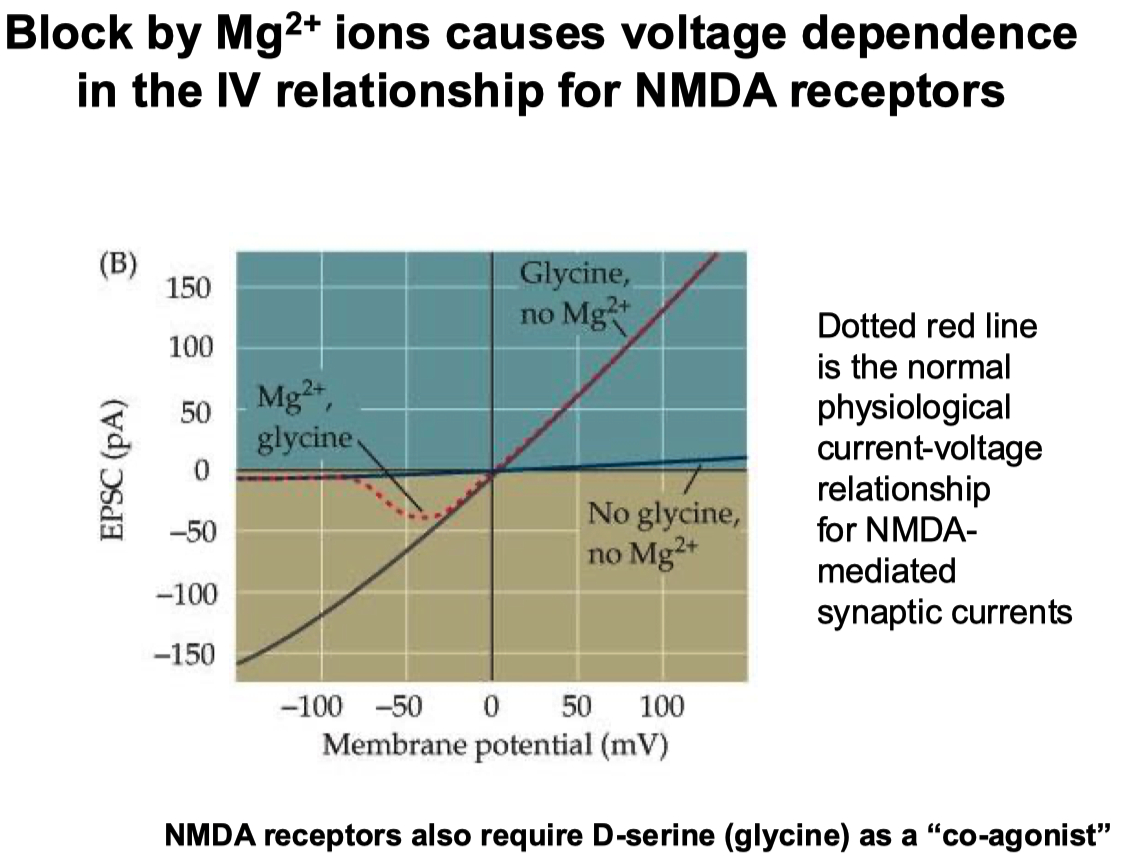
Extracellular magnesium is used to block / inactivate NMDA channels at negative potentials
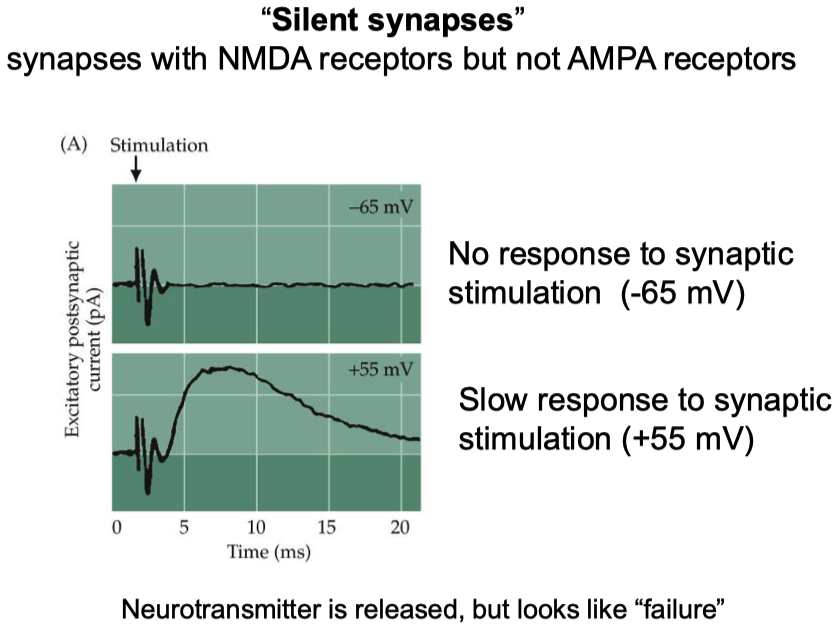
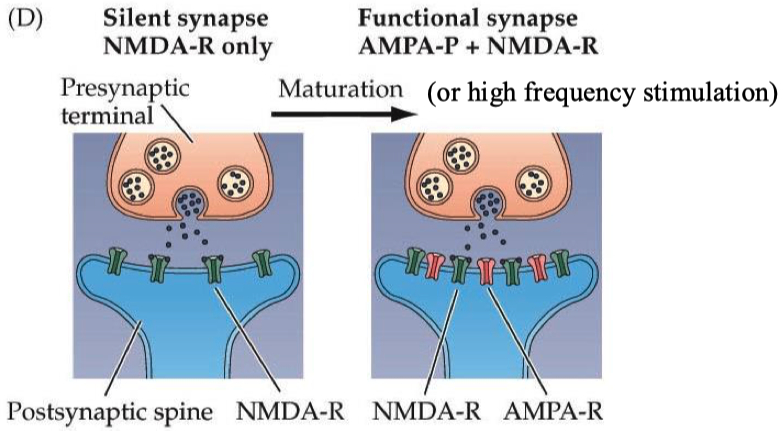
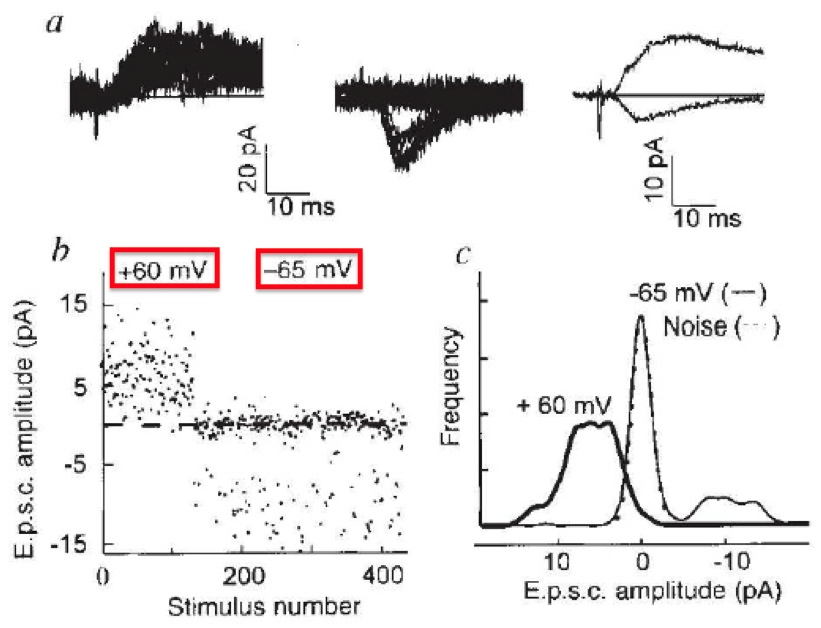
Engisch
Heuser Paper :
Hypothesis = synaptic vesicle exocytosis underlies quantal neurotransmitter release
one synaptic vesicle fusing with the membrane corresponds to one quantum of neurotransmitter release,
and these events occur independently at the presynaptic active zone
Conclusions :
Each vesicle undergoing exocytosis corresponds to a quantum of neurotransmitter release
Vesicle fusion events are independent, not cooperative
Quick-freezing successfully captures exocytosis events.
6At low levels of release, collapsed vesicles must be included to match quantal output
Evidence :
Freeze-fracture electron microscopy captured vesicles fusing with the membrane 5–6 ms after nerve stimulation, matching the timing of neurotransmitter release.
4-aminopyridine (4-AP) was used to increase quantal output, showing a dose-dependent increase in vesicle exocytosis events.
Quantitative correlation: The number of vesicles fusing matched the number of quanta released, across low to high 4-AP concentrations.
Spatial analysis showed vesicle openings occur precisely at active zones, not elsewhere, and are randomly distributed, supporting independent vesicle fusion.
At lower 4-AP doses, inclusion of collapsed vesicles (early fusion stages) improved correlation with physiological data.
No evidence for synchronized or cooperative fusion — each vesicle acts independently.
Together, this evidence strongly supports the hypothesis that synaptic vesicles fuse with the membrane to release neurotransmitter quanta, and that this can be directly visualized and quantified using high-resolution, time-locked quick-freezing techniques.
How to calculate

| Feature | Curare | 4-AP (4-Aminopyridine) |
|---|---|---|
| Mechanism | Blocks nicotinic ACh receptors at neuromuscular junction | Blocks voltage-gated K⁺ channels |
| Effect | Prevents muscle contraction (paralysis) | Enhances neurotransmitter release (prolongs action potential) |
| Site of Action | Postsynaptic side (muscle) | Presynaptic side (nerve terminal) |
| Main Result | Flaccid paralysis (no depolarization) | Increased ACh release, improves muscle strength |
| Use | Historically hunting (poison darts); research; anesthetic adjunct | Treatment for disorders like Lambert-Eaton myasthenic syndrome; MS symptoms |
| Overall | Inhibits neuromuscular transmission | Facilitates neuromuscular transmission |
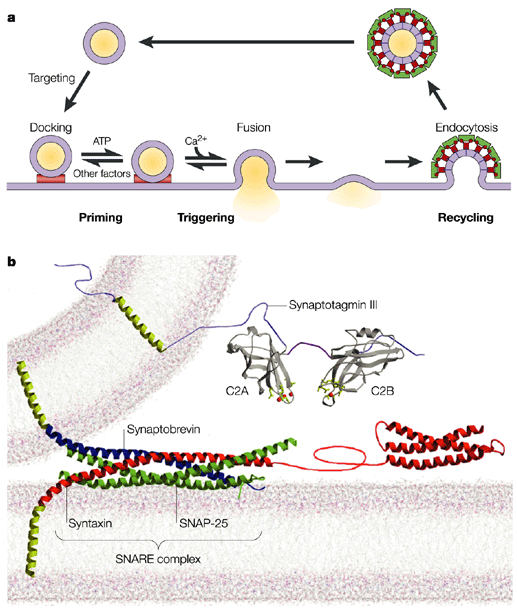
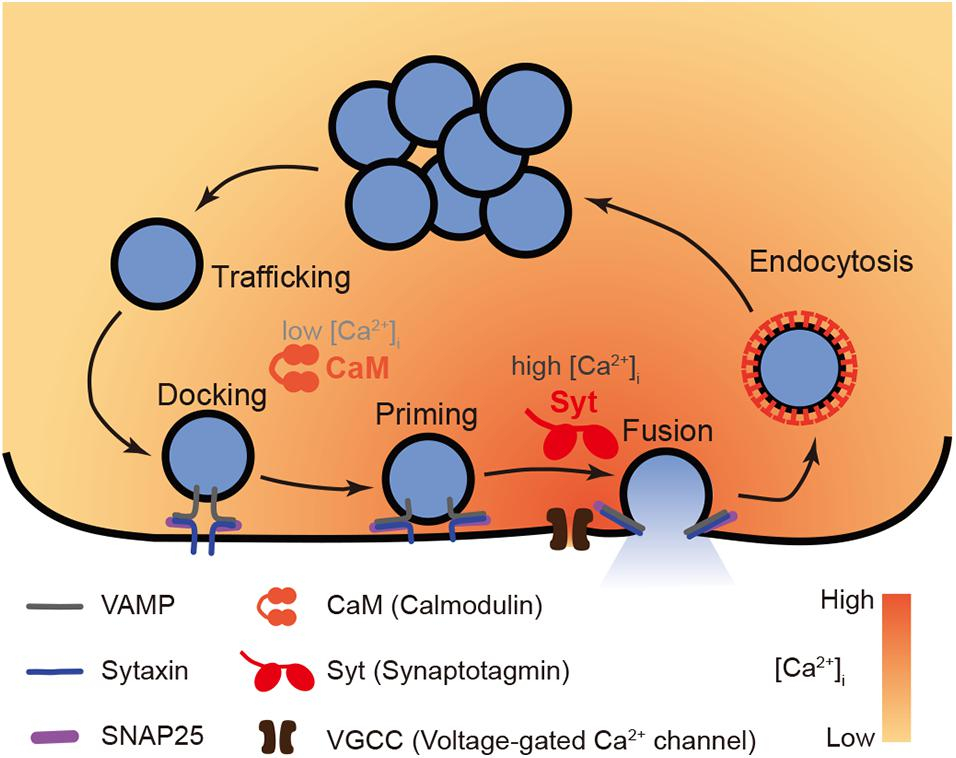
Compare and Contrast synaptotagmin vs synaptobrevin knockouts
| Feature | Synaptobrevin ( zipper ) KO (VAMP KO) | Synaptotagmin ( sensor ) KO |
|---|---|---|
| Evoked Amplitude | ↓↓↓ (almost abolished) | ↓↓↓ (almost abolished) |
| Evoked Frequency | ↓↓↓ | ↓↓↓ |
| Mini Amplitude | ↓ (reduced) | Increased |
| Mini Frequency | ↓↓↓ (very rare) | ↑ (increased spontaneous fusion) |
| Docking | ↓ (reduced docking, fewer primed vesicles) | Normal docking |
| Fusion | ↓↓↓ (both evoked and spontaneous fusion defective) | Ca²⁺-triggered fusion defective; spontaneous fusion increased |
Halm