Synaptic vesicles of cholinergic neurons are loaded with the neurotransmitter acetylcholine (
) The membrane of these synaptic vesicles contains an electrogenic
that pumps into the vesicle , together with -exchangers ( vAChT ) and -exchangers ( CLC ) The coupling stoichiometry of the vAChT is one ACh+ exchanged for
, and of the CLC is exchanged for , with the hydrolyzing for pumped These vesicles exhibit a membrane potential
, inside positive , and a pH more acidic than cytoplasm by units
Assume that the cytosol of the pre-synaptic terminal has the following properties :
You predict that the vesicular transporters constitute a secondarily active system maintaining a high vesicular
Draw a diagram of the synaptic vesicle that includes the transporters and indicates the relative rates for each that will achieve a steady-state
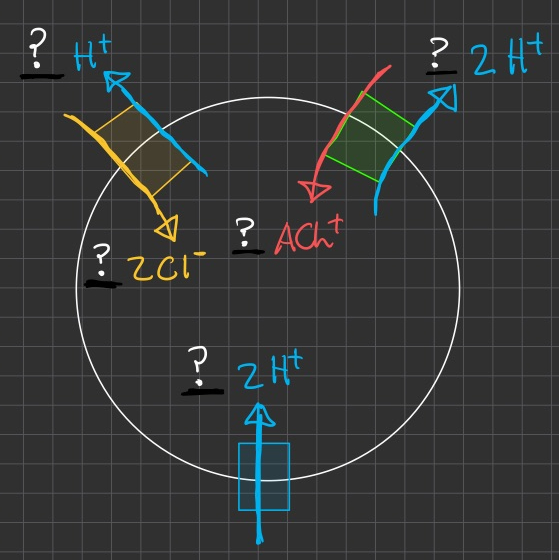
in order to know what "steady-state" looks like , we have to balance the stoichiometry numbers for all of the inputs and outputs
we don't need to worry about balancing ACh , because we want and expect it to accumulate in the synaptic vesicle
and since in our simplified model we don't have a way to export chloride , we want to then minimize the number of cycles that transporter
so lets just balance hydrogen :
at steady-state , we have more leaving ( 3 ) the cell than entering ( 2 )
if we run the ATPase
so then inputs = 3 , and outputs = 3
but we can't run the ATPase half-way , its all or none.
double
but now total hydrogen inputs = 6 , and total hydrogen outputs = 3
so make them also run an additional cycle
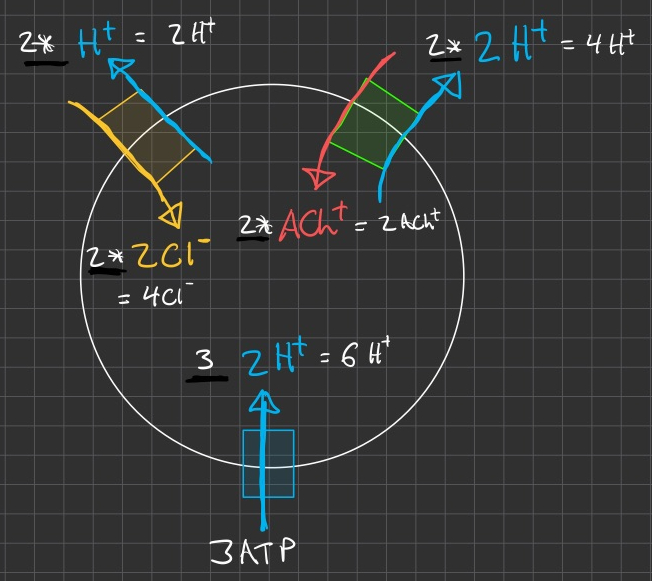
ok , but the charges are messed up and its not electrically neutral
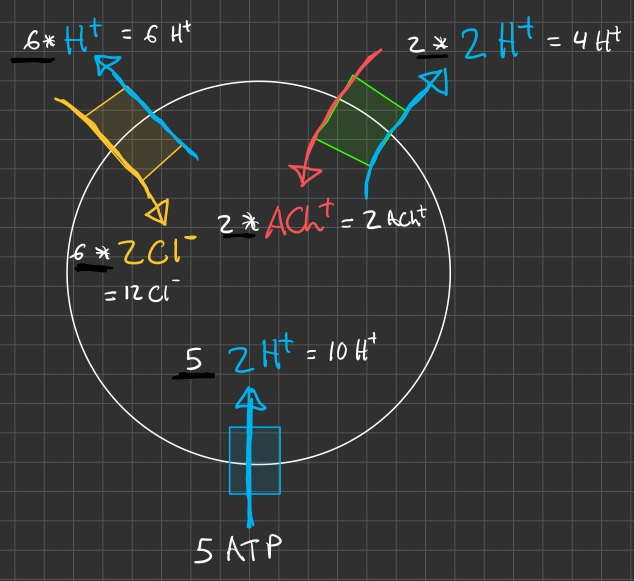
now
You decide to calculate how high the vesicular
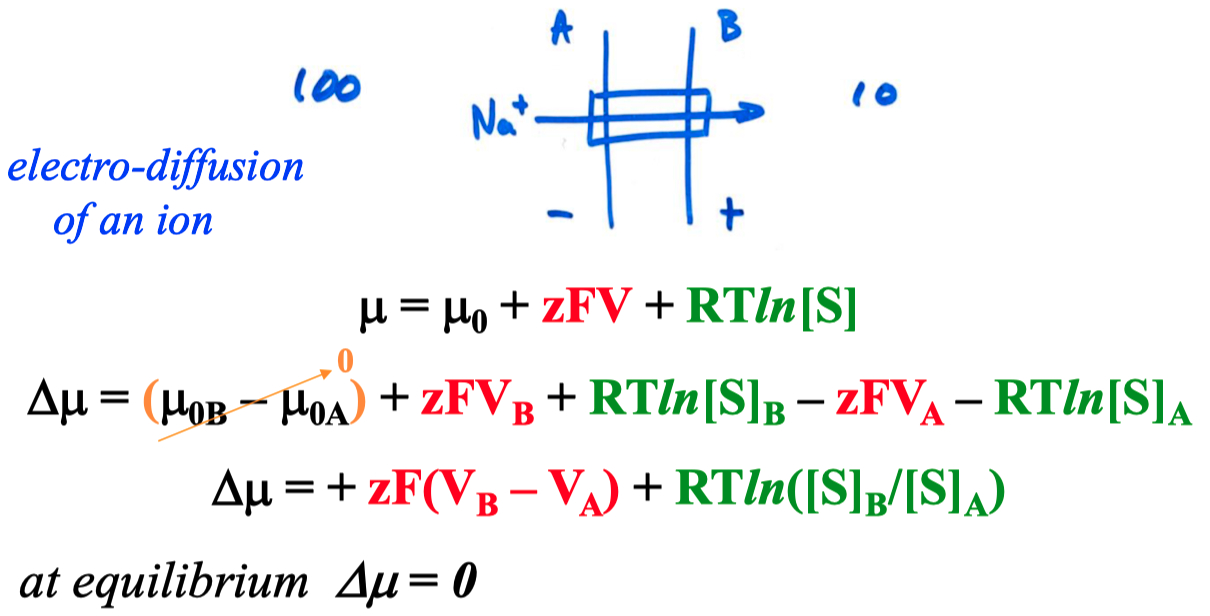
so we were given a pH difference instead of concentrations
and we also need the potential across the membrane (
we can simplify the natural log to :
ok , but
so , we have to flip the order of
being explicit :
finally solving for
xxxxxxxxxximport mathfrom decimal import Decimal as D( D('1') * D('96484') * D(-80.0 * 10**-3) ) + ( D('8.314') * D('311.15') * D('1.5') * D(math.log(10)) )but now we need
and its coupled to hydrogen in the vAChT transporter at 1 : 2 ratio
now we can plug this into the nersnt equation , and solve for the vesicular concentration of ACh :
Changing the membrane electrical potential difference (
) requires separation of charges between the inside and outside of the cell You predict that generating
for a large cell will require more charge separation than for a smaller cell You also are curious about the amount of charge separation needed compared with the total number of charges inside of each cell
Calculate how many charges you have to separate across the cell membrane to achieve
Membrane Specific Capacitance =
Surface Area :
back to capacitance :
total charge :
and finally , converting to number of elementary charges :
Calculate how many charges likely are inside of this cell. Assume a
volume of a sphere :
convert concentration of
now into total number of particles :
but KCl breaks apart into
For synaptic vesicles
surface area of the
capacitance :
charge :
number of charges :
now the same thing for
capacitance :
charge :
number of charges :
Calculate how many charges likely are inside of these synaptic vesicles
volume :
moles :
total particles :
times 2 for van't Hoff factor :
volume :
moles :
total particles :
times 2 for van't Hoff factor :
Review Questions
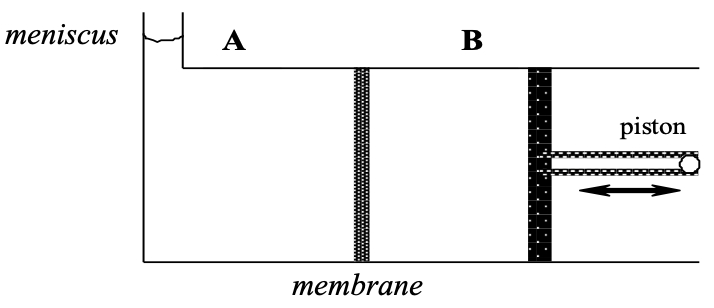
Consider a system with two compartments separated by a rigid membrane that is freely permeable to water but impermeant to sucrose.
Both compartments contain pure water and pressure is applied to the piston establishing a pressure difference ,
, across the membrane
Describe the relation between volume flow across the membrane and the hydrostatic pressure difference ,
No force is applied to the piston and 100mM sucrose is placed in compartment B.
In what direction will the meniscus ( in compartment A ) move?
asdf
What is the driving force for this volume flow?
asdf
Adding NaCl ( also impermeant ) to what compartment could prevent volume displacement?
asdf
What concentration of NaCl would have to be added to prevent volume displacement?
asdf
What concentration of MgCl2 would prevent volume displacement?
asdf
What pressure ( and orientation ) must be applied to the piston to prevent volume flow?
asdf
Consider the membrane permeable to glycerol , but less permeable to glycerol than to water
No force is applied to the piston and
glycerol is placed in compartment B
How will the meniscus in the capillary of A move?
asdf
How will the rate of movement compare with that observed when
asdf
What pressure ( and orientation ) must be applied to the piston to prevent volume flow?