1.1 The table below lists , for a particular cell type , the intracellular ( i ) and extracellular ( e ) concentrations for a number of solutes , X [ in mM ] , including monovalent and divalent ions.
The electrical potential difference across the membrane (
1.A : Enter your estimate of the Nernst potential ,
see table
1.B : Considering only the extracellular concentration [X]e , enter your prediction for the intracellular concentration [X]i of each solute, assuming a passive distribution across the membrane ( electrochemical equilibrium according to the measured
example using
xxxxxxxxxx104 / ( 10**( -0.06 / ( ( 8.314 * ( 29.5 + 274.15 ) ) / ( 1 * 96484 ) ) ) )1.C : Enter your predictions about the physiological cellular properties concerning these solutes, especially those that are not distributed at electrochemical equilibrium.
asdf
| Solute X | Predictions | ||||
|---|---|---|---|---|---|
| 104 | 7 | 1030.184 | 70.316 mV | ||
| 8 | 110 | 1571.055 | -68.298 mV | ||
| 0.01 | 1 | 385.658 | -60.0 mV | ||
| 10 | 5 | 0.0509 | -18.062 mV | ||
| 100 | 10 | 0.5092 | -60.0 mV | ||
| 2 | 2 | 0.01018 | 0 mV | ||
| Q | 4 | 4 | 0 mV | ||
| R | 1 | 3 | 0 mV |
Use T = 29.25 Celsius
1.2 : Prior to conducting a series of electrophysiological experiments on your favorite cell type, you consider the possible influences on the membrane electrical potential difference (
Before changing the bathing solution so that
1.2.i : Calculate your estimates for the values of
xxxxxxxxxx( 60 / 1 ) * math.log10( 145 / 10 )1.2.ii : From Ohm / Kirchhoff rules for membrane conductances , calculate
start by calculating each driving force
the driving force is negative , think of inside of the cell as negative
sodium is positive charged cation , it will be attracted electrically into the cell
the driving force is positive , think of inside of the cell as positive
potassium is positive charged cation , it will be repelled electrically out of the cell
We are assuming the cell is in steady-state , no net flow of ions across the membrane. So set all of the currents equal to zero.
write current equation for each
set equal to zero
solve for the ratio of
multiply both sides by
plug in values
( -1 * ( ( -64 ) - ( ( 60 / 1 ) * math.log10( 4.5 / 150 ) ) ) ) / ( ( ( -64 ) - ( ( 60 / 1 ) * math.log10( 145 / 10 ) ) ) )
since
if the result of the ratio were greater than
1.2.A : Calculate your prediction for
new nernst :
"change in
new driving force :
write balanced equation :
we use the same conductance ration as before , and solve for one of them , so we can simplify the equation :
replace
divide both sides of equation by
plug numbers in , solve for
*** also , instead of replacing
we also could find
aka , we let the numerator be the answer
which means , the denominator ,
therefore ,
now we just use the full parallel conductance equation :
1.2.B : List assumptions that might complicate your estimate.
steady-state with no net ionic current
conductances are constant
ignores other ions ( calcium , chloride )
1.2.C : You apply an experimental stimulus to the cell that quickly depolarizes
Calculate your estimate for gNa / gK during this period of depolarization
If the stimulus only altered
If the stimulus only altered
1.2.D : After you add a hormone to the bathing solution ,
assuming conductances stay constant after
then
let
if only
therefore ,
1.3 : Changing the membrane electrical potential difference (
1.3.A : You decide to calculate how many charges you have to separate across the cell membrane to achieve
compute surface area assuming sphere shape :
capacitance units is just Farads , but for some "specific membrane capacitance"
we are going to assume
capacitance is also
a Coulomb is also the total charge of around
so that was about
now to calculate cell volume ( assuming sphere ) :
convert
convert to moles of potassium ions :
convert to total ions :
xxxxxxxxxx( ( ( 4.0 / 3.0 ) * math.pi * ( ( 5.0 * 10**-6 )**3 ) ) * 1000 ) * ( 0.15 ) * ( 6.02214076 * 10**23 )
1.3.B : You decide to calculate how many charges you have to separate across the cell membrane to achieve
surface area :
capacitance , again assuming
charge needed to establish
converting to elementary charges :
xxxxxxxxxx( ( 4.0 * math.pi * ( 0.5 )**2 ) * ( 10**-2 ) * ( 10**-6 ) * ( 60 * 10**-3 ) ) / ( 1.602176634 * 10**-19 )
now the volume :
converting to Liters :
converting to moles using same concentration as before :
converting to total ions :
R1 : Consider two compartments of equal volume separated by a membrane ( shown below )
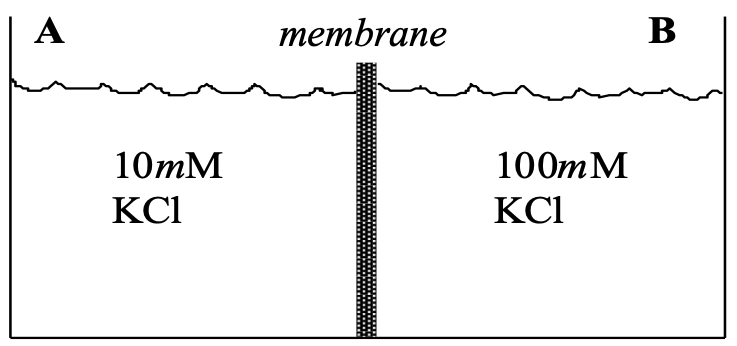
Evaluate the following issues for each permeability condition of the membrane :
Comparing side A with side B, predict the orientation of the electrical potential difference (ePD) across the membrane during the initial moments of flow (before noticeable changes in chemical composition have occurred)
Predict the magnitude of the ePD across the membrane during the initial moments of flow.
Predict the ePD and chemical composition of compartments A and B at electrochemical equilibrium.
Condition A : The membrane is equally permeable to both K+ and Cl-.
asdf
Condition B : The permeability of the membrane to K+ is made greater than that to Cl-
asdf
Condition C : The membrane is made impermeant to Cl-, but remains permeable to K+.
asdf
R2 : Consider two compartments of equal volume separated by a membrane (shown below).
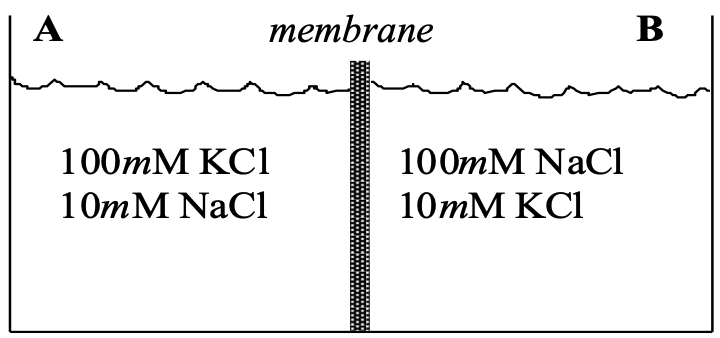
Evaluate the following issues for each permeability condition of the membrane :
Comparing side A with side B, predict the orientation of the electrical potential difference (ePD) across the membrane during the initial moments of flow (before noticeable changes in chemical composition have occurred)
Predict the magnitude of the ePD across the membrane during the initial moments of flow.
Predict the ePD and chemical composition of compartments A and B at electrochemical equilibrium.
Condition D : The membrane is permeable to K+, but not to Na+ or anions.
asdf
Condition E : The membrane becomes permeable only to Na+
asdf
Also, compare these outcomes with those for condition D.
asdf
Condition F : The membrane becomes permeable to both Na+ and K+ , with Na+ permeability greater than K+ permeability
asdf
Also, indicate the factors that the magnitude of the ePD depend on.
asdf